Abstract
Purpose
Replication error is an important mechanism in carcinogenesis. The microsatellite instability (MSI-H) of colorectal cancers is associated with the development of multiple cancers. The influence of MSI-H on the development of multiple gastric cancers in sporadic gastric cancer patients has not been defined. This study was performed to reveal the association between the clinicopathologic features and MSI in sporadic gastric cancers.
Materials and Methods
Between July 2004 and March 2009, the clinicopathologic characteristics, including MSI status, were evaluated in 128 consecutive patients with sporadic gastric cancers. None of the patients had hereditary non-polyposis colorectal cancer of familial gastric cancer. The markers that were recommended by the NCI to determine the MSI status for colorectal cancers were used.
Results
MSI-H cancers were found in 10.9% of the patients (14/128). Synchronous gastric cancers were shown in 4 patients (3.1%). Synchronous cancers were found in 2 of 14 patients with MSI-H gastric cancer (14.3%) and 2 of 114 patients with MSS gastric cancer (1.8%; P=0.059, Fisher's exact test). Among the patients with synchronous cancer 50% (2/4) had MSI-H cancer, but 9.7% of the patients (12/124) without synchronous cancer had MSI-H cancer. MSI-H (RR, 24.7; 95% CI, 1.5~398.9; P=0.024) was related with to synchronous gastric cancer, but age, gender, family history, histologic type, location, gross morphology, size, and stage were not related to synchronous gastric cancer.
Conclusions
MSI is associated with the intestinal-type gastric cancer and the presence of multiple gastric cancers in patients with sporadic gastric cancer. Special attention to the presence of synchronous and the development of metachronous multiple cancer in patients with MSI-H gastric cancer is needed.
Keywords: Microsatellite instability, Stomach neoplasms, Synchronous gastric cancer
Introduction
Attention has recently focused on the role of microsatellite instability (MSI) in the development of digestive tract cancer, and a number of studies have been conducted on the association with clinicopathologic features. MSI has been reported to be closely associated with hereditary non-polypopsis colorectal cancer (HNPCC). MSI is detected in approximately 15% of patients with non-HNPCC.(1) In patients with MSI, the incidence of metachronous colon cancer is increased.(2) In gastric cancer, MSI is shown in 7~50% cases.(3) Multiple gastric cancer is detected in 4~15% of all gastric cancer patients.(4) Nevertheless, in gastric cancer patients, it is not clear whether or not multiple development is associated with hereditary factors. It has been shown that the development of metachronous gastric cancer is more frequent in multiple gastric cancer than single gastric cancer.(5) Therefore, by the examination of genetic factors associated with multiple gastric cancer, development of metachronous cancer could be predicted, and the early diagnosis by appropriate screening tests could be anticipated. A high frequency of MSI was observed in multiple gastric cancer in comparison with single gastric cancer, which suggests an association.(6) In Korea, studies on MSI have not been conducted, and thus we examined the clinicopathologic characteristics of gastric cancer, including the association of MSI with multiple gastric cancer.
Materials and Methods
1. The patients
One hundred twenty-eight patients who were diagnosed with gastric cancer between July 2004 and November 2009, underwent surgical resection, and were pathologically-diagnosed with gastric adenocarcinoma were enrolled in the study. Eighty-eight patients were male and 40 patients were female. The mean age of the patients was 60.57±11.80 years. Eighty-six patients had advanced gastric cancer and 42 patients had early gastric cancer. Gastric adenocarcinoma was classified according to the Japanese Gastric Cancer Association (JGCA) classification as well differentiated, moderately differentiated, poorly differentiated, mucinous, and signet ring cell. (7) According to Lauren's classification, gastric adenocarcinoma was classified as intestinal, diffuse, and mixed.(8) For the determination of the stage of gastric cancer, the AJCC cancer staging manuals (6th edition) was applied. By examining the medical records of patients and the results of histologic tests, age, gender, tumor site, macroscopic classification, and tumor size and stage were evaluated. The family history was obtained by telephone interviews, and patients with a family history of gastric cancer or HNPCC were excluded. Based on the time of diagnosis, patients diagnosed at the same time or within 6 months were considered to be synchronous cancer, and cases diagnosed 6 months after the diagnosis of primary cancer were classified as metachronous cancer.(9) The mean duration of follow-up was 4.12±1.13 years. During the follow-up period, none of patients developed metachronous gastric cancer or secondary cancers in other organs.
2. Sampling and DNA extraction
Tissue slides of 128 patients were examined under a light microscope, and DNA was extracted from normal and tumor tissues of tissue sections stained with HE. The tissues were sonicated in 600 µl of nucleic lysis solution, and the lysate was collected and incubated at 65℃ for approximately 30 minutes. Three microliters of RNase was added and incubated again at 37℃ for approximately 30 minutes. Two hundred microliters of protein precipitation solution was added, centrifuged, the supernatant was discarded, 70% ethanol was added, centrifuged, and the pellets were dried. DNA was dissolved by adding 100 µl of DNA rehydration solution. PCR was performed with the solution containing 60 ng of genomic DNA, 2 pmol each primers, 50 mM KCL, 10 mM Tris, 0.4 mM dNTP, 1.5 mM MgCl2, and 0.6 unit Taq DNA polymerase (Promega, Madison, Fitchburg, WI, USA). The final volume was 10 µl. As the first step of PCR, the initial denaturation was performed for 5 minutes at 94℃, and 30 cycles of the procedure, 94℃ for 1 minute, 55℃ for 1 minute, 72℃ for 1 minute, and the final amplification procedure 72℃ for 10 minutes. Amplified product (0.7 µl) was added to the mixture of 0.3 µl Genescan 500 size standard and 9 µl of HiDi formamide, and analyzed by an ABI Prism 3100 Genetic analyzer.
3. Analysis of MSI
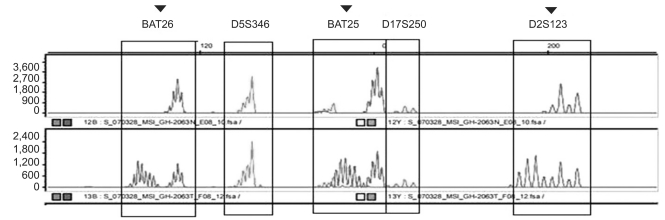
BAT25 and BAT26 with the repeated sequence of a single nucleotide, and D5S346, D2S123, and D17S250 with the repeated sequence of two nucleotides, were selected as PCR primers for the assessment of MSI.(10) The microsatellite loci were amplified by PCR using radiolabeled oligonucleotide primers, the length was analyzed by gel electrophoresis or autoradiography, and the difference between normal tissues and cancer tissues was examined. With respect to MSI, if the DNA of tumor tissues show new bands in comparison with normal tissues, it was considered to be MSI. The loss of an allelic gene was not considered to be MSI (Fig. 1). In our study, according to the guideline of the National Cancer Institute (Bethesda, Maryland, MD, USA), 5 markers were selected. Cases positive for >2 markers were considered to be high-frequency MSI (MSI-H), while cases positive for <2 markers were considered to be low-frequency MSI (MSI-L) or microsatellite stable (MSS).(11) Only MSI-H was considered to be the satellite instability group, and MSI-L or MSS was referred to as the satellite stability group.
Fig. 1.
An example of PCR analysis of microsatellite instability. Tumor DNA shows alterations of allele lengths in BAT25, BAT26, D5S346, D2S123, and D17S250. ▾ = arrow heads indicate microsatellite instability. Microsatellite instability is associated with the clinicopathologic features of gastric cancer in sporadic gastric cancer patients.
4. Data analysis and statistics
In the comparison of clinicopathologic characteristics for univariate analysis, a chi-square test was performed; for multivariate analysis, multivariate logistic regression analysis was performed. Among univariate factors, age and tumor size were consecutive variables, and other variables were categorical. The common analysis factors for multivariate analysis were gender and age.(12) In the current study, MSI, the location of the tumor, disease stages reported to be statistically significant in other studies,(13) differentiation,(14) and advanced or early gastric cancer were included. SPSS (ver. 17.0; SPSS, Inc., Chicago, IL, USA) was used. A P-value <0.05 was considered to be statistically significant.
Results
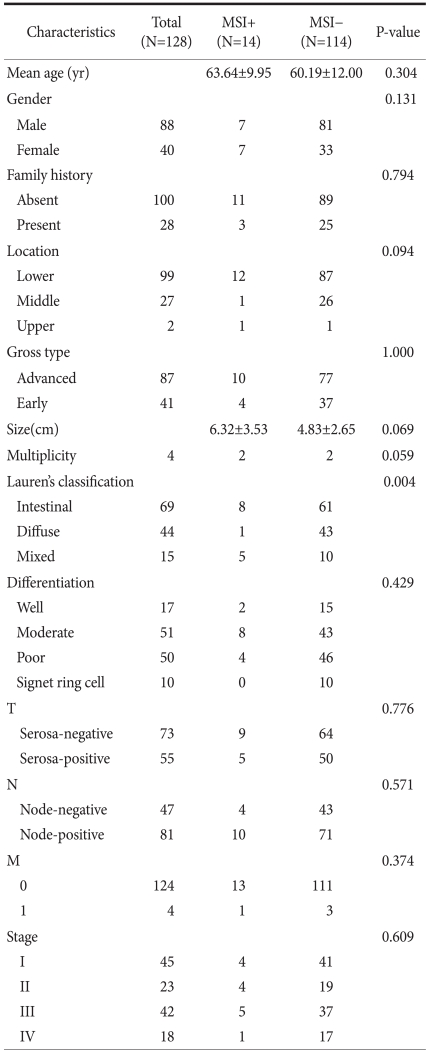
One hundred twenty-eight gastric cancer patients underwent gastrectomies and were diagnosed by histologic evaluation; MSI was detected in 14 patients (10.9%) and 114 patients (89.1%) were in the MSS group. Four patients (3.1%) had multiple gastric cancer and 124 patients (96.8%) had single gastric cancer. In Lauren's classification of the MSI group, 8 patients had the intestinal type, 1 patient had the diffuse type, and 5 patients had the mixed type. In the MSS group, 61 patients had the intestinal type, 43 patients had the diffuse type, and 10 patients had the mixed type. In the MSI group, intestinal-type gastric cancer was dominant, and a significant difference between MSI-H and MSI-L was shown (P=0.004). Age, gender, and clinical factors, such as family history, tumor size, tumor location, lymph node metastasis, distant metastasis, and gross findings, did not show any difference between MSI-H and MSI-L (Table 1).
Table 1.
Clinicopathologic characteristics of gastric cancers positive for microsatellite instability
R.R. = relative risk; C.I. = confidence interval.
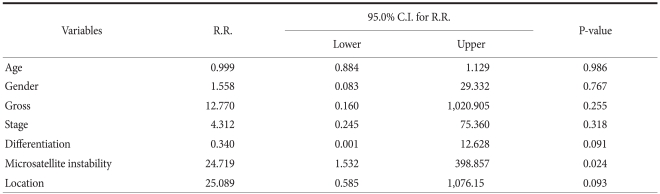
Among the 14 patients in the MSI group, 2 patients (14.3%) had multiple gastric cancer. Among the 114 patients in the MSS group, 2 patients (1.8%) had multiple gastric cancer. Of the 4 patients with multiple gastric cancer, 2 (50%) had MSI. Based on logistic regression analysis, only MSI was shown to be an independent risk factor associated with the development of multiple gastric cancer (P=0.024; RR, 24.71; 95% CI, 1.532~98.857) (Table 2). In multiple gastric cancer, the rate of MSI was 50%, which was shown to be significantly higher than single gastric carcinoma (9.6%).
Table 2.
Multivariate logistic regression analysis for the variables associated with synchronous cancers in the patients with gastric cancer
MSI+ = high-frequency microsatellite instability; MSI- = low-frequency microsatellite instability or microsatellite stability.
