Abstract
Purpose
In an effort to examine the clinicopathological characteristics of GC and the status of its surgical treatment, the Korean Gastric Cancer Association (KGCA) conducted a nationwide survey targeting surgically-treated gastric cancer patients in 2009.
Materials and Methods
A standardized electrical case report was sent to every member institution of the KGCA via E-mail with detailed instructions regarding the survey data. Completed data forms were retrieved from each institution and analyzed by the KGCA information committee.
Results
Data on 14,658 patients was collected from 59 institutions. The mean patient age was 59.2±11.9 years with a male to female ratio of 2.05 : 1. Lower third cancer (56.0%) was the most common among all gastric cancers. The histological type revealed poorly differentiated adenocarcinoma (34.1%) to be the most common, and the Lauren classification revealed the intestinal type (50.0%) to be the most prevalent. Curative surgery was performed in 92.4% of patients with laparoscopic surgery in 25.8% of patients. A Billroth I reconstruction was performed most frequently after a distal gastrectomy (63.4% of distal gastrectomy). T1 cancers accounted for 57.6% of all cases, and 62.6% of patients showed no lymph node metastasis. Compared to previous reports, it was found that patients are becoming older, laparoscopic surgery is being performed increasingly, and the proportion of T1 cancer is increasing with time.
Conclusions
This survey presented the clinicopathological characteristics and current status of the surgical treatment of gastric cancer in Korea. This survey is expected aid research studies as well as planning and evaluation programs targeting cancer control.
Keywords: Stomach neoplasms, Data collection, Republic of Korea, Demography, Surgical procedures
Introduction
Despite the decreasing global incidence, gastric cancer (GC) remains one of the most common forms of malignancy around the world.(1) GC is now the fourth most common malignancy after lung, breast, and colorectal cancer, and the second leading cause of cancer death worldwide. Furthermore, age-standardized incidence rates are twice as high for men.(2) GC is the fourth most common male cancer after lung, prostate, and colorectal cancer, and the fifth most common female cancer after breast, uterine cervix, colorectum, and lung cancer. The geographical distribution of GC is characterized by wide international variations. More than 70% of cases occur in developing countries, and half of the world's case total occurs in Eastern Asia.(2)
In South Korea, GC is now the most prevalent malignant neoplasm and annually affects over 25,000 patients. In addition, it is the second leading cause of cancer death after lung cancer, and is responsible for over 10,000 deaths per year.(3) Despite the publication of numerous single institutional experiences of GC, relatively little nationwide data is available that describes the demographic and clinicopathological features of GC in Korea. Accurate statistics based on nationwide data are essential both for research and for the planning and evaluation of cancer control programs. In an effort to investigate the clinicopathological characteristics of GC and the status of its surgical treatment, the Korean Gastric Cancer Association (KGCA) has conducted a nationwide survey targeting surgically treated gastric cancer patients every five years since 1999. The latest 2004 nationwide survey results were published in 2007 after the first report was published in 2002.(4,5) Here, we present the results of a nationwide survey of 14,658 gastric cancer patients who were surgically treated for gastric cancer in 2009.
