Abstract
Purpose
Most surgeons administer prophylactic antibiotics for 3 to 5 days postoperatively. However, the Center for Disease Control (CDC) guideline recommends antibiotic therapy for 24 hours or less in clean/uncontaminated surgery. Thus, we prospectively studied the use of short term prophylactic antibiotic therapy after gastric cancer surgery.
Materials and Methods
A total of 103 patients who underwent gastric cancer surgery between October 2007 and June 2008 were prospectively enrolled in a short term prophylactic antibiotics program. One gram of cefoxitin was administered 30 minutes before the incision, and one additional gram was administered intraoperatively for cases with an operation time over 3 hours. Postoperatively, one gram was administered 3 times, every 8 hours. Patients were checked routinely for fever. All cases received open surgery, and the surgical wounds were dressed and checked for Surgical Site Infection (SSI) daily.
Results
Of the 103 patients, 15 were dropped based on exclusion criteria (severe organ dysfunction, combined resection of the colon, etc). The remaining 88 patients were included in the short-term program of prophylactic antibiotic use. Of these patients, SSIs were detected in 8 (9.1%) and fever after 2 postoperative days was detected in 11 (12.5%). The incidence of SSIs increased with patient age, and postoperative fever correlated with operation time.
Conclusions
Short term prophylactic antibiotic usage is feasible in patients who undergo gastric cancer surgery, and where there are no grave comorbidities or combined resection.
Keywords: Stomach neoplasm, Antibiotic prophylaxis, Anti-bacterial agents
Introduction
The Surgical Site Infection (SSI) is one of the most important postoperative complications. In 1992, the Surgical Wound Infection Task Force defined SSIs as infections of all the surgery-related sites, including the surgical wound and the organ that is operated on. The mortality of patients is 2~3 times higher when SSI occurs, and the readmission rate is also higher.(1) In 1999, the ASHP (American Society of Health-System Pharmacists) guideline recommended that prophylactic antibiotics be administered within only the first 24 hours after surgery.(2) Long-term antibiotic use may encourage the growth of antibiotic-resistant strains and cause anaphylaxis, and it may also delay making a proper diagnosis by masking pre-existing infections.(3) Despite these problems, long term use is still very common. A study evaluating 302 hospitals regarding prophylactic antibiotics use in Korea revealed that antibiotics were being administered for an average of 7.9 days after surgery. This study also showed that approximately 23% of patients receiving gastrectomy had inappropriate antibiotics administered.(4) Also, many thoracic surgeons and orthopedic surgeons administer antibiotics until all the drainage catheters are removed. There is no evidence that administration of antibiotics according to the duration of the catheter drainage prevents SSI.(5,6) This kind of practice may be due to insufficient knowledge/information and a lack of an organized system that may facilitate reliable antibiotic administration.
There have been studies that have recommended 24-hr short-term prophylactic antibiotic use in appendectomy cases or colon surgery cases.(7-10) Further, it has been proven that for other gastrointestinal surgeries, 24 hr short term prophylactic antibiotics use is as effective as administering prophylactic antibiotics for 3 days or longer.(11) However, most of the research on this in Korea has been limited to hepatobiliary surgery or colon surgery.(7-12) We have previously reported the safety of short term prophylactic antibiotics after gastric cancer surgery as compared to that of longer usage,(13) and we have been using short term prophylactic antibiotics since the time of that study. However, studies that have focused on the inclusion/exclusion criteria of short term antibiotics for gastric cancer patients are insufficient. We present here the clinical outcome of the patients who underwent short term use of prophylactic antibiotics after gastric cancer surgery in order to confirm the rationality of short-term prophylactic antibiotic usage and to determine the specific inclusion/exclusion criteria for this therapy.
Materials and Methods
1. Study group
One hundred three patients were diagnosed with stomach cancer from Oct 2007 to June 2008, and these patients were prospectively evaluated to be resectable according to preoperative study, and they all underwent gastrectomy combined with lymphadenectomy. The preoperative exclusion criteria were preoperative wound infection, severe organ dysfunction, an ASA (American Society of Anesthesia) score >3, they were being treated with insulin or they had complicated DM, bleeding, perforation, obstruction from stomach cancer or a fever over 38℃ within 24 hours before surgery. The intraoperative exclusion criteria were emergency surgery, a co-operation of other organs, combined resection of the pancreas, liver or colon, open thoracic surgery, intraoperative organ damage and intraoperative transfusion. The postoperative exclusion criteria were a re-operation within 24 hours of the first surgery, and major complications not due to infection, requiring further antibiotics therapy.
2. Perioperative care
Open surgery was performed on all the patients. Central venous catheters were inserted preoperatively and they were kept inserted until the time of discharge. For the cases with a suspicion of venous catheter infection, the catheter was removed and it was cultured for phlebitis. Foley catheters were inserted after anesthesia and these were removed 1 day postoperatively after urinary catheter training. All patients were checked daily for signs of infection. Erythema or suppurative fluid discharge at the wound was classified as incisional SSI. Fever, tenderness, and radiologic evidence of fluid collection below the tender point was classified as organ space SSI. Other fever focuses such as respiratory tract infection, urinary tract infection, phlebitis, etc. were classified as fever focuses not due to SSI.
3. Antibiotics injection
The 2nd generation cephalosporin cefoxitin (Pacetin; Choongwae Pharma Corp., Seoul, Korea) was used as a prophylactic antibiotic. One gram was administered within 30 minutes before the first incision, and an additional 1 gram was administered intraoperatively when the operation time exceeded 3 hours. Postoperatively, 1 gram every 8 hours was given for 24 hours. The antibiotics were restarted after the initial 24 hours when there was fever over 38℃, leukocytosis or clear evidence of infection.
4. Statistical analysis
Surgical site infection and variables were analyzed using the chi-square test or Fisher's exact test for proportions. Statistical analysis was performed by SPSS (Statistical Package for Social Science version 13.0, Chicago, IL, USA) and P-value <0.05 was deemed statistically significant.
Results
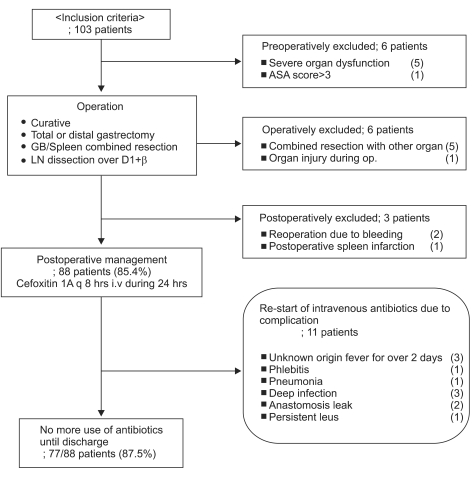
Fifteen of the 103 patients were excluded from study. Of the 6 subjects excluded preoperatively, the 5 cases with severe organ dysfunction had end-stage renal disease, a history of recent myocardial infarction or cerebral infarction, liver cirrhosis or atrial fibrillation. The patient with a high ASA score (4 points) had Parkinson's disease. Of the 6 patients who were excluded intraoperatively, 5 had combined surgery of other organs and 1 had damage to another organ. In cases with combined surgery, 4 had colon resection and 1 had combined distal pancreatectomy. The patient with organ damage experienced intestinal damage during adhesiolysis. For the 3 patients excluded postoperatively, two had re-operations due to postoperative bleeding and one had postoperative spleen infarction. As a result, 88 patients (85.4%) were chosen for the study. As noted before, 1 gram of cefoxitin was administered every 8 hours postoperatively for 24 hours. Eleven patients had antibiotics therapy restarted after the initial 24 hours. Of these 11 patients, 3 had fever of unknown origin for over 2 days, 1 had phlebitis, 1 had pneumonia, 3 had incisional deep infection of the fascia and muscles, 2 had anastomosis leakage and 1 had persistent ileus. A possible cause of fever for the 3 patients who had fever for over 2 days was atelectasis, so chest radiographs were taken. Chest radiographs and physical examination did not show any findings suggestive of atelectasis. The 3 patients were thoroughly evaluated for other possible reasons, but no origin was found. In all 3 cases, the fever spontaneously subsided between postoperative day 3 and 4. Seventy seven of the 88 (87.5%) subjects did not require further antibiotics therapy (Fig. 1).
Fig. 1.
Inclusion criteria and exclusion critera. 103 patients had curative gastrectomy performed. 15 patients were excluded and 88 patients were chosen. 11 patients had antibiotics therapy restarted after the initial 24 hours. 78 patients did not need additional antibiotics therapy until discharge.
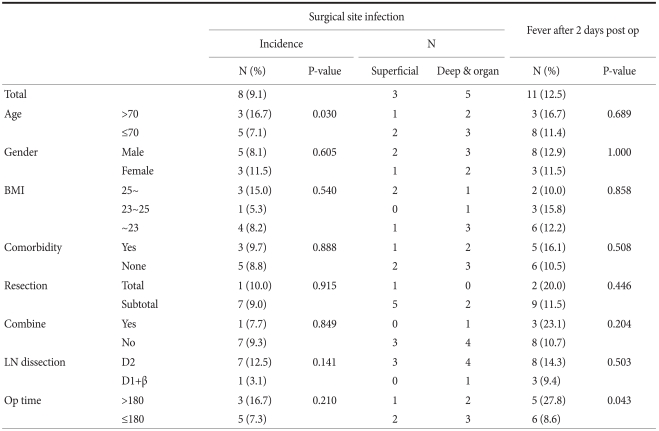
The 88 subjects were classified by age, gender, the BMI, the complications, the extent of gastric resection, combined surgery, the extent of lymph node dissection and the operation time. The presence of SSI and fever after postoperative 2 days was assessed (Table 1). Of the 88 subjects, 18 were over the age of 70, and 70 were under the age of 70. In the over 70 group, there were 3 cases of SSI (16.7%), and there were 8 cases of SSI (11.4%) in the under 70 group. There were higher rates of SSI in the older group, but these results were statistically insignificant (P=0.689). Fifty six subjects had D2 dissection performed, and 32 subjects had D1+β dissection performed. Seven cases had SSI (12.5%) in the D2 dissection group, and 1 case had SSI in the D1+β group (3.1%). There were higher SSI rates with wider node dissection, but the difference between the 2 groups for SSI was statistically insignificant (P=0.503). Of the 88 subjects, 70 subjects had an operation time under 180 minutes and 18 patients had an operation time over 180 minutes. In the over 180 minute group, 5 patients (27.8%) had fever, and 6 patients (8.6%) in the under 180 minute group had fever. These results were statistically significant (P=0.043), and they showed that the subjects with a longer operation time had a higher rate of fever.
Table 1.
Results of analyzing the 88 patients who were injected with short term prophylactic antibiotics
N = number; BMI = body mass index; LN = lymph node.
Discussion
The SSI is an important complication after surgery. As the importance of patient factors related to SSI has recently come to light, there have been trials of individualized postoperative antibiotic therapy according to certain patient factors.(14-17) Maintaining a normal body temperature before and after surgery, and providing a FiO2 over 80% in the operating room and the recovery unit have proven to increase the O2 saturation in the operation wound and to improve the WBC function, and so this decreases the rate of SSI. Also, previous studies have shown that controlling the blood glucose levels, regardless of a previous history of DM, decreases SSI and other surgical complications.(15-17)
It has been proven that prophylactic antibiotics are also effective for preventing SSIs.(18) The use of prophylactic antibiotics is already widely acknowledged and this is being commonly practiced, but the route of administration, the duration of therapy and many other factors such as the site and method of surgery, the patient age, the operation time and the degree of intraoperative contamination must be considered when choosing the type of antibiotics. Antibiotic therapy given in accordance with various patient factors may have a result that greatly differs from that of the antibiotic therapy that is given without considering the patient.
In this study, all the patients had pathologically confirmed stomach cancer, and they all underwent gastrectomy. The operation wounds of gastrectomies are considered clean-uncontaminated wounds, and all these patients require prophylactic antibiotics. We found that of the 88 subjects, 77 subjects did not require additional antibiotic therapy after 1 postoperative day, and this confirms the rationality of short-term prophylactic antibiotic therapy. Also, when analyzing the patients who required additional antibiotics after the initial 24 hours, we found that patients who had received longer operations showed fever after the postoperative second day. Also, older patients and patients who had received wider node dissection tended to have fever, but these properties did not show statistical significance.
At the beginning of the study, we started with an exclusion criteria. These criteria were based on exclusion criteria commonly used for other types of surgery. As for the intraoperative criteria, we feared that prospectively enrolling patients who may be potential candidates of grave co-morbidities may have negative results.
Among the 11 patients who required additional antibiotics, 3 patients had unexplained fever for over 2 days. As discussed earlier, all 3 patients were evaluated for possible causes of postop fever, but no origin was documented. In these cases the antibiotics were restarted to avoid possible aggravation of hidden causes. Whether unexplained fever warrants long term antibiotics is arguable.
A limitation of this study is the one-armed study design. Before initiation of the study, our institution, like many others in Korea, administered prophylactic antibiotics quite empirically. Although this study lacks control group data (data of patients who received long term antibiotics), overall results show no significant discrepancies with the results of our previous patients, who had received long term antibiotics (data not shown). After confirming the feasibility of short term prophylactic antibiotics, we have started following the short term principle. Also, the choice of antibiotics is a limitation. For gastric cancer surgery, 1st generation cephalosporin is sufficient.(19) Before this study, we administered 2nd generation cephalosporin as prophylactic antibiotics. In controlling the parameter of administration duration, we decided to lessen a variable by continuing the 2nd generation cephalosporins. Feasibility of short term antibiotics with the recommended 1st generation cephalosporins is unevaluated in this study.
Despite the problems of long term antibiotics use discussed earlier, unwarranted long-term use is still very common. There are several factors that cause unnecessary long term antibiotics usage. This may be the result of a lack of an organized system for guiding prophylactic antibiotics administration. When there is no clinical pathway that guides the doctor when administering specific antibiotics for specific types of surgery, the antibiotic therapy is at risk of being changed on a case-by-case basis and at the whim of the ordering physician. Previous studies have shown that long term antibiotics use is largely the result of the uninformed orders given by residents or interns, and this in turn is the result of improper education.(20) In 2005, the SIPP (Surgical Infection Prevention Project) was launched in the U.S. to lower the rate of SSI. The preliminary research has shown that during 32,000 cases of surgery, only in 55.7% of the cases were the prophylactic antibiotics given within 1 hour of the first incision. Based on these findings, an education program lasting about one year was started in over 50 centers, and the SSI rates were lowered by about 27%.(19,21) This shows that if the ordering doctor does not have accurate information on prophylactic antibiotics, then the preventable SSIs may not be prevented. To overcome these shortcomings, the duration of antibiotic therapy has recently become one of the factors for judging the quality of medical service in Korea. Many centers are also making guidelines and recommendations regarding antibiotics administration. As proven in this study, when the appropriate subjects are chosen, short-term prophylactic antibiotic therapy may reduce the adverse effects of long term antibiotics use and it may effectively prevent SSI.
In conclusion, short-term prophylactic antibiotics therapy is effective when performing stomach cancer surgery, in cases without grave comorbidities or significant combined resection. The feasibility of short-term antibiotics when 1st generation cephalosporins are used warrants further study.
References
- 1.Mayer AD, Brennan SS, Pickford IR, Evans M, Pollock AV. The prophylaxis of surgical wound infection: is cefuroxime any better than cephaloridine? J Hosp Infect. 1982;3:143–148. doi: 10.1016/0195-6701(82)90006-8. [DOI] [PubMed] [Google Scholar]
- 2. [January 21, 2004]. Accessed http://www.ashp.org/DocLibrary/BestPractices/TGSurgery.aspx.