Discussion
The mechanism by which gastric cancer develops is a multi-step process. Indeed, cancer develops by the accumulation of genetic or environmental changes inducing the dysfunction of genes.(15) The genetic mechanisms involved in the development of gastric cancer include mutations or inactivation of various tumor suppressor genes. Nevertheless, genetic instability is the most basic change, as well as an essential factor. Gastric cancer exhibits particular clinical and pathologic characteristics depending on the presence or absence of microsatellite changes, and thus the molecular biological characteristics of gastric cancer is assessed by examining microsatellite changes.
In the MSI group, genetic changes accumulate and exert effects on the multistep tumor development process. Thus, the incidence of multiple cancer, such as HNPCC is high. Among the characteristic of HNPCC, genetic instability is associated with the development of multiple colon carcinoma.(2) Therefore, the same theory could be applied to gastric cancer, which is one of the organs preferentially developing HNPCC, although it has not been proven.(16) In other words, in sporadic gastric cancer, systemic studies on the genetic factors inducing multiple gastric cancer have not been conducted. Numerous studies on the role of the mismatch repair (MMR) of DNA during the oncogenic process of gastric cancer have been reported; however, the results are contradictory in many cases, and since factors involved in the oncogenesis are abundant, definitive conclusions cannot be drawn.
MSI is detected in 10~45% gastric cancer, reflecting differences in definition of MSI.(17) Specifically, in previous studies on MSI of gastric cancer, diverse definitions of MSI have been applied, such as the 5 NCI consensus markers,(10) or BAT-26.(18) In colorectal cancer, the consensus is the standard of markers chosen by the National Cancer Institute in 1977 (BAT25, BAT26, D5S346, D2S123, and D17S250). In the current study, according to the guidelines of the National Cancer Institute, the presence or absence of MSI was defined. In the low-frequency MSI group, clinical differences from the MSS group were not detected. Noticeable differences of the high-frequency MSI group from the two groups were detected from the clinical features and survival rate.(19) Therefore, MSI-H could be considered as MSI-positive gastric cancer. Similarly, in the current study, only MSI-H was classified as the MSI group. The results show that among 128 gastric cancer cases, only 14 patients (10.9%) had MSI, which is comparable to the incidence reported in other studies. Although the guidelines of the National Cancer Institute for colorectal cancer were followed, among 14 patients in the MSI group, 13 patients were positive for the BAT-26 marker that has been shown to determine the phenotype of MSI, and it is considered that the criteria of the MSI-H group selected in our study were sufficient to be classified as the MSI group.
In the current study, four cases of multiple gastric cancer were all double tumors, thus the number of total tumors was eight. MSI of all tumors was tested, and even if MSI was detected in only one tumor, the case was classified as MSI. Among the 4 cases (8 tumors), MSI-H was detected in 2 tumors (25%) in 2 cases (50%), and in the cases of multiple cancer, all individual tumors were MSI-H were not detected. This was interpreted to be that under the same environmental and genetic backgrounds in multiple cancer, the mechanisms underlying tumor suppressor genes and mutations for each tumor acted differently and induced individual tumors, and the sum was shown as multiple tumors. This implies the heterogeneity of MSI in multiple gastric cancer, and other studies also have shown a similar characteristic.(20)
Synchronous gastric cancer is detected in 4~10% of all gastric cancers.(3) In addition, the probability of patients treated with primary cancer developing a secondary cancer within 10 years is known to be approximately 10%.(21) The incidence of metachronous cancer is higher in multiple gastric cancer than single gastric cancer,(22) and thus if predictive factors are identified, which would be of help to improve the prognosis of patients. In addition, if multiple lesions are overlooked at the time of initial treatment, the incidence of metachronous gastric cancer could be increased after treatment, which becomes an important issue.(23) The results of the current study show that in the MSI group, the incidence of multiple gastric center was higher. Therefore, it is thought that MSI is a factor exerting effects on the development of multiple cancer not only in HNPCC, but also in sporadic gastric cancer.
According to the study reported by Nakashima,(20) in cases of MSI, the incidence of synchronous multiple gastric cancer was shown to be higher. Nevertheless, HNPCC was not excluded, and cancer in other organs, such as liver and colorectal cancer, were considered to be multiple cancer, and even MSI-L was considered as the MSI group. According to the study reported by Ribeiro,(13) the incidence of synchronous gastric cancer is high in MSI-H cases. However, in their study, hMLH1 was considered to be a marker for MSI-H, and instead of PCR-based mutational studies, the presence or absence of microsatellite changes was assessed by immunohistochemical staining.
It has been reported when MSI-positive cases are compared with -negative cases, generally, clinical as well as pathologic characteristics are shown, and the prognosis is also different. Hence, in attempts to use it in clinics as markers for the early detection of cancer, as well as to predict prognosis or response, numerous studies have been conducted. The clinicopathologic characteristics of MSI colorectal cancer are well-characterized. In contrast, relatively few studies on gastric cancer have been conducted, and thus except few characteristics, none of them are characterized. Recently, it has been reported that in gastric cancer, the MSI cases generally occur in older age groups, and the development of cancer in the lower body, intestinal type, few lymph node metastasis regardless of T disease stage, and prognosis was relatively good.(24)
Our study had limitations. The proportion of multiple gastric cancer in all cases of gastric cancer was 4/124 cases (3.1%), which was comparable to other studies conducted in other countries (4~15%) or slightly less.(3) This is thought due to the lack of interests on multiple lesions during the initial period of treatment, insufficient comprehensive record of resected specimens and histologic tests, which could be inferred from the finding that during 6 years of the entire study period, cases corresponding to 75% (3/4) were detected during the last 2 years. In addition, based on research results that generally, the onset time of metachronous gastric cancer after treatment is within an average of 10 years,(21) it is thought that 5.12±1.13 years of the follow-up observation period of our study is somewhat short to discuss the development of metachronous gastric cancer. It is thought that in the future, studies on metachronous cancer and primary cancer in other organs in a large scale patient group are needed.
In summary, the results of the current study show that the MSI group has the characteristic of intestinal type as reported previously, and particularly, in sporadic gastric cancer, it is thought to be associated with the development of synchronous multiple cancer. Nevertheless, in order to apply it as a molecular biological factor that could predict the development of multiple gastric cancer, additional studies are needed. Therefore, in the MSI group, the possibility of the presence of synchronous multiple cancer as well as the possibility of the development of metachronous cancer in the future should be monitored carefully and the follow-up observation is required. In addition, it could be speculated that in multiple and single gastric cancer, the genetic pathologic mechanism due to genetic factors different from each other is involved.
Footnotes
This work was supported by a research grant from Hanyang University (2007-6301).
References
- 1.Pedroni M, Tamassia MG, Percesepe A, Roncucci L, Benatti P, Lanza G, Jr, et al. Microsatellite instability in multiple colorectal tumors. Int J Cancer. 1999;81:1–5. doi: 10.1002/(sici)1097-0215(19990331)81:1<1::aid-ijc1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Sengupta SB, Yiu CY, Boulos PB, De Silva M, Sams VR, Delhanty JD. Genetic instability in patients with metachronous colorectal cancers. Br J Surg. 1997;84:996–1000. doi: 10.1002/bjs.1800840725. [DOI] [PubMed] [Google Scholar]
- 3.Bae JM, Won YJ, Jung KW, Suh KA, Ahn DH, Park JG. Annual report of the central cancer registry in Korea-1999: based on registered data from 128 hospitals. Cancer Res Treat. 2001;33:367–372. doi: 10.4143/crt.2001.33.5.367. [DOI] [PubMed] [Google Scholar]
- 4.Honmyo U, Misumi A, Murakami A, Haga Y, Akagi M. Clinicopathological analysis of synchronous multiple gastric carcinoma. Eur J Surg Oncol. 1989;15:316–321. [PubMed] [Google Scholar]
- 5.Kaibara N, Maeta M, Ikeguchi M. Patients with multiple primary gastric cancers tend to develop second primaries in organs other than the stomach. Surg Today. 1993;23:186–188. doi: 10.1007/BF00311241. [DOI] [PubMed] [Google Scholar]
- 6.Lynch HT, Smyrk TC, Watson P, Lanspa SJ, Lynch JF, Lynch PM, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993;104:1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma - 2nd english edition - Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 8.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a HISTO-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Cleary JB, Kazarian KK, Mersheimer WL. Multiple primary cancer. Thirty patients with three or more primary cancers. Am J Surg. 1975;129:686–690. doi: 10.1016/0002-9610(75)90346-3. [DOI] [PubMed] [Google Scholar]
- 10.Suraweera N, Duval A, Reperant M, Vaury C, Furlan D, Leroy K, et al. Evaluation of tumor microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology. 2002;123:1804–1811. doi: 10.1053/gast.2002.37070. [DOI] [PubMed] [Google Scholar]
- 11.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 12.Ahn YJ, Oh SJ, Song JW, Kang WH, Hyung WJ, Choi SH, et al. The clinicopathologic features and prognosis of multiple early gastric cancer. J Korean Gastric Cancer Assoc. 2008;8:198–203. [Google Scholar]
- 13.Ribeiro U, Jr, Jorge UM, Safatle-Ribeiro AV, Yagi OK, Scapulatempo C, Perez RO, et al. Clinicopathologic and immunohistochemistry characterization of synchronous multiple primary gastric adenocarcinoma. J Gastrointest Surg. 2007;11:233–239. doi: 10.1007/s11605-007-0101-7. [DOI] [PubMed] [Google Scholar]
- 14.Bae JS, Lee JH, Ryu KW, Kim YW, Bae JM. Characteristics of synchronous cancers in gastric cancer patients. Cancer Res Treat. 2006;38:25–29. doi: 10.4143/crt.2006.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol. 1993;119:265–272. doi: 10.1007/BF01212724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong JM, Fukayama M, Hayashi Y, Takizawa T, Koike M, Konishi M, et al. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 1994;54:4595–4597. [PubMed] [Google Scholar]
- 17.Sepulveda AR, Santos AC, Yamaoka Y, Wu L, Gutierrez O, Kim JG, et al. Marked differences in the frequency of microsatellite instability in gastric cancer from different countries. Am J Gastroenterol. 1999;94:3034–3038. doi: 10.1111/j.1572-0241.1999.01453.x. [DOI] [PubMed] [Google Scholar]
- 18.Lim S, Lee HS, Kim HS, Kim YI, Kim WH. Alteration of E-cadherin-mediated adhesion protein is common, but microsatellite instability is uncommon in young age gastric cancers. Histopathology. 2003;42:128–136. doi: 10.1046/j.1365-2559.2003.01546.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayden JD, Cawkwell L, Quirke P, Dixon MF, Goldstone AR, Sue-Ling H, et al. Prognostic significance of microsatellite instability in patients with gastric carcinoma. Eur J Cancer. 1997;33:2342–2346. doi: 10.1016/s0959-8049(97)00343-2. [DOI] [PubMed] [Google Scholar]
- 20.Nakashima H, Inoue H, Honda M, Shibuta K, Arinaga S, Mori M, et al. The heterogeneity of microsatellite instability in multiple gastric cancers. Am J Gastroenterol. 1995;90:653–656. [PubMed] [Google Scholar]
- 21.Oliveira C, Seruca R, Seixas M, Sobrinho-Simões M. The clinicopathological features of gastric carcinomas with microsatellite instability may be mediated by mutations of different "target genes": a study of the TGFbeta RII, IGFII R, and BAX genes. Am J Pathol. 1998;153:1211–1219. doi: 10.1016/s0002-9440(10)65665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyoshi E, Haruma K, Hiyama T, Tanaka S, Yoshihara M, Shimamoto F, et al. Microsatellite instability is a genetic marker for the development of multiple gastric cancers. Int J Cancer. 2001;95:350–353. doi: 10.1002/1097-0215(20011120)95:6<350::aid-ijc1061>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, et al. Incidence, diagnosis and significance of multiple gastric cancer. Br J Surg. 1995;82:1540–1543. doi: 10.1002/bjs.1800821127. [DOI] [PubMed] [Google Scholar]
- 24.Kim HC, Roh SA, Yook JH, Oh ST, Kim BS, Yu CS, et al. Microsatellite instability and promoter methylation of hMLH1 in sporadic gastric carcinoma. J Korean Gastric Cancer Assoc. 2003;3:50–55. [Google Scholar]