Abstract
Purpose
Infection with Helicobacter pylori is an important risk factor for gastric cancer in humans. We compared the clinicopathologic features of gastric cancer patients based on H. pylori infection.
Materials and Methods
We prospectively studied 155 patients who had gastric cancer and underwent gastrectomies in 1 hospital in Korea. We examined H. pylori infections using the rapid urease test (RUT) with gastrectomy specimens and collected clinical and pathologic data.
Results
The number of H. pylori infections based on the RUT was 137 (88%). The H. pylori-negative group was significantly associated with AGC and tumor histology. H. pylori infection was significantly correlated with type I/IIa in EGC and type III/IV/V in AGC. AGC was significantly correlated with larger tumor size, lymphatic invasion, perineural invasion, and H. pylori infection based on univariate and multivariate analyses.
Conclusions
We report the prevalence of H. pylori based on the RUT in gastric cancer patients. H. pylori infection influences the tumor histology, progression, and growth type of gastric cancer.
Keywords: Helicobacter pylori, Stomach neoplasms, Phenotype
Introduction
Helicobacter was discovered in 1983,(1) but it was not until 1989 that it was suggested that H. pylori plays a role in the development of precancerous lesions.(2) Further, it has been suggested that H. pylori is associated with gastric cancer based on the findings that H. pylori is more prevalent in patients with atrophic gastritis than a control group.(3) In 1994, the International Agency for Research on Cancer (IARC) classified H. pylori as a definite carcinogenic factor (Group I) in humans.(4) In a meta-analysis, it was reported that the risk of gastric cancer in the population with H. pylori infection increased from 1.81- to 2.24-fold and greater differences were shown in the young age groups.(5) It has been reported that in the population with H. pylori infection, the incidence of gastric cancer is higher in the cytotoxin-associated gene A (CagA)-positive group.(6) In Japanese and Korean patients with gastric cancer, the CagA-positive rate has been reported to be high.(7) It has been reported that the mechanism by which H. pylori induces gastric cancer involves the induction of chronic inflammation in the gastric mucosa, and while progressing to through pre-cancerous lesions (atrophic gastritis, metaplasia, and dysplasia), H. pylori induces mutation of genes.(8) It has been hypothesized that a similar mechanism is involved in the development of colorectal cancer from adenoma to adenocarcinoma; specifically, the process of activation of oncogenes, suppression of tumor suppressor genes, and mismatch repair of DNA occurs, resulting in the development of gastric cancer.(9) Korea is an area where the H. pylori infection rate is high, and thus to examine the effect of H. pylori on gastric cancer, various clinical factors of patients with gastric cancer according to the status of infection and the pathologic factors of gastric cancer were analyzed.
Materials and Methods
Among patients diagnosed with gastric adenocarcinoma who underwent gastrectomies at the Korea Cancer Center Hospital between September 2006 and May 2010, 161 patients in whom H. pylori infection was confirmed by the rapid urease test (RUT) method performed on the gastric mucosa obtained by biopsy immediately after gastrectomy were recruited. Among them 161 patients, 2 patients who received neoadjuvant chemotherapy, 1 patient with remnant gastric cancer, and 3 patients who did not undergo radical resection were excluded; thus, the study was conducted on 155 patients. A past history of H. pylori and eradication treatments were assessed by questionnaire, and patients with a past history of infection were excluded.
In the current study, the status of H. pylori infection was assessed by the RUT. As we reported previously,(10) this method is to biopsy the mucosa of the antrum and body of the stomach (one each), from resected specimens immediately after gastrectomy, add to a RUT kit (Pronto Dry® kit; Medical Instruments Corp., Solothum, Switzerland), fix, and read the positivity based on color changes within 24 hours.
The clinical characteristic of the patients (age, gender, and carcinoembryonic antigen and CA19-9 levels) were recorded. Using an enzyme immunoassay kit (Pyloriset EIA-G; Orion Diagnostica, Espoo, Finland), serum anti-H. pylori immunoglobulin G (anti-HP IgG) was measured, and a value <20 U/ml was read as negative. Early gastric cancer (EGC) or advanced gastric cancer (AGC), the gross type, tumor size, vertical and horizontal size of tumor, histologic type according to the WHO classification, tissue type according to the Lauren's classification, lymphatic invasion, venous invasion, and perineural invasion were examined. In addition, the depth of invasion, lymph node metastasis status, peritoneal washing cytology results, and the TNM disease stage according to the 6th(11) and 7th editions of the UICC(12) were examined.