- 3.Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, Jr, et al. Infectious Diseases Society of America. Quality standard for antimicrobial prophylaxis in surgical procedures. Infectious Diseases Society of America. Clin Infect Dis. 1994;18:422–427. doi: 10.1093/clinids/18.3.422. [DOI] [PubMed] [Google Scholar]
- 4.Kim KH, Park CS, Chang JH, Kim NS, Lee JS, Choi BR, et al. Association between prophylactic antibiotic use and surgical site infection based on quality assessment data in Korea. J Prev Med Public Health. 2010;43:235–244. doi: 10.3961/jpmph.2010.43.3.235. [DOI] [PubMed] [Google Scholar]
- 5.Gorecki P, Schein M, Rucinski JC, Wise L. Antibiotic administration in patients undergoing common surgical procedures in a community teaching hospital: the chaos continues. World J Surg. 1999;23:429–432. doi: 10.1007/pl00012319. [DOI] [PubMed] [Google Scholar]
- 6.Polk HC, Jr, Christmas AB. Prophylactic antibiotics in surgery and surgical wound infections. Am Surg. 2000;66:105–111. [PubMed] [Google Scholar]
- 7.Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds; Guidelines for clinical care. Arch Surg. 1993;128:79–88. doi: 10.1001/archsurg.1993.01420130087014. [DOI] [PubMed] [Google Scholar]
- 8.Andersen BR, Kallehave FL, Andersen HK. Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst Rev. 2005;(3):CD001439. doi: 10.1002/14651858.CD001439.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang SH, Kim SW, Jo IH, Hwang KS, Park SZ, Kim CD, et al. Prospective clinical study of prophylactic antibiotic therapy in nonperforated appendicitis. J Korean Surg Soc. 2000;58:824–833. [Google Scholar]
- 10.Jo JH, Lee SH, Ahn BK, Baek SU. Efficacy of 24 Hour-administration of antibiotic prophylaxis after elective colorectal surgery. J Korean Surg Soc. 2008;74:129–133. [Google Scholar]
- 11.Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 12.Sohn BH, Lim JS, Jo DH, Kang KJ, Lim TJ. Clinical effect of an intraoperative bile culture and antibiotic prophylaxis in biliary tract surgery. J Korean Surg Soc. 1998;54:109–116. [Google Scholar]
- 13.Si Y, Hur H, Kim SK, Jun KH, Chin HM, Kim W, et al. The use of short-term antimicrobial prophylaxis in elective surgery for gastric cancer. J Korean Gastric Cancer Assoc. 2008;8:154–159. [Google Scholar]
- 14.Walz JM, Paterson CA, Seligowski JM, Heard SO. Surgical site infection following bowel surgery: a retrospective analysis of 1446 patients. Arch Surg. 2006;141:1014–1018. doi: 10.1001/archsurg.141.10.1014. [DOI] [PubMed] [Google Scholar]
- 15.Kurz A, Sessler DI, Lenhardt R Wound Infection and Temperature Group. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. N Engl J Med. 1996;334:1209–1215. doi: 10.1056/NEJM199605093341901. [DOI] [PubMed] [Google Scholar]
- 16.Greif R, Akça O, Horn EP, Kurz A, Sessler DI Outcomes Research Group. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 17.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 18.Stone HH, Hooper CA, Kolb LD, Geheber CE, Dawkins EJ. Antibiotic prophylaxis in gastric, biliary and colonic surgery. Ann Surg. 1976;184:443–452. doi: 10.1097/00000658-197610000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bratzler DW, Houck PM, Richards C, Steele L, Dellinger EP, Fry DE, et al. Use of antimicrobial prophylaxis for major surgery: baseline results from the National Surgical Infection Prevention Project. Arch Surg. 2005;140:174–182. doi: 10.1001/archsurg.140.2.174. [DOI] [PubMed] [Google Scholar]
- 20.Wasey N, Baughan J, de Gara CJ. Prophylaxis in elective colorectal surgery: the cost of ignoring the evidence. Can J Surg. 2003;46:279–284. [PMC free article] [PubMed] [Google Scholar]
- 21.Dellinger EP, Hausmann SM, Bratzler DW, Johnson RM, Daniel DM, Bunt KM, et al. Hospitals collaborate to decrease surgical site infections. Am J Surg. 2005;190:9–15. doi: 10.1016/j.amjsurg.2004.12.001. [DOI] [PubMed] [Google Scholar]