Abstract
Purpose
Laparoscopy-assisted gastrectomy (LAG) has become a technically feasible and safe procedure for early gastric cancer treatment. LAG is being increasingly performed in many centers; however, there have been few reports regarding LAG at low-volume centers. The aim of this study was to report our early experience with LAG in patients with gastric cancer at a low-volume center.
Materials and Methods
The clinicopathologic data and surgical outcomes of 39 patients who underwent LAG for gastric cancer between April 2007 and March 2010 were retrospectively reviewed.
Results
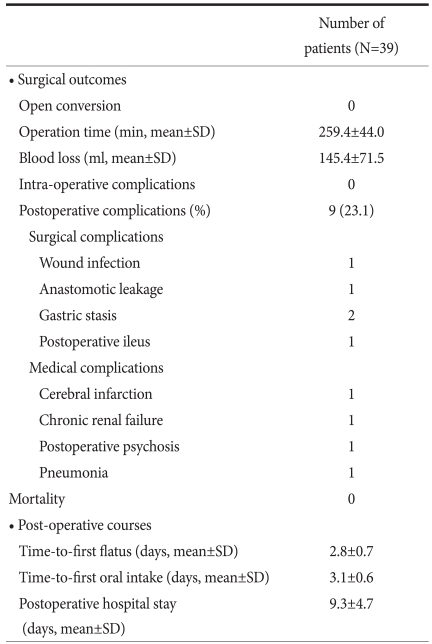
The mean age was 68.3 years. Thirty-one patients had medical co-morbidities. The mean patient ASA score was 2.0. Among the 39 patients, 4 patients underwent total gastrectomies and 35 patients underwent distal gastrectomies. The mean blood loss was 145.4 ml and the mean operative time was 259.4 minutes. The mean time-to-first flatus, first oral intake, and the postoperative hospital stay was 2.8, 3.1, and 9.3 days, respectively. The 30-day mortality rate was 0%. Postoperative complications developed in 9 patients, as follows: anastomotic leakage, 1; wound infection, 1; gastric stasis, 2; postoperative ileus, 1; pneumonia, 1; cerebral infarction, 1; chronic renal failure, 1; and postoperative psychosis, 1.
Conclusions
LAG is technically feasible and can be performed safely at a low-volume center, but an experienced surgical team and careful patient selection are necessary. Furthermore, for early mastery of the learning curve for LAG, surgeons need education and training in addition to an accumulation of cases.
Keywords: Stomach neoplasms, Laparoscopy, Gastrectomy
Introduction
For the treatment for early gastric cancer (EGC), laparoscopy-assisted gastrectomy (LAG) is echnically feasible and accepted to be a safe method. Recently, Kitano et al.(1) reported the 5-year survival rate in a prospective, multicenter study, and excellent oncologic outcomes of LAG have been established. With the increased performance of routine health examinations, the incidence of EGC is on the rise. Recently, the incidence of EGC has been reported to be >50%, and thus the application of LAG is correspondingly on the rise. In comparison with laparotomy, laparoscopic surgery has advantages the following advantages: rapid recovery of gastrointestinal function; short hospitalization period; decreased pain due to a reduction in wound size; and cosmetic advantages. Thus, LAG is being performed in more institutions. Large institutions performing >100 cases annually have reported outcome data; however, reports on LAG at small institutions performing <50 cases annually are limited.(2,3) Therefore, we analyzed the early mastery of the learning curve for LAG with respect to the operative time and safety when performed at low-volume centers.
Materials and Methods
1. The subjects
We retrospectively reviewed the database of 39 patients who underwent LAG at the National Medical Center between April 2007 and March 2010. Distal gastrectomy were performed on 35 patients and total gastrectomy were performed on 4 patients. All patients were thoroughly informed about LAG prior to surgery, and a consent for surgery was obtained. An endoscopic biopsy and abdominal computed tomography were performed on all patients. At our hospital, the indication for LAG in gastric cancer patients is limited to pre-operative stage T1N0M0, T1N1M0, or T2N0M0. Patients with an ASA score >4 points and patients suitable for endoscopic mucosal resection were excluded.(4) The indication for endoscopic mucosal resection was mucosal carcinoma without ulceration and a lesion <2 cm. Disease stage was classified according to the UICC TNM classification (6th edition). The characteristics of patients, such as age, gender, body mass index (kg/m2), history of abdominal surgery, ASA score, co-morbidities, and surgical outcomes (operative methods, pathologic results, operative time, blood loss, complications, time-to-first flatus, time-to-first oral intake, and post-operative hospitalization period), were examined.