Univariate analysis was performed for nominal variables by a chi-square test. For the categories in which >5 expected values of cells were <70% were analyzed by the Fisher's exact test. For multivariate analysis, multiple logistic regression model was used. A P-value<0.05 was considered to be statistically significant. SPSS (ver.14; SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
Among 155 patients, 99 were male, 56 were female, and the mean age was 61.6 and 58.1 years, respectively. Ninety-seven patients were >60 years of age and 58 patients were <60 years of age. One hundred thirty-seven patients were H. pylori-positive based on the RUT test, for an 88.4% infection rate.
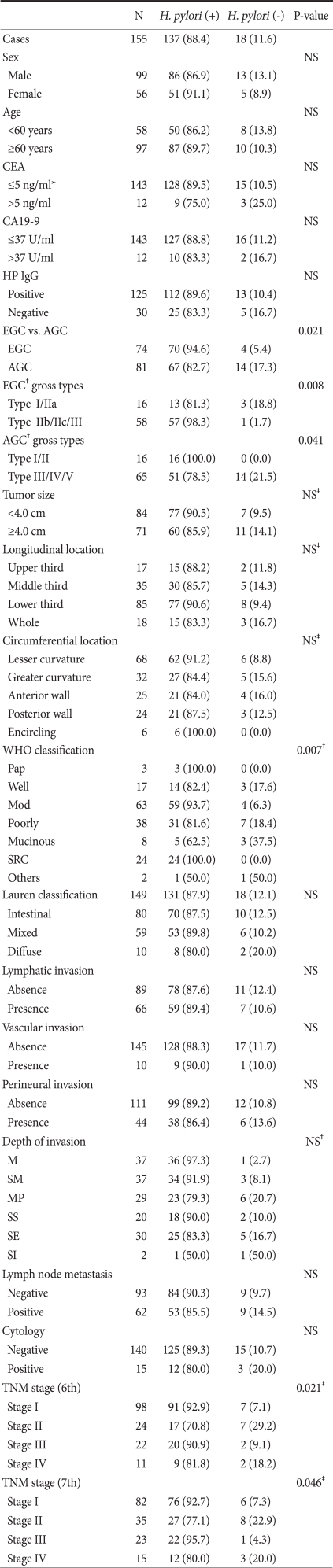
The clinicopathologic factors of H. pylori-positive and -negative gastric cancer patients were compared (Table 1). In the H. pylori-negative group, AGC was prevalent (P=0.021), and the histologic type according to the WHO classification also showed a significant difference between the two groups (P=0.007). The signet ring cell-type was present in 24 patients, all of whom were in the H. pylori-positive group. The poorly differentiated tubular- and mucinous-type were more prevalent in the H. pylori-negative group. Based on macroscopic findings, tumors were divided into EGC and AGC and compared. It was shown that in the H. pylori-negative group, type I or IIa EGC was abundant (P=0.005) and the frequency of types III, IV, and V AGC was high (P=0.041). In addition, the TNM disease stage according to the classification of the 6th (P=0.021) and 7th editions of the UICC (P=0.046) showed a significant difference. The patient gender, age, size or location of the tumor, tumor markers, and the results of immunoserological tests for H. pylori between the two groups were not significantly different.
Table 1.
Comparison of clinicopathologic features between HP positive gastric cancer and HP negative gastric cancer patients in Rapid Urease Test
HP = H. pylori ; NS = not significant; CEA = carcinoembrionic antigen; CA= carbohydrate antigen; EGC = early gastric cancer; AGC = advanced gastric cancer; SRC = signet ring cell; M = mucosa; SM = submucosa; MP = muscularis propria; SS = subserosa; SE = serosa exposure; SI = serosa invasion. *CEA≤10 ng/ml in smoker; †EGC and AGC were categorized according to the histological depth of invasion; Values in parentheses are percentages; ‡P-value in Fisher's exact test.
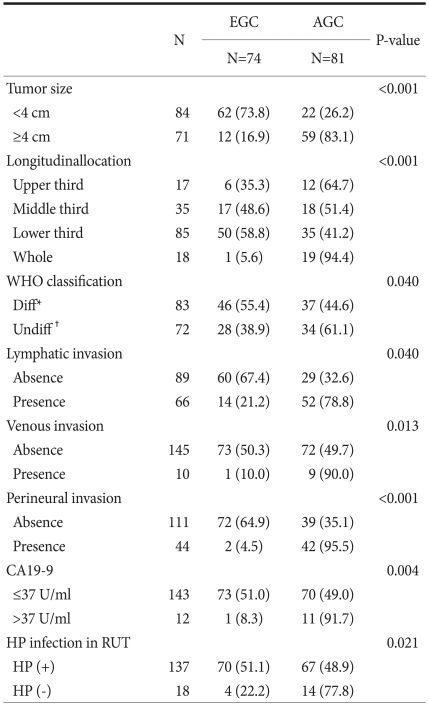
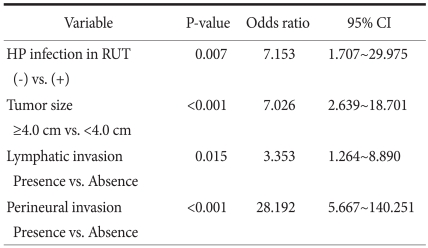
The patients were divided into EGC (74 patients) and AGC groups (81 patients) (Table 2). There were no differences in gender, age, carcinoembryonal antigen level, and H. pylori immunoserological tests between the two groups. Significant factors were tumor size (P<0.001), location (P<0.001), histologic type (P=0.040), lymphatic invasion (P=0.040), venous invasion (P=0.013), and perineural invasion (P<0.001). In addition, the elevation of CA19-9 (P=0.004) and H. pylori infection (P=0.021) also showed a significant difference, and the ratio of AGC was high in the H. pylori-negative group (77.8%). Multivariate analysis was performed on the variables shown to be significant based on univariate analysis (Table 3). Independent risk factors for AGC included lymphatic invasion (odds ratio [OR], 3.353; 95% confidence interval [CI], 1.264~8.890), perineural invasion (OR, 28.192; 95% CI, 5.667~140.251), tumor size (OR, 7.026; 95% CI, 2.639~18.701), and H. pylori-negative (OR, 7.153; 95% CI, 1.707~29.975).
Table 2.
Comparison of clinicopathologic features between EGC and AGC
EGC = early gastric cancer; AGC = advanced gastric cancer; CA= carbohydrate antigen; HP = H. pylori ; RUT = rapid urease test. *Papillary, tubular adenocarcinoma, well andmoderately differentiated; †Tubular adenocarcinoma poorly differentiated, Mucinuous adenocarcinoma, signet ring cell carcinoma, undifferentiated adenocarcinoma and others; Values in parentheses are percentages.
Table 3.
Multivariate analysis between EGC and AGC. Binary logistic regression, backward Conditional method
EGC = early gastric cancer; AGC = advanced gastric cancer; CI = confidence interval; HP = H. pylori; RUT = rapid urease test.
Discussion
In a nested-case control study conducted in many countries, the rate of H. pylori infection in the gastric cancer and control groups was reported to be 81.2% and 66.1%, respectively.(13) In a Japanese study, the infection rate in the gastric cancer and control groups was reported to be 91.1% and 75.6%, respectively.(14) In a prospective study, gastric cancer patients existed in the H. pylori-positive group only, and the infection rate in the gastric cancer group was shown to be 100%.(15) In a nested-case control study conducted in Korea, the status of H. pylori infection in gastric cancer patients was examined, and 83.7% patients were positive.(16) This was not greatly different from the results of the current study (88.4%) that measured the rate of H. pylori infection by RUT only. In the current study, analyzed according to age and gender, the rate of H. pylori infection did not show a significant difference, and other cinical factors also did not show significant differences. In the current study, the status of H. pylori infection was determined by the results of the RUT only. In comparison with the status of H. pylori infection assessed by serological tests, a significant difference was not shown. In the 155 patients, the positive prediction rate by the RUT was shown to be high, but a significant difference was not shown, which may may be due to patients in whom the serological tests were false-negative, false-positive RUT, or harvested specimens that were H. pylori-positive, but H. pylori was absent. Since each study assessed the infection rate of H. pylori using different methods, a direct comparison was difficult.