Materials and Methods
1. Survey data
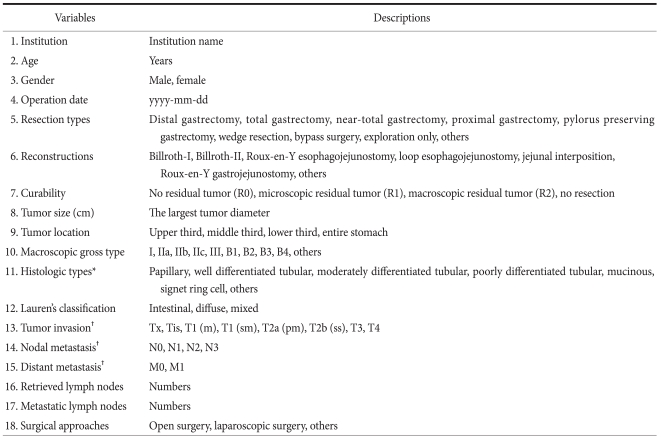
In 1999, KGCA started a nationwide, hospital-based survey targeting patients treated surgically for GC. This survey requested patients' information regarding demographic and clinicopathological features and the surgical treatment administered at voluntarily participating institutions. Patients that received treatments other than surgery, such as, endoscopic resection or systemic chemotherapy, were not included. Survey data included age, gender, resection type, reconstruction method, curability, surgical approach, tumor size, tumor location, macroscopic type, histologic type, Lauren's classification, tumor depth, presence of distant metastasis, and numbers of metastatic and harvested lymph nodes (Appendix 1). Histopathological characteristics are described according to the guidelines issued by the Korean Gastric Cancer Association.(6) For example, tumor locations were described as lower, middle, upper third, or whole stomach, and when a large tumor occupied more than two stomach regions, primary tumor site location was defined as the tumor center. Macroscopic gross types were classified as types I, IIa, IIb, IIc, or III for early gastric cancers, and as B1, B2, B3, or B4 for advanced gastric cancers. For combined superficial macroscopic types, the type occupying the largest area was recorded as the primary macroscopic type. Histologic types were classified as papillary adenocarcinoma, well differentiated, moderately differentiated and poorly differentiated tubular adenocarcinoma, signet ring cell, and mucinous adenocarcinoma. Pathological staging, including tumor (T), node (N), and metastasis (M), was based on the sixth edition of the UICC TNM classification.(7) Surgical approaches were classified as either open or laparoscopic, and robotic assisted surgery was considered as laparoscopic surgery.
2. Data collection and processing
A standardized electrical case report form prepared using spread sheet program (Microsoft Excel®) was sent to every member institution of the KGCA via E-mail with detailed instructions regarding survey data. Completed data forms were retrieved from each institution and analyzed by the KGCA information committee. Retrieved data was reexamined for its correctness, for example, correct codes, and underwent a data validation process at the KGCA data center. During this process, if any question was raised about the data, an appropriate query was forwarded with the original data to the institution concerned. Data received on non-standard forms were converted into standardized format coded data. Missing values were either treated as 'unknowns' during the analysis of categorical data or were excluded from the analysis of continuous data. From February to April 2010, the KGCA information committee collected data from 59 institutions on 14,658 patients who had undergone surgery for GC during 2009.
3. Statistical analysis
Continuous data are presented as means and standard deviations, and were analyzed using the student's t-test. The chi-square test was used to analyze categorical data. Statistical analyses were performed using SPSS for Windows ver. 12.0 (SPSS Inc., Chicago, IL, USA), and P-value of <0.05 were regarded statistically significant.
Results
1. Institutions and patients
Fifty-nine institutions participated in the survey and data was collected on 14,658 patients, which represents a significant increase compared to the 29 institutions and 5,380 patients in the first survey conducted in 1999. Of the 59 institutions, 23 institutions performed fewer than 100 operations, 18 institutions performed 100 to 200 operations, and 12 institutions 200 to 500 operations. Six institutions performed more than 500 operations, and 44.8% of all GC surgeries were conducted at these six institutions in 2009.
2. Age and sex
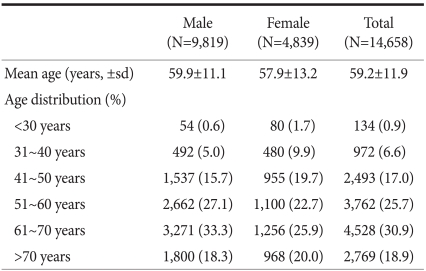
Table 1 shows patients' age and sex, and the distribution of patients according to age groups. There were 9,819 males and 4,839 females with a mean age of 59.2±11.9 years. Male patients were significantly older than female patients (59.9 vs. 57.9 years, P<0.001). For both sexes, the most common age was the sixth decade, which accounted for 30.9% of all cases, followed by the fifth decade (25.7%). Patients' mean age has continued to increase for both sexes since the first survey conducted in 1995, and consequently the proportion of patients aged >70 significantly increased from 9.1% in 1995 to 18.9% in 2009.(5)
Table 1.
Age and sex
The overall incidence of GC was as twice as high among men than women. However, when gender ratios were examined in age subgroups, the incidence of GC was found to be higher in women than in men in patient group aged <30 (male to female ratio of 1 : 1.5). The proportion of male patients gradually increased with age and peaked in the sixth decade (male to female ratio of 2.6 : 1 in the sixth decade) (Table 1).
3. Histopathological characteristics
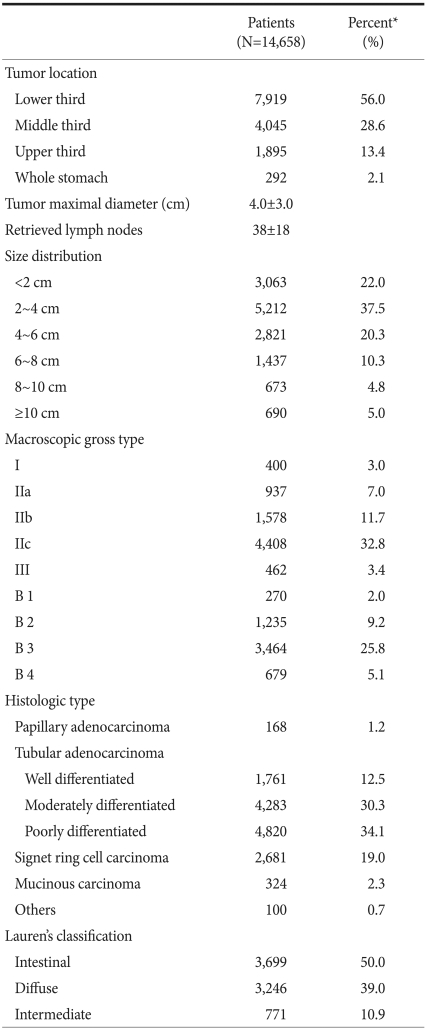
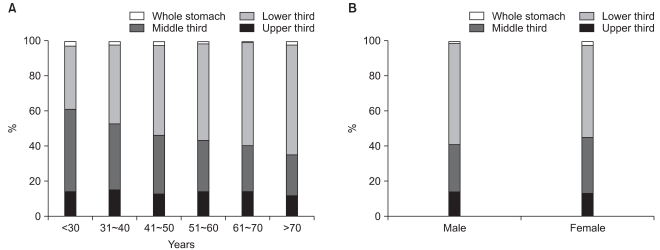
Table 2 summarizes the histopathological characteristics of GCs. Tumors located in the lower third of the stomach were the most common and accounted for 56.0% of all cases. The percentages of tumors of the middle, upper, and whole stomach were 28.6%, 13.4%, and 2.1%, respectively. When tumor location was investigated in age and sex subgroups, middle third cancers were found to be more common in younger patients and to decrease gradually with age from 46.7% in patients aged <30 to 23.3% in patients aged >70 (Fig. 1). On the other hand, lower third cancers showed the opposite tendency and increased with age from 35.8% in patients aged <30 to 62.4% in patients aged >70. No significant difference was observed between men and women in terms of the distribution of tumor locations.
Table 2.
Histopathological characteristics of gastric cancer
*Unknown or missing values were excluded from the calculation of percentages.
Fig. 1.
Tumor location stratified by age (A) and sex (B). The proportion of middle third cancers gradually decreased with age, while that of lower third cancers increased (A). No significant differences were found between men and women in terms of tumor location (B).