2. Surgical methods
Patients were placed in the supine position, and the surgeon stood on the right side of the patient. A camera assistant who also served as the first assistant was positioned on the left side of the patient. The second assistant was positioned on the right side of the camera assistant. After general anesthesia, a 10-mm trocar was inserted at the sub-umbilical area, and a pneumoperitoneum was formed by insufflation of carbon dioxide. The patient was placed in reverse. Trendelenburg position and five additional trocars were inserted. The intraperitoneal pressure was maintained as 12~13 mmHg. A 12-mm trocar was inserted on the right side of the umbilicus, and lateral to the rectus abdominis muscle. A 5-mm trocar was inserted in the subcostal area on the lateral side of the rectus abdominis muscle for use by the surgeon. A 10-mm trocar was inserted at the subxiphoid process area and used for insertion of a fan-shaped retractor. A 10-mm trocar was inserted at the left subcostal area on the lateral side of the rectus abdominis muscle. A 5-mm trocar was inserted on the left side of the umbilicus and the lateral side of the rectus abdominis muscle for use by the assistant in retracting organs. The EXERA® laparo-thoraco videoscope (Olympus, Tokyo, Japan) was used. For dissection of tissues, an ultrasonic cautery Harmonic scalpel® (Ethicon-Endo Surgery, Cincinnati, OH, USA) was used. For gastroduodenostomy cases, a 4~6 cm transverse mini-laparotomy was made in the subcostal area. For other anastomoses, a vertical 4~6 cm mini-laparotomy was made in the subxiphoid process area, and an ALEXIS® wound retractor was installed (2.5~6 cm; Applied Medical, Rancho Santa Margarita, CA, USA), and the procedure was performed. For gastroduodenostomies after distal gastrectomy or esophagojejunostomy after total gastrectomy, circular staplers were used. For Billroth-II gastrojejunostomy and Roux-en-Y gastrojejunostomy after distal gastrectomy, linear staplers were used.
Results
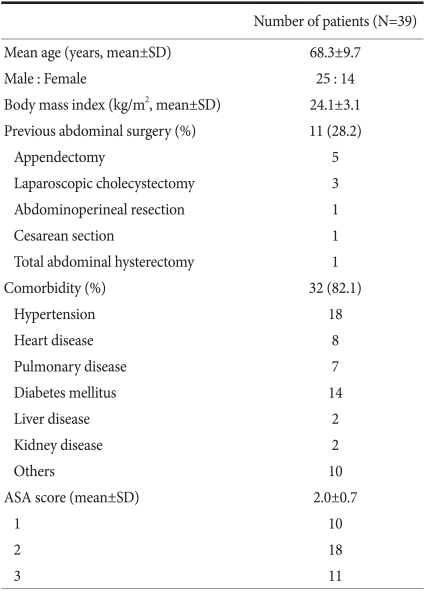
In our study, surgery was performed by one surgeon without LAG experience for gastric cancer. However, the surgeon had considerable experience in LAG for gastric cancer as the first assistant and during conventional open gastrectomy. Furthermore, the surgeon had performed numerous laparoscopic procedures for diverse benign diseases, such as laparoscopic cholecystectomy, laparoscopic appendectomy, laparoscopic adrenalectomy, and laparoscopic surgery for peptic ulcers. None of the procedures were converted to open gastrectomy. No intra-operative complications or mortalities occurred. In 1 stage IA patient, hepatic metastasis was detected 8 months after surgery. The characteristics of the subject patients are summarized in Table 1. The mean age of the patients was 68.3 years. There were 25 male and 14 female patients. The mean BMI was 24.1 kg/m2. Thirty-two patients had co-morbidities, and the mean ASA score was 2.0.
Table 1.
Characteristics of patients
SD = standard deviation; ASA= American Society of Anesthesiologists.
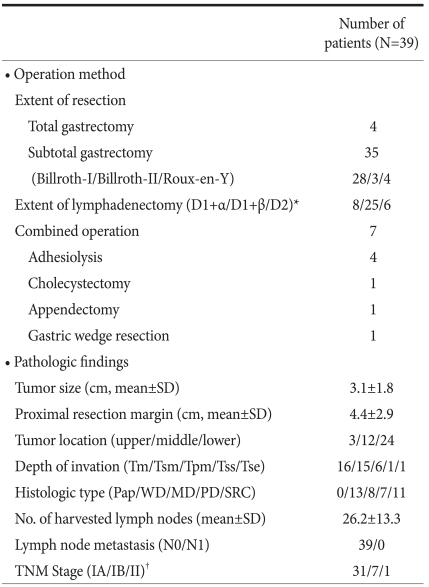
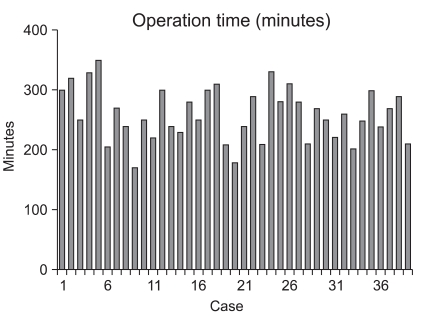
The operative methods and the pathologic results are summarized in Table 2. The mean tumor size was 3.1 cm. The location of the tumor was the upper body in 3 patients, the mid-body in 12 patients, and the lower body in 24 patients. Four patients underwent total gastrectomy and 35 patients underwent distal gastrectomy. As the reconstruction method after distal gastrectomy, 28 patients had gastroduodenostomy, 3 patients had Billroth-II gastrojejunostomy, and 4 patients had Roux-en-Y gastrojejunostomy. Combined surgical procedures were performed in 7 patients. Four patients had adhesiolysis, 1 patient had a cholecystectomy, 1 patient had an appendectomy, and 1 patient with GIST in a low-risk group had a gastric wedge resection of the greater curvature of the upper body. Lymphadenectomies were performed as follows: D1+α, 8 patients; D1+β, 25 patients; and D2, 6 patients. The mean proximal resection margin was 4.4 cm. The depth of invasion was the mucosa in 16 patients, the submucosa in 15 patients, the proper muscle in 6 patients, the subserosa in 1 patient, and the serosa in 1 patient. In the patient with serosal invasion, serosal invasion was not detected in the operative field, but diagnosed as focal serosal invasion based on pathologic evaluation. With respect to histologic type, 13 patients had well-differentiated carcinoma, 8 patients had moderately-differentiated carcinoma, 7 patients had poorly-differentiated carcinoma, and 11 patients had signet ring cell carcinoma. The mean number of retrieved lymph nodes was 26.2. Lymph node metastasis was not detected in any of the patients. Thirty-one patients were stage IA, 7 patients were stage IB, and 1 patient was stage II according to the 6th UICC TNM staging. The operative times and post-operative hospital stays are shown in Fig. 1 and 2 for each patient, respectively. The mean operative time was 259.4 minutes, the mean blood loss was 145.4 ml, and none of the cases were converted to open gastrectomy. Post-operative complications developed in 9 patients, as follows: wound infection, 1; anastomostic leakage, 1; gastric stasis, 2; post-operative ileus, 1; pneumonia, 1; cerebral infarction, 1; chronic renal failure, 1; and post-operative psychosis, 1. The patient with a cerebral infarction had hypertension and diabetes and was in a high-risk group with a history of myocardial infarction and cerebral infarction.
Table 2.
Operation method and pathologic findings
SD = standard deviation; Tm = mucosa; Tsm = submucosa; Tpm = proper muscle; Tss = subserosa; Tse = serosa; Pap = papillary adenocarcinoma; WD = well-differentiated adenocarcinoma; MD = moderately-differentiated adenocarcinoma; PD = poorly-differentiated adeno carcinoma; SRC = signet ring cell carcinoma.
*Extent of lymph adenectomy classified according to the Guidelines of the Japanese Gastric Cancer Association; †Stage classified by the 6th the edition of the International Union Against Cancer (UICC).
Fig. 1.
Operative time of the each case.
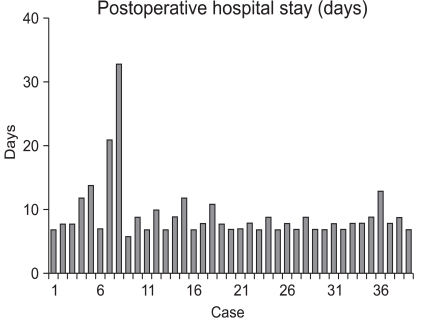
Fig. 2.
Postoperative hospital stay of the each case.
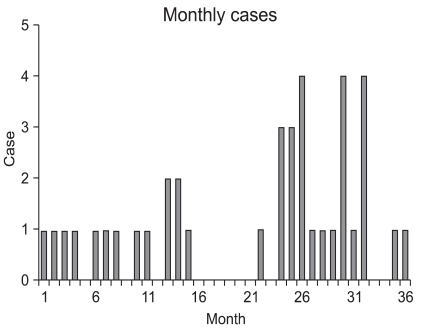
The patient with chronic renal failure had a number of co-morbidities, such as hypertension, diabetes, and diabetic nephropathy. The patient developed renal failure post-operatively, thus hemodialysis was performed. In the patient with an anastomostic leakage, bile juice leaked into the drainage tube 2 days post-operatively, thus an anastomostic leakage was confirmed; the anastomotic leakage closed spontaneously 4 days post-operatively. For the two patients with abdominal distention and vomiting post-operatively, an endoscopic examination and contrast study were performed, gastric stasis was noted, and the symptoms improved with conservative treatment. The time-to-first flatus was a mean of 2.8 days. The time-to-first oral intake was an average of 3.1 days, and the mean post-operative hospital stay was 9.3 days (Table 3). Fig. 3 shows the number of LAGs performed in each month. On average, 1 LAG was performed per month.
Table 3.
Surgical outcomes and postoperative courses
SD = standard deviatation
Fