Abstract
Purpose
In resectable gastric cancer, choice regarding the extent of resection depends on tumor size, location, and distance from resection margin. However, there remains controversy for choice of resection for tumors in the middle third of the stomach. This study investigated patients who underwent gastrectomy in order to analyze the differences between total gastrectomy (TG) and subtotal gastrectomy (STG).
Materials and Methods
From 2000 to 2006, 125 patients with a tumor in the middle third of the stomach underwent radical gastric resection at EUMC. We retrospectively conducted comparative analysis for the differences in clinicopathological characteristics and prognosis between TG and STG.
Results
The average tumor size was 6.7 cm for TG, and 4.1 cm for STG. The number of metastatic lymph nodes were 13.3 for TG, and 3.7 for STG. Patients with more advanced cancer were more likely to receive TG. The 5-year survival rate for TG was lower (38.1%) than STG (69.0%). However, if tumor stages were stratified, there was no significant difference in the survival rate. Histologically, for the undifferentiated type of cancer (Stage 1, 2), the 5-year survival rate of STG was higher (88.1%) than TG (75.0%).
Conclusions
Comparing patients with tumors in the middle third of the stomach who underwent TG and STG, there was no statistically significant difference in the 5-year survival rate. If stages were stratified, the clinicopathological characteristic becomes a key factor in deciding the prognosis, rather than the choice of resection. Thus if the radical resection margin can be obtained for a tumor in the middle third of the stomach, STG is considered instead of TG.
Keywords: Gastric neoplasms, Gastrectomy, Prognosis
Introduction
Recently, in gastric cancer, the diagnosis of early gastric cancer is on the rise due to the improvement of diagnostic techniques and physical examinations, and the survival rate is also on the rise due to attempts such as individualized combination therapy.(1,2) Nevertheless, gastric cancer has been reported to be a leading cause of death following lung cancer and liver cancer in Korea.(3) Since Billroth successfully treated gastric cancer patients by gastrectomy and gastroduodenostomy in 1881, until today surgical resection is the only curative treatment for gastric cancer.(4) In gastric cancer treatments, the resection range is determined by the size and location of the lesion, and the distance to the resection margin.(5-7) Nonetheless, the resection range for middle third gastric cancer cases is controversial. Numerous studies recommend a total gastrectomy as the standard surgery for middle third gastric cancer.(6,7) Nevertheless, with the increased frequency of early gastric cancer and thus prolonged post-surgical survival period, considering post-surgical complications, nutritional conditions, and quality of life, also distal subtotal gastrectomy has been reported to be an effective curative treatment.(8,9) In our study, we examined whether or not factors exerting effects on prognosis are present besides the resection margin that is known to be a factor determining the extent of surgery for middle-third gastric cancer,(8) and whether or not surgical techniques exert effects on survival rate.
Materials and Methods
The study was conducted on 125 middle-third gastric cancer patients recruited among patients that received curative gastrectomy in the Department of Surgery, Ewha Womans University Medical School, from January 2000 to December 2006. Middle third gastric cancer was defined as follows. Among patients suspected to have middle-third gastric cancer in pre-surgical tests, the points anatomically dividing the gastric lesser curvature and the greater curvature to three parts equally were connected, and cancer was present in the area excluding the upper 1/3 and the lower 1/3 parts. Among the patients during the study period, cases of laparoscopic surgery, cases in which curative resection could not be performed and thus conservative surgery was performed, cases of proximal subtotal gastrectomy, and cases that died within 30 days after surgery were excluded. 125 patients were divided according to surgical methods as the group received a total gastrectomy (63 patients, total resection group) and the group received distal subtotal gastrectomy (62 patients, subtotal resection group), and their clinical characteristics and prognosis were analyzed retrospectively.
The disease stage of gastric cancer was analyzed according to the 6th and 7th edition TNM classification of the Union for International Cancer Control (UICC).(10) Histological types were classified as differentiated carcinomas (papillary adenocarcinoma, well-differentiated tubular adenocarcinoma, and moderate differentiated tubular adenocarcinoma) and undifferentiated carcinoma (poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma), and analyzed. The survival period was defined as the period from the day of surgery to the day of death or the last day of follow-up observation. Statistical analysis was performed by a Student t-test, chi-square test and Kaplan-Meier survival analysis using the SPSS version 12.0 statistics program (SPSS Inc., Chicago, IL, USA). Significance between survival rates was analyzed by log-rank test. To see whether surgical methods mediate effects on survival rates independently even after the adjustment of various factors mediating effects on survival rate, multivariate analysis was performed. For multivariate analysis, Cox proportional hazards mode was applied. In all statistics of our study, a P-value less than 0.05 was determined to be statistically significant.
Results
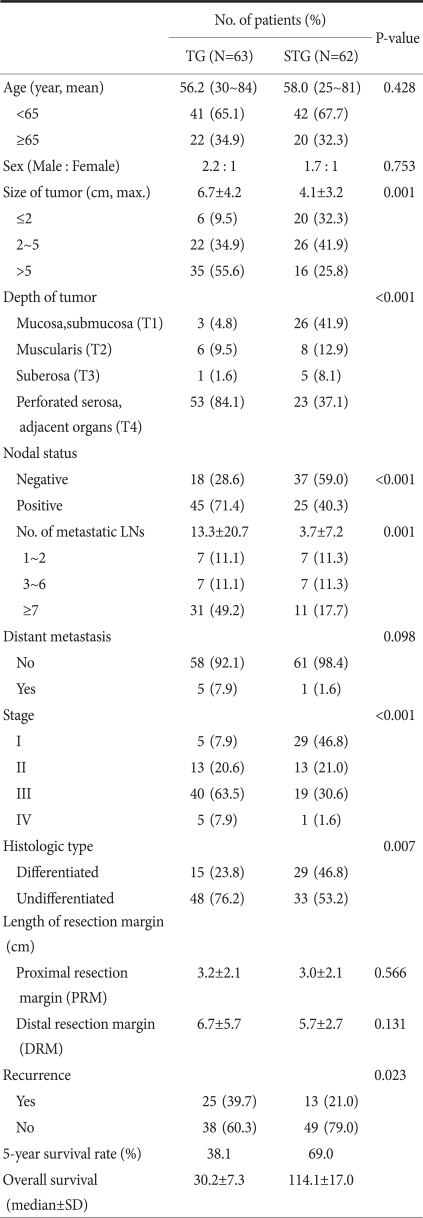
The average age of all 125 patients was 56.8 years (25~84 years), and the male to female ratio was 1.9 : 1 (82 male patients, 43 female patients). The average age of 63 patients of the total resection group was 56.2 years (30~84 years), and the subtotal resection group (62 cases) was 58.0 years (25~81 years). The age and the gender ratio according to surgery were not statistically different (P=0.428) (Table 1).
Table 1.
Clinicopathologic characteristics of patients who underwent operation by TG or STG in mid-body gastric cancer
TG = total gastrectomy; STG = subtotal gastrectomy; SD = standard deviation.
In comparison of clinicopathological characteristics, the size of the lesion (P=0.001), the level of infiltration (P<0.001), the number of lymph node metastasis (P=0.001), and the disease stage (P<0.001) of the two surgical techniques were not different. In comparison of histological differentiation grade, undifferentiated adenocarcinoma was prevalent in the total resection group (48 cases, 76.2%). In the subtotal resection group, the difference of the frequency of differentiated cancer (29 cases, 46.8%) and undifferentiated cancer (33 cases, 53.2%) was small (P=0.007). In regards to the distance to the resection margin, in both the proximal area and the distal area, differences between the two groups were not shown (P=0.566, P=0.131). 5 patients had distant metastasis in the total resection group (7.9%), 1 case in the subtotal resection group (1.6%), and curative resection was performed on all cases.
Recurrence after surgery was 25 cases in the total resection group (39.7%), 13 cases in the subtotal resection group (21.0%), and it was higher in the total resection group (P=0.023) (Table 1). Nonetheless, in the comparison of stage-stratified disease, recurrence rates were not different (P>0.05). Among recurrent cases after surgery, cases recurred in the resection margin was 5 cases in the total resection group (7.9%) and 2 cases in the subtotal resection group (3.2%), and a statistical difference between the two groups was not detected (P=0.252).