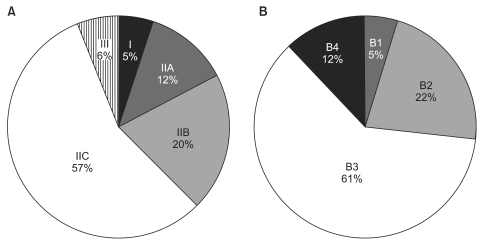
Mean tumor diameter was 4.0±3.0 cm, and tumors with diameters between 2 and 4 cm were most common. The mean number of retrieved lymph nodes per patient was 38±18. As for macroscopic type, Type IIc (56.6%) was the most prevalent gross type among early gastric cancers, followed by type IIb (20.3%). For advanced gastric cancers, B3 (61.3%) type was the most common gross type, followed by B2 type (21.9%) (Fig. 2).
Fig. 2.
Macroscopic gross type in EGC (A) and in AGC (B). EGC = early gastric cancer; AGC = advanced gastric cancer.
Histologic classifications showed poorly differentiated adenocarcinoma was the most common histologic type and that it accounted for 34.1% of all cases. Lauren's classification showed that the intestinal, diffuse and mixed types accounted for 50.0%, 39.0% and 10.9%, respectively. When histologic type was compared between young and elderly patients (aged <40 vs. ≥40 years), the undifferentiated type (poorly differentiated tubular adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma) were found to be significantly more common in young than in elderly patients (86.1% vs. 53.9%, P<0.001). Diffuse type carcinomas were also significantly more common in young patients (70.5% vs. 37.0%, P<0.001).
4. Treatment details
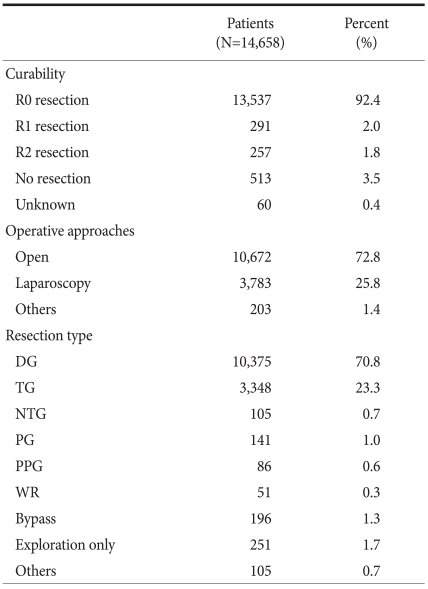
Table 3 presents a summary of the operative procedures performed. Of the 14,658 patients, 10,672 (72.8%) underwent open surgery and 3,783 (25.8%) laparoscopic surgery. Curative surgery was performed in 92.4% of patients. The number of laparoscopic surgeries showed a marked increase as compared with the 740 cases (6.6% of all gastric cancer surgeries) encountered in 2004.(5) As for resection types, 10,375 (70.8%) patients underwent distal gastrectomy and 3,248 (23.3%) total gastrectomy. Near total gastrectomy, proximal gastrectomy, pylorus preserving gastrectomy, and wedge resection were performed in 105 (0.7%), 141 (1.0%), 86 (0.6%), and 51 (0.3%) patients, respectively.
Table 3.
Details of operative procedures
DG = distal gastrectomy; TG = total gastrectomy; NTG = near total gastrectomy; PG = proximal gastrectomy; PPG = pylorus preserving gastrectomy; WR = wedge resection.
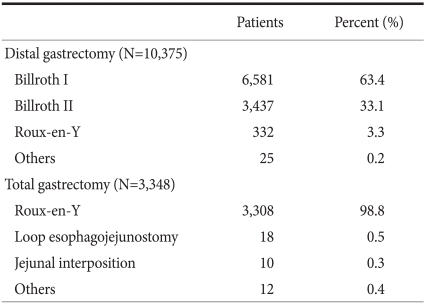
Table 4 details the reconstruction methods used after distal and total gastrectomy. The Billroth I procedure was most frequently performed after distal gastrectomy (in 63.4%), followed by Billroth II (33.1%). Roux-en-Y gastrojejunostomy was only performed in 3.3% of patients after distal gastrectomy. The most common reconstruction procedure used after total gastrectomy was Roux-en-Y esophagojejunostomy, which was performed in 98.8% (3,308/3,348) of all total gastrectomy patients. Loop-esophagojejunostomy and jejunal interposition were performed in only in 18 (0.5%) and 10 (0.3%) patients, respectively, after total gastrectomy.
Table 4.
Reconstruction methods
5. Tumor stage
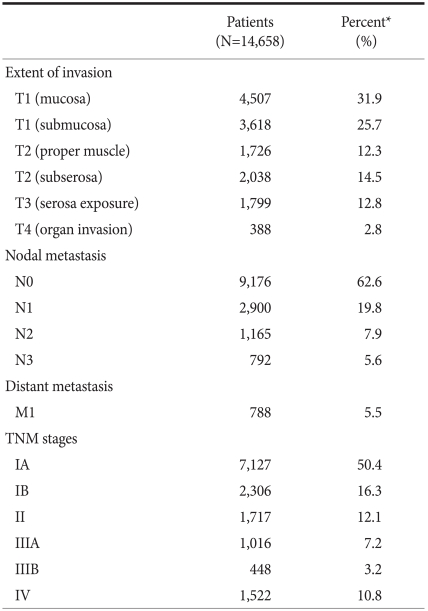
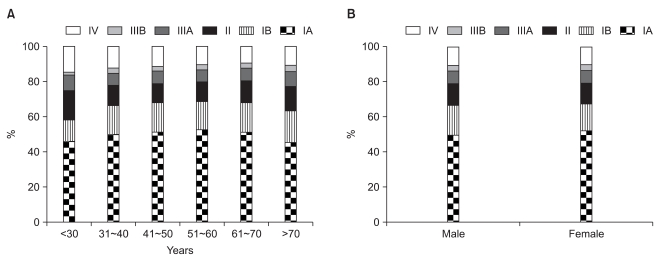
Tumor stage, including tumor (T), node (N), and metastasis (M) stages, is illustrated in Table 5. As for the tumor depth, T1 cancers were the most common and accounted for 57.6% of cases, followed by T2 (26.8%), T3 (12.8%), and T4 (2.8%). N stages showed N0, N1, N2, and N3 in 62.6%, 19.8%, 7.9% and 5.6% of patients, respectively. In the final TNM stages, there were IA, IB, II, IIIA, IIIB, and IV in 50.4%, 16.3%, 12.1%, 7.2%, 3.2%, and 10.8% of patients, respectively. Analysis of TNM stages by age or sex revealed no significant differences between the groups (Fig. 3).
Table 5.
Gastric cancer stages
Tumor (T), node (N), and metastasis (M) were defined as described in the sixth edition of UICC TNM classification of gastric carcinoma. *Unknown or missing values were excluded from the calculation of percentages.
Fig. 3.
TNM stage according to age (A) and sex (B). TNM stage distributions were independent of age and sex.
When tumor stages in the serial cohorts of GC patients were compared, the proportion of T1 cancers was found to have increased from 28.6% in 1995 to 57.7% in 2009. Similarly, the proportion of node negative cancers was also found to have increased from 49.3% in 1995 to 62.6% in 2009. Consequently, while the proportions of stage IA and IB patients has increased with time, the proportion of stage II or more patients has decreased.