When infected with H. pylori, inflammation develops in the gastric mucosa due to the infiltration and activation of various inflammatory cells resulting in the injury and degeneration of the epithelium of the stomach. Initially, H. pylori induces the production of various cytokines through the direct contact with gastric epithelial cells, and among them, IL-8 strongly induces leucocytes.(17) In addition, H. pylori infection induces diverse immunologic reactions. H. pylori induces the synthesis of auto-antibodies in parietal cells,(18) which is thought to be a factor inducing the development of atrophic gastritis after H. pylori infection and the progression of gastric cancer. H. pylori infection induces the infiltration of various inflammatory cells, and through this, a large amount of active oxidants are released, NO production is accelerated by the elevation of the expression of iNOS of infected epithelial cells, ammonia is degraded by urease, the reactive oxidant NH2Cl is produced, NH2Cl penetrates cell membranes freely and oxidizes intracellular components, and consequently induces gastric cancer.(19)
Parsonnet et al.(20) reported that gastric cancer was classified into intestinal- and diffuse-type gastric cancer and found that in the H. pylori infection group, intestinal-type gastric cancer was significantly more abundant. In the current study, the association between H. pylori infection and the diffuse-type gastric cancer was not significantly different from the intestinal-type gastric cancer classified according to the Lauren's classification. In the current study, when gastric cancer was classified by histologic morphology according to the WHO classification, a significant difference between the two groups was shown. The poorly undifferentiated tubular- and mucious-type in the negative group was 38.9% (7/18) and 16.7% (3/18), respectively, and it was shown to be relatively more prevalent. Interestingly, 100% signet ring cell-type was detected in the H. pylori-positive group, and such results could be considered in connection to the high incidence of the signet cell-type in Korean patients with gastric cancer.(21) Tatsuta et al.(22) reported that H. pylori infection was significantly more abundant in well-differentiated tumors in patients with EGC. In the current study, the WHO classification was broadly divided into the differentiation group (papillary-, well differentiated-, and moderately differentiated tubular-type) and the undifferentiated group (poorly differentiated tubular-, mucinous-, and the signet ring cell-type) and compared; such a difference was not detected.
The signet cell-type occurs only in the H. pylori-positive group. Therefore, such results are in agreement with the theory that H. pylori infection is associated with the development of the intestinal-type gastric cancer, as shown previously, and it also shows that H. pylori infection is closely associated with the development of signet cell-type gastric cancer. Thus, it suggests that H. pylori infection could exert effects on the phenotype of gastric cancer cells.
In a meta-analysis that compared EGC with AGC, AGC was significantly more prevalent in the H. pylori-negative group in comparison with EGC,(5) and our results were similar. In the current study, when EGC was examined according to morphology, in the H. pylori-negative group, 3 cases were the elevated shape (I/IIa) and 1 case was the flat/depressed shape (IIb/IIc/III), and the H. pylori-positive group 13 of 70 cases had the elevated shape, thus the elevated shape was more prevalent.
In a study analyzed EGC according to H. pylori infection, the infection rate was significantly low in depressed-type EGC, including ulcers.(22) In the current study, in the H. pylori-positive group, type IIc EGC was most prevalent, followed by type IIb, IIa, I, and III in order. In the negative group, the order was IIa>I=IIc. Nonetheless, in the current study, the association of H. pylori infection with the depressed-type EGC was examined and significant results were not shown. In the current study, the number of the H. pylori-negative group, as well as the depressed-type was detected, and patients who had an indication for endoscopic submucosal dissection were excluded, and thus it is difficult to compare directly with the extant literatures.
The ratio of H. pylori infection in EGC and AGC was described previously. In a study which analyzed the status of H. pylori infection exclusively in AGC, it has been reported that in the positive group, Borrmann type I and II were significantly increased.(23) In the current study, in the H. pylori-positive group, Borrmann type I and II of AGC was 16 of 16 cases (100%), and similar results were shown. During the process of the development of gastric cancer, when the gastric mucosa progresses to the precancerous lesion metaplasia, the pyloric mucosa becomes the environment in which H. pylori could not survive. To overcome this, in the current study, a biopsy was also performed on the mucosa of the body of the stomach. Examining the causes of such differences, first, it could be hypothesized that as the area of advanced cancer becomes wide, most areas of the pyloric mucosa where H. pylori harbors is substituted with gastric cancer, and thus becomes the environment where H. pylori could not survive, and such a hypothesis could be applicable to Borrmann type IV gastric cancer infiltrating the entire stomach.
Second, it could be hypothesized that H. pylori infection itself mediates effects on the progression of gastric cancer, and thus in H. pylori-positive gastric cancer, the progression is slow; however, in H. pylori-negative gastric cancer, rapid progression was shown and thus the ratio of advanced gastric cancer becomes high, and the biological characteristic of gastric cancer may be aggressive. This concurs well to the fact that the prognosis of H. pylori-negative patients is poor.(24) In addition, it was observed that in Borrmann type I and II gastric cancer, the rate of the H. pylori-positive rate was high, which shows the close association of the relatively slow progression with H. pylori infection.