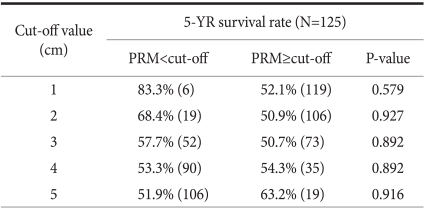
The distance to the margin area of the carcinoma and the proximal resection margin were divided by 1 cm intervals, and defining 1 cm, 2 cm, 3 cm, 4 cm, and 5 cm as the standards, the 5-year survival rate of the group less than the standard value was compared with the group larger than the standard value. The 5-year survival rate of the group whose proximal resection margin was less than 1cm was 83.3% and the group larger than 1 cm was 52.1%, P=0.579, which was not different. The prognosis of the group less than 2 cm, 3 cm, 4 cm, or 5 cm was not different from the group larger than the standard values (P>0.05) (Table 2). In our results, the difference of the survival rate according to the margin of carcinoma and the proximal resection margin was not shown.
Table 2.
5 year-survival rates of patients with middle-third gastric cancer according to the length of PRM
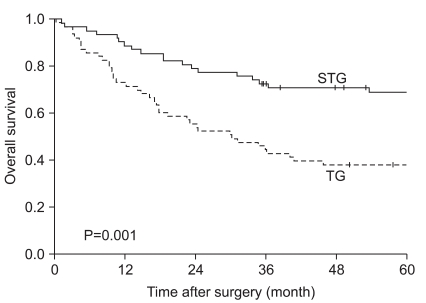
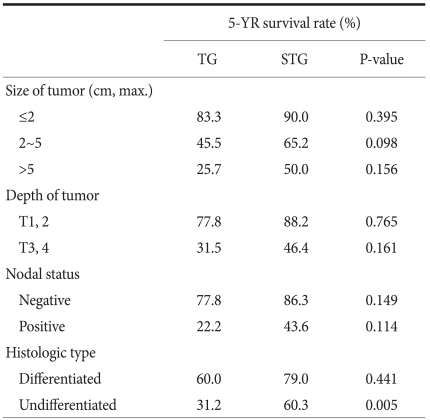
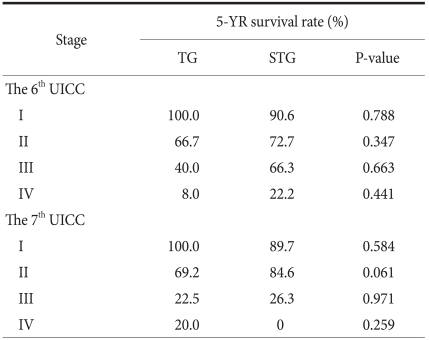
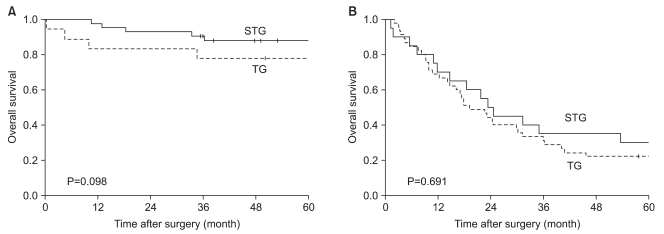
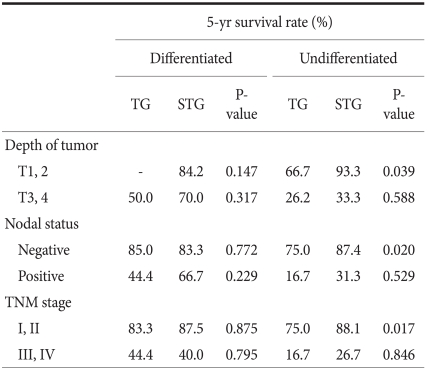
When the 5-year survival rate of the total resection group and the subtotal resection group was analyzed, the total resection group was 38.1%, the subtotal resection group was 69.0%, and a statistically significant difference was shown (P=0.001) (Fig. 1). Nevertheless, when it was analyzed under the identical condition of the size of lesions, the infiltration level, the presence or absence of lymph node metastasis, and the disease stage, a significant difference between the two groups was not detected (P>0.05) (Table 3, 4, Fig. 2). In the comparison according to histological differentiation grade, and similarly in differentiated carcinoma cases, a difference between the two groups was not shown (P=0.441). In undifferentiated cancer, the total resection group was 31.2%, the subtotal resection group was 60.3%, and the subtotal resection group showed higher 5-year survival rate (P=0.005) (Table 3).
Fig. 1.
Overall survival rate of patients after TG and STG of mid-third gastric cancer. TG = total gastrectomy; STG = subtotal gastrectomy.
Table 3.
Factor-stratified 5 year-survival rate for patients after TG and STG of mid-body gastric cancer
TG = total gastrectomy; STG = subtotal gastrectomy
Table 4.
Stage-stratified 5 year-survival analysis for patients after TG and STG of mid-body gastric cancer
TG = total gastrectomy; STG = subtotal gastrectomy; UICC = Union for International Cancer Control.
Fig. 2.
Stage-stratified (7th UlCC) 5 year-survival analysis for patients after TG and STG of mid-third gastric cancer. (A) Stage 1, 2 (B) Stage 3, 4. STG = subtotal gastrectomy; TG = total gastrectomy; UICC = Union for International Cancer Control.
It was divided into more detail to the presence or absence of the infiltration to the lamina propria, the presence or absence of lymph node metastasis, and the disease stages were divided to stage 1, 2, 3, and 4, and the 5-year survival rate according to the differentiation grade and surgical techniques was examined. The results show that only in undifferentiated carcinoma, cases with the infiltration level lower than the lamina propria (≤T2, P=0.039), cases without lymph node metastasis (N0, P=0.020), and stage 1 and stage 2 (P=0.017), in other words, in early gastric cancer, the subtotal resection group showed higher 5-year survival rates (Table 5). This is considered to be due to the points that the total resection group was relatively older patients (the total resection group: 57.0 years, the subtotal resection group: 55.3 years, P=0.733), the size of lesion was different (the total resection group: 4.8 cm, the subtotal resection group: 3.4 cm, P=0.328), and the tendency of a larger number of lymph node metastasis (the total resection group: 0.8 lymph nodes, the subtotal resection group: 0.2 lymph nodes, P=0.659) acting in combination.
Table 5.
Histologic type-stratified 5 year-survival analysis for patients after TG and STG of mid-body gastric cancer
TG = total gastrectomy; STG = subtotal gastrectomy.
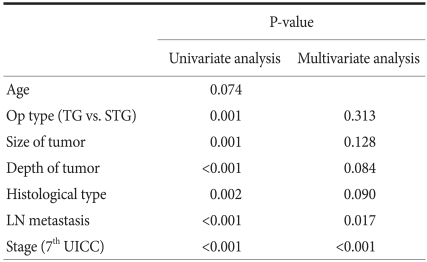
In univariate analysis, factors associated with survival rate was surgical techniques, the size of the lesion, the infiltration level, histological differentiation grade, the presence or absence of lymph node metastasis and disease stage. The factors were analyzed by multivariate analysis, and it was found that independent prognostic factors exerting effects on survival rate were the presence or absence of lymph node metastasis and disease stage (Table 6).
Table 6.
Result of univariate and multivariate analysis of prognostic factors associated with survival in mid-body gastric cancer
TG = total gastrectomy; STG = subtotal gastrectomy; UICC = Union for International Cancer Control.
Discussion
Curative resection for gastric cancer could be considered to be an ideal surgical method that shows not only high survival rate after surgery but also causes less complications, and maintenance of a high quality of life.(6,7) In addition, on account of diagnostic methods and physical examination being performed widely, gastric cancer is diagnosed early in many cases, and survival rate was improved owing to individualized combination therapy, and thus the safety associated with surgery or quality of life after surgery are considered inevitably. From such a point of view, if long-term survival rate is not different, surgeries that allow to maintain good quality of life or inducing less post-surgical complications should be considered first.