Discussion
GC is the most prevalent malignant neoplasm in South Korea and now accounts for about 16% of all cancer sites. According to the latest Korean cancer statistics, as of 2007, GC is the most common male cancer and the third most common female cancer after breast and thyroid cancer. Furthermore, GC is the third leading cause of cancer death after lung and liver cancer in men and the second leading cause of cancer death after lung cancer in women.(3) Despite the continuing decline in the incidence of GC in the West, the incidence of GC is higher in South Korea and Japan than in any other country, and this has remained unchanged over the past decade or more.(8) On the other hand, mortality due to GC continues to decrease in Korea, for example, the age-standardized mortality rate of GC decreased from 63 for men and 29 for women in the early 1980's to 25 for men and 9 for women in 2007.(9)
As has been widely reported, GC patients are getting older and the proportion of elderly patients continues to increase in South Korea.(10,11) A similar trend has also been observed in Japan.(12) Our report showed that the mean age of GC patients was 59.2 years and the proportion of patients aged >70 was as high as 19% in 2009, which is a substantial increase over the mean age of 55.5 years and the proportion of patients aged >70 of 9% reported in 1995.(4) However, the mean age of GC patients in South Korea and Japan is still younger than that reported in the West, where the incidence of gastric cancer is much lower and nationwide screening program for GC is not performed.(13) Surgical treatment of elderly patients with GC requires comprehensive perioperative care, including the proper management of coexistent medical illnesses, which is essential to achieve optimal surgical outcomes. However, advances in operative techniques and perioperative care mean that chronologic age alone is no longer regarded as a crucial determinant on deciding the surgical treatment.(14)
The introduction of a national screening program in Korea for gastric cancer has resulted in the diagnosis of many gastric cancer patients with early stage disease.(15) In the present survey, the proportion of patients with early gastric cancer (EGC) was found to have markedly increased from 28.6% in 1995 to 57.7% in 2009. In addition to advances in treatment techniques, this early detection is believed to be primarily responsible for marked improvement in survival of GC in Korea and Japan.(16) With the excellent long term survival of EGC, treatment strategy for EGC is now moving to the direction of enhancing functional outcomes and postoperative quality of life.(17) For example, laparoscopic surgery, function-preserving surgery, and endoscopic resection provide good alternatives to radical surgery for the treatment of EGC. In a recent randomized trial, it was shown that laparoscopic surgery results in a better postoperative quality of life than open surgery in addition to short term benefits during the immediate postoperative period.(18) Endoscopic resection is currently widely used to treat mucosal cancers without lymph node metastasis in Korea and Japan.(19) However, accurate preoperative diagnosis of tumor invasion extent and lymph node metastasis remain pivotal problems that must be adequately resolved to enable proper individualized treatments to be chosen for EGC patients.
In the present study, tumors located in the lower third were most common, and accounted for more than 50% of cases, whereas the proportion of upper third cancers was the lowest at 13.4%. Contrary to the increasing incidence of distal esophageal adenocarcinoma and gastric cardia cancer in the West, proximal GCs, including gastroesophageal junction (GEJ) cancers, are uncommon in Korea, where most gastric cancers are located in the distal stomach.(20) Although the incidence of proximal GCs in 2009 was 2.2% higher than in 1995, it is unclear whether upper GCs are truly increasing in Korea. In the literature, some authors have concluded that proximal GC shows an increasing trend in Korea,(10,11) but others disagree.(21)
Some researchers have demonstrated clinicopathological differences of GC between young and old patients. In the present study, younger patients (<40) contained larger proportions of females, middle third cancers, and undifferentiated and diffuse type carcinomas as compared with older patients (>40). These age-related clinicopathological differences appear to sound because of the consistency of the reports on the subject.(22,23) However, the causes of these differences are unclear, and investigations of these difference at the epidemiologic and molecular level are required to enhance our understanding of gastric carcinogenesis.