H. pylori is a carcinogenic factor for gastric cancer, and depending on the infection status, differences of the frequency of EGC from AGC were shown, and differences of the growth pattern between AGC and EGC were shown. In the histologic classification, differences were also shown, and thus it appears that the status of H. pylori infection exerts effects on the development of gastric cancer and the progression pattern. Nonetheless, the rate of H. pylori infection is high in Korea, consequently, the number of the control group in the current study was small, and thus significant differences were not detected in several categories. In the current study, the status of H. pylori infection according to serological and RUT tests showed great differences, and thus it is thought that studies on the method of the diagnosis of H. pylori infection is required. If more studies are conducted, the clinical course, as well as the pattern of progression of gastric cancer according to H. pylori infection, could be better elucidated.
References
- 1.Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273–1275. [PubMed] [Google Scholar]
- 2.Fox JG, Correa P, Taylor NS, Zavala D, Fontham E, Janney F, et al. Campylobacter pylori-associated gastritis and immune response in a population at increased risk of gastric carcinoma. Am J Gastroenterol. 1989;84:775–781. [PubMed] [Google Scholar]
- 3.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 4.Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 5.Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114:1169–1179. doi: 10.1016/s0016-5085(98)70422-6. [DOI] [PubMed] [Google Scholar]
- 6.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 7.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Stoicov C, Saffari R, Cai X, Hasyagar C, Houghton J. Molecular biology of gastric cancer: Helicobacter infection and gastric adenocarcinoma: bacterial and host factors responsible for altered growth signaling. Gene. 2004;341:1–17. doi: 10.1016/j.gene.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Kim SI, Jin SH, Lee JH, Min JS, Bang HY, Lee JY. An alternative method for a rapid urease test using back-table gastric mucosal biopsies from gastrectomy specimen for making the diagnosis of Helicobacter pylori infection on patients with gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:172–176. [Google Scholar]
- 11.AJCC, editors. AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 12.AJCC, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2009. [Google Scholar]
- 13.Helicobacter and Cancer Collaborative Group, editors. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49:347–353. doi: 10.1136/gut.49.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe Y, Kurata JH, Mizuno S, Mukai M, Inokuchi H, Miki K, et al. Helicobacter pylori infection and gastric cancer. A nested case-control study in a rural area of Japan. Dig Dis Sci. 1997;42:1383–1387. doi: 10.1023/a:1018833819860. [DOI] [PubMed] [Google Scholar]
- 15.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 16.Shin A, Shin HR, Kang D, Park SK, Kim CS, Yoo KY. A nested case-control study of the association of Helicobacter pylori infection with gastric adenocarcinoma in Korea. Br J Cancer. 2005;92:1273–1275. doi: 10.1038/sj.bjc.6602467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crabtree JE, Wyatt JI, Trejdosiewicz LK, Peichl P, Nichols PH, Ramsay N, et al. Interleukin-8 expression in Helicobacter pylori infected, normal, and neoplastic gastroduodenal mucosa. J Clin Pathol. 1994;47:61–66. doi: 10.1136/jcp.47.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negrini R, Savio A, Appelmelk BJ. Autoantibodies to gastric mucosa in Helicobacter pylori infection. Helicobacter. 1997;2(Suppl 1):S13–S16. doi: 10.1111/j.1523-5378.1997.06b05.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Hibi T. Oxidative stress in Helicobacter pylori-associated gastroduodenal disease. J Clin Biochem Nutr. 2006;39:56–63. [Google Scholar]
- 20.Parsonnet J, Vandersteen D, Goates J, Sibley RK, Pritikin J, Chang Y. Helicobacter pylori infection in intestinal- and diffuse-type gastric adenocarcinomas. J Natl Cancer Inst. 1991;83:640–643. doi: 10.1093/jnci/83.9.640. [DOI] [PubMed] [Google Scholar]
- 21.Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060–1064. doi: 10.1111/j.1445-1433.2004.03268.x. [DOI] [PubMed] [Google Scholar]
- 22.Tatsuta M, Iishi H, Okuda S, Taniguchi H, Yokota Y. The association of Helicobacter pylori with differentiated-type early gastric cancer. Cancer. 1993;72:1841–1845. doi: 10.1002/1097-0142(19930915)72:6<1841::aid-cncr2820720608>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Lee WJ, Lin JT, Lee WC, Shun CT, Hong RL, Cheng AL, et al. Clinicopathologic characteristics of Helicobacter pyloric seropositive gastric adenocarcinomas. J Clin Gastroenterol. 1995;21:203–207. doi: 10.1097/00004836-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Meimarakis G, Winter H, Assmann I, Kopp R, Lehn N, Kist M, et al. Helicobacter pylori as a prognostic indicator after curative resection of gastric carcinoma: a prospective study. Lancet Oncol. 2006;7:211–222. doi: 10.1016/S1470-2045(06)70586-1. [DOI] [PubMed] [Google Scholar]