In surgery for gastric cancer, the basic point in curative surgery is that microscopic residual cancer cells should not be detected in the resection margin, which has been well known as a factor exerting effects on post-surgical prognoses. Nonetheless, the gold standard that shows the distance from tumor margin to the resection margin has not been established. In our study, the distance from tumor margin to proximal resection margin was divided by 1cm, 2 cm, 3 cm, 4 cm, and 5 cm, defined as the standards, and 5-year survival rate of the group less than the standard was compared with the group greater than the standard. Differences according to the distance of the proximal resection margin was not detected, and thus the distance to the proximal resection margin was not a prognostic factor (Table 2). Therefore, in regards to the resection range, it could be concluded if a safe resection margin allowing curative surgery could be secured, the prognosis of subtotal resection and total resection is not different. However, diverse studies on appropriate resection margins based on tumor infiltration level or macroscopic findings have been reported,(8,9) hence, additional studies by dividing into more in detail are required.
In previous studies compared to the resection range, techniques were compared without differentiating the location of lesions,(11-13) or compared upper gastric(14,15) or lower gastric lesions(16) in most cases. Among them, studies that compared total gastrectomy with proximal subtotal gastrectomy in upper gastric carcinoma(14,15) are different from our study that compared total gastrectomy with distal subtotal gastrectomy in middle-third gastric cancer. In studies on middle-third gastric cancer, as factors determining surgical techniques, only the distance to the resection margin was suggested.(9) Hence, in our study, it was examined whether factors other than the distance are present as factors exerting effects on prognosis. In most studies, the survival rate of the two groups was not shown(13,16) Even in studies showing higher 5-year survival rates of the subtotal resection group, in the comparison of the survival rate of the same disease stage, the survival rate of the two groups was not different,(9) and results concur to our study were shown. In a study reported by Gouzi et al.,(16) in addition to the comparison of the same disease stage, patients were divided according to the presence or absence of the invasion to the serosa and the presence or absence of lymph node metastasis, and the survival of the two groups was compared. It was found that under the same condition, the survival rate of the two groups was not different. In our study, except that the infiltration level was divided based on the lamina propria instead of the invasion to the serosa, results concur to the study reported by Gouzi et al.(16) were obtained.
In studies that compared complications or mortality caused by surgery, it has been reported that total resection is no longer more dangerous than subtotal resection.(12,16,17) Nonetheless, in comparison of post-surgical nutrition or quality of life, cases that had subtotal resection showed better outcomes.(11,17-19) Taken together, previous studies and the results of our study between the total resection group and the subtotal resection group, prognosis such as post-surgical complications, mortality, and long-term survival rate were not different. Nonetheless, after subtotal resection, nutrition condition or quality of life showed better outcomes. It thus was thought if the required condition for curative resection is satisfied, subtotal resection should be considered first.
In our study, for advanced gastric cancer based on the size of the lesion, infiltration level, lymph node metastasis and disease stage, total resection was performed in many cases, and even in undifferentiated cancer of which the prognosis is known to be relatively poor,(20) total resection was performed in many cases. It could be speculated that such results were obtained because this study has a limitation of retrospective studies. Hence, the possibility that during surgery surgeons performing total resection is high for advanced gastric cancer of which the prognosis is anticipated to be poor, in order to secure safe resection margins.
According to the UICC TNM, the purposes of the classification of TNM are that first, to help clinicians planning treatments, and second, to point out indication for prognosis, third, to play an assistant role in the evaluation of treatment outcomes, fourth, to facilitate the exchange of objective information among institutions, and fifth, to facilitate to continue studies on human malignant tumors. (21,22) The 7th edition UICC TNM disease classification published in 2009 is controversial. It has been reported that in comparison with the 6th edition UICC, the difference of the survival rate according to disease stage became weakened.(21,23) It also has been reported that generally, it classified more evenly than previous editions.(24) In our study, survival rate was classified simultaneously by the application of the 6th and 7th editions according to the resection range in middle-third gastric cancer cases (Table 4). In comparison with the previous 6th edition, survival rate according to the 7th edition UICC TNM classification method was not greatly different, and thus it is determined that the new disease stage classification method was also significant. In the classification according to the 7th edition only, in stage 2, the total resection group was 69.2%, the subtotal resection group was 84.6% (P=0.061), marginal significance was shown, and the 5-year survival rate showed a tendency to be high in the subtotal resection group. Nevertheless, the number of the subtotal resection groups corresponding to stage 2 of our study was only 26 cases, and thus it is thought that because of the limitation of the number according to the size of the groups, it is difficult to consider it statistically significant.