The standard surgery for gastric carcinoma is radical gastrectomy with regional lymph node dissection, although the extent of lymph node dissection continues to be the subject of long standing debate. In Korea and Japan, D2 lymph node dissection is regarded as a standard surgery,(24) but the best form of reconstruction after distal gastrectomy has remained the subject of debate. Our survey showed that the most commonly performed reconstruction after distal gastrectomy is Billroth I reconstruction in Korea, which is probably because it is a simple and safe method to be performed using mechanical staplers. Gastrojejunostomy is often adopted when gastroduodenostomy is not technically feasible due concerns of anastomosis failure or the need for wide stomach resection. In Korea, Billroth II reconstruction is most widely used for gastrojejunostomy, and Roux-en Y reconstruction is seldom performed. The best reconstruction method after distal gastrectomy remains to be determined because most studies on reconstruction after gastrectomy have been small or non-randomized.(25) A large multicenter trial is required to compare the different reconstruction options after gastrectomy in terms of oncologic and physiologic functions.
Summarizing, this survey describes the clinicopathological features and surgical treatment of GC in Korea. Our survey showed that the number of elderly patients with GC has increased significantly, as has the proportion of patient with EGC. Furthermore, laparoscopic surgery is being more widely used for the surgical treatment of EGC. The present study also presents the histopathological characteristics of GC in Korea, which can be compared with those of other countries. Finally, we hope that this survey will aid research studies and planning and evaluation programs that target cancer control.
The Information Committee of the Korean Gastric Cancer Association
Information committee members are as follows: Oh Jeong, Young-Kyu Park (Chonnam National University Hwasun Hospital, Hwasun, Korea), Jun Ho Lee (National Cancer Center), Ho Young Jeong (Kyungpook National University), Jin Jo Kim (Catholic University Incheon St. Mary's Hospital), Do Joong Park (Seoul University Bundang Hospital), Kab Joong Kim (Asan Medical Center).
Participating Institutions
The participating institutions are as follows: The Catholic University of Korea, Daejeon St. Mary's Hospital; The Catholic University of Korea, St. Vincent's Hospital; The Catholic University of Korea, Incheon St. Mary's Hospital; The Catholic University of Korea, Seoul St. Mary's Hospital; The Catholic University of Korea, Yeoido St. Mary's Hospital; Konkuk University Medical Center; Konyang University Hospital; Kyungpook National University Hospital; Gyeongsang National University Hospital; Kyunghee University Medical Center; Keimyung University Dongsan Hospital; Korea University Guro Hospital; Korea University Ansan Hospital; Kosin University Gospel Hospital; National Cancer Center; National Medical Center; Daegu Catholic University Medical Center; Daegu Fatima Hospital; Saptist Hospital: Pusan National University Hospital; Pusan National University Yangsan Hospital; Pusan St. Mary's Hospital; Busan Medical Center; Seoul National University Bundang Hospital; Daejin Medical Center; Bundang Cha Hospital; Samsung Medical Center; Seoul National University Hospital; Seoul National University Boramae Medical Center; Asan Medical Center; Seoul Medical Center; Handong University Sunlin Hospital; Saegyero Hospital; Soonchunhyang University Pucheon Hospital; Soonchunhyang University Cheonan Hospital; Ajou University Medical Center; Yeonsei University Severance Hospital; Yeonsei University Gangnam Severance Hospital; Wonju Christian Hospital; Yeungnam University Medical Center; Presbyterian Medical Center; Dongkang Medical Center; Wonkang University Hospital; Korean Cancer Center Hospital; Ewha Woman's University Mokdong Hospital; Inje University Dongnae Paik Hospital; Chonnam National University Hospital; Chonnam National University Hwasun Hospital; Chonbuk National University Hospital; Jeju National University Hospital; Chosun University Hospital; Chungang University Hospital; Chungang University Yongsan Hospital; Chungnam National University Hospital; Chungbuk National University Hospital; Hallym University Medical Center; Hallym University Chuncheon Medical Center; Hallym University Hangang Medical Center; Hanyang University Hospital.
Appendix 1
Survey data
*WHO histologic classification of gastric cancer; †Based on the sixth edition of the UICC TNM classification of gastric carcinoma
References
- 1.Parkin DM, Ferlay J, Curado MP, Bray F, Edwards B, Shin HR, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127:2918–2927. doi: 10.1002/ijc.25517. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Jung KW, Park S, Kong HJ, Won YJ, Boo YK, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1321. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korea Gastric Cancer Association. Nationwide Gastric Cancer Report in Korea. J Korean Gastric Cancer Assoc. 2002;2:105–114. [Google Scholar]
- 5.The Information Committee of the Korean Gastric Cancer Association. 2004 Nationwide Gastric Cancer Report in Korea. J Korean Gastric Cancer Assoc. 2007;7:47–54. [Google Scholar]
- 6. http://www.kgca-i.or.kr/ [Accessed January 1, 2011].
- 7.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours (UICC) 6th ed. New York: Wiley-Liss; 2002. [Google Scholar]
- 8.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 9. http://www.cancer.go.kr/ [Accessed Jan 1, 2011].
- 10.Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36–41. doi: 10.1016/j.ejso.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Ahn HS, Lee HJ, Yoo MW, Jeong SH, Park DJ, Kim HH, et al. Changes in clinicopathological features and survival after gastrectomy for gastric cancer over a 20-year period. Br J Surg. 2011;98:255–260. doi: 10.1002/bjs.7310. [DOI] [PubMed] [Google Scholar]
- 12.Maehara Y, Kakeji Y, Oda S, Takahashi I, Akazawa K, Sugimachi K. Time trends of surgical treatment and the prognosis for Japanese patients with gastric cancer. Br J Cancer. 2000;83:986–991. doi: 10.1054/bjoc.2000.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borch K, Jönsson B, Tarpila E, Franzén T, Berglund J, Kullman E, et al. Changing pattern of histological type, location, stage and outcome of surgical treatment of gastric carcinoma. Br J Surg. 2000;87:618–626. doi: 10.1046/j.1365-2168.2000.01425.x. [DOI] [PubMed] [Google Scholar]
- 14.Jeong O, Park YK, Ryu SY, Kim YJ. Effect of age on surgical outcomes of extended gastrectomy with D2 lymph node dissection in gastric carcinoma: prospective cohort study. Ann Surg Oncol. 2010;17:1589–1596. doi: 10.1245/s10434-010-0916-4. [DOI] [PubMed] [Google Scholar]
- 15.Choi IJ. Gastric cancer screening and diagnosis. Korean J Gastroenterol. 2009;54:67–76. doi: 10.4166/kjg.2009.54.2.67. [DOI] [PubMed] [Google Scholar]
- 16.Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243–255. doi: 10.1053/ctrv.2000.0164. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333–339. doi: 10.1159/000065829. [DOI] [PubMed] [Google Scholar]
- 18.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 19.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 20.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30:1415–1425. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Kim HY, Kim KH, Jang HJ, Kim JB, Lee JH, et al. No changing trends in incidence of gastric cardia cancer in Korea. J Korean Med Sci. 2003;18:53–57. doi: 10.3346/jkms.2003.18.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguchi T, Takahashi Y, Yamagata M, Kasahara M, Fujii M. Gastric cancer in young patients. J Am Coll Surg. 1999;188:22–26. doi: 10.1016/s1072-7515(98)00268-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim DY, Ryu SY, Kim YJ, Kim SK. Clinicopathological characteristics of gastric carcinoma in young patients. Langenbecks Arch Surg. 2003;388:245–249. doi: 10.1007/s00423-003-0387-1. [DOI] [PubMed] [Google Scholar]
- 24.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 25.Piessen G, Triboulet JP, Mariette C. Reconstruction after gastrectomy: which technique is best? J Visc Surg. 2010;147:e273–e283. doi: 10.1016/j.jviscsurg.2010.09.004. [DOI] [PubMed] [Google Scholar]