In our study, comparative analysis was performed not only on the TNM disease stage that could be determined only during surgery but also on histological differentiation grade that could be evaluated prior to surgery. Histological differentiation grade could be assessed prior to surgery by endoscopic biopsy, because it is a prognostic factor, and it provides information for the determination of the resection range. In relatively early gastric cancer such as a lesion infiltrated below the lamina propria, cases without lymph node metastasis, or stage 1 and stage 2, etc., if it is undifferentiated cancer, the prognosis of the total resection group was poor. In contrast, if the infiltration level was higher than the subserosal layer, cases with lymph node metastasis or disease stage 3 and disease stage 4, regardless of undifferentiated cancer or differentiated cancer, the 5-year survival rate of the total resection group was not different from the differentiation group (Table 5). McNeer et al.(25) stated that the basic surgery for gastric cancer is curative total resection that secures the resection margin safely. According to his study, it is predicted that the prognosis of the total resection group whose distance to the resection margin is secured more safely would be better than the subtotal resection group. However, in our study, in advanced gastric cancer cases, the 5-year survival rate between the two groups was not different. Hence, in cases performed curative resection for advanced gastric cancer, the resection range was not an independent prognostic factor exerting effects on long-term prognosis. In our study, among middle-third gastric cancer patients corresponding to relatively early stage, in cases whose histological differentiation degree was undifferentiated, the total resection group showed the result of poor prognosis than the subtotal resection group. This is considered to be due to the age of the total resection group was relatively high, the size of lesion was slightly different, and a trend of the increased number of lymph node metastasis was shown. Nonetheless, in the analysis of each factor, statistical significance could not be observed. Therefore, it is thought that to elucidate the cause, a large scale prospective study should be conducted. In addition, in our study, the effect of additional treatments such as pre-surgical and post-surgical chemotherapy, radiation therapy, etc. was not considered, and thus it is thought that the evaluation of the effect of pre-surgical and post-surgical treatments on surgical techniques is required.
In middle-third gastric cancer, between patients that received curative total gastrectomy and subtotal gastrectomy, the 5-year survival rate of the same disease stage was not statistically significant. In the same disease stage of middle-third gastric cancer, prognosis is determined by clinicopathological characteristics of the lesion itself rather than the difference of prognosis according to the resection range, and thus if curative resection margin could be secured, subtotal resection instead of total resection is feasible.
References
- 1.Sim YK, Kim CY, Jeong YJ, Kim JH, Hwang Y, Yang DH. Changes of the clinicopathological characteristics and survival rates of gastric cancer with gastrectomy: 1990s vs early 2000s. J Korean Gastric Cancer Assoc. 2009;9:200–206. [Google Scholar]
- 2.Kim W, Park CH, Park SM, Park WB, Lim KW, Kim SN. Prognostic significance of lymphatic and perineural invasions in patients with gastric cancer who have no lymph node and serosal involvement. J Korean Gastric Cancer Assoc. 2001;1:77–82. [Google Scholar]
- 3. [Accessed July 4, 2010]. http://www.cancer.go.kr/cms/statics/mortality/index.html/
- 4.Billroth T. Offenes schreiben an Herrn Dr. L. Wittelshofer. Wien Med Wochenschr. 1881;31:161–165. [Google Scholar]
- 5.Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. doi: 10.1097/01.sla.0000149300.28588.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein HJ, Sendler A, Siewert JR. Site-dependent resection techniques for gastric cancer. Surg Oncol Clin N Am. 2002;11:405–414. doi: 10.1016/s1055-3207(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 7.Clark CJ, Thirlby RC, Picozzi V, Jr, Schembre DB, Cummings FP, Lin E. Current problems in surgery: gastric cancer. Curr Probl Surg. 2006;43:566–670. doi: 10.1067/j.cpsurg.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bozzetti F, Bonfanti G, Bufalino R, Menotti V, Persano S, Andreola S, et al. Adequacy of margins of resection in gastrectomy for cancer. Ann Surg. 1982;196:685–690. doi: 10.1097/00000658-198212001-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang YJ, Park MS, Kim JH, Park SS, Park SH, Kim SJ, et al. Advanced gastric cancer in the middle one-third of the stomach: Should surgeons perform total gastrectomy? J Surg Oncol. 2010;101:451–456. doi: 10.1002/jso.21431. [DOI] [PubMed] [Google Scholar]
- 10.Sobin L, Gospodarowicz M, Wittekind C, editors. International Union Against Cancer (UICC) TNM Classifi cation of Malignant Tumors. 7th ed. New York: Weiley; 2009. [Google Scholar]
- 11.Davies J, Johnston D, Sue-Ling H, Young S, May J, Griffith J, et al. Total or subtotal gastrectomy for gastric carcinoma? A study of quality of life. World J Surg. 1998;22:1048–1055. doi: 10.1007/s002689900515. [DOI] [PubMed] [Google Scholar]
- 12.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Crose N, et al. The Italian Gastrointestinal Tumor Study Group. Total versus subtotal gastrectomy: surgical morbidity and mortality rates in a multicenter Italian randomized trial. Ann Surg. 1997;226:613–620. doi: 10.1097/00000658-199711000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, Gennari L Italian Gastrointestinal Tumor Study Group. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Ann Surg. 1999;230:170–178. doi: 10.1097/00000658-199908000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim IH, Kim YC, Kim SM. Prognosis of total and proximal gastrectomy for gastric cancer. J Korean Surg Soc. 1986;31:545–555. [Google Scholar]
- 15.Lim HJ, Jeong YJ, Yang DH. A comparative study on the outcomes of total and proximal gastrectomies performed for gastric cancer. Korean J Gastroenterol. 2002;40:364–370. [Google Scholar]
- 16.Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, Lacaine F, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg. 1989;209:162–166. doi: 10.1097/00000658-198902000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braga M, Molinari M, Zuliani W, Foppa L, Gianotti L, Radaelli G, et al. Surgical treatment of gastric adenocarcinoma: impact on survival and quality of life. A prospective ten year study. Hepatogastroenterology. 1996;43:187–193. [PubMed] [Google Scholar]
- 18.Oh CA, Kim DH, Oh SJ, Choi MG, Noh JH, Sohn TS, et al. Changes of the preoperative and postoperative nutritional statuses in patients with gastric cancer and assessment of the nutritional factors that are correlated with short-term postoperative complications. J Korean Gastric Cancer Assoc. 2010;10:5–12. [Google Scholar]
- 19.Bozzetti F, Ravera E, Cozzaglio L, Dossena G, Agradi E, Bonfanti G, et al. Comparison of nutritional status after total or subtotal gastrectomy. Nutrition. 1990;6:371–375. [PubMed] [Google Scholar]
- 20.Rohatgi PR, Yao JC, Hess K, Schnirer I, Rashid A, Mansfield PF, et al. Outcome of gastric cancer patients aft er successful gastrectomy: influence of the type of recurrence and histology on survival. Cancer. 2006;107:2576–2580. doi: 10.1002/cncr.22317. [DOI] [PubMed] [Google Scholar]
- 21.Gospodarowicz M, Benedet L, Hutter RV, Fleming I, Henson DE, Sobin LH. History and international developments in cancer staging. Cancer Prev Control. 1998;2:262–268. [PubMed] [Google Scholar]
- 22.Sobin LH. TNM: principles, history, and relation to other prognostic factors. Cancer. 2001;91(8 suppl):1589–1592. doi: 10.1002/1097-0142(20010415)91:8+<1589::aid-cncr1170>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Ha TK, Kim HJ, Kwon SJ. Does the new UICC/AJCC TNM staging system (7th Edition) improve assessing prognosis in gastric cancer compared to the old system (6th Edition)? J Korean Gastric Cancer Assoc. 2009;9:159–166. [Google Scholar]
- 24.Deng J, Liang H, Sun D, Wang D, Pan Y. Suitability of 7th UICC N stage for predicting the overall survival of gastric cancer patients after curative resection in China. Ann Surg Oncol. 2010;17:1259–1266. doi: 10.1245/s10434-010-0939-x. [DOI] [PubMed] [Google Scholar]
- 25.McNeer G, Bowden L, Booner RJ, McPeak CJ. Elective total gastrectomy for cancer of the stomach: end results. Ann Surg. 1974;180:252–256. doi: 10.1097/00000658-197408000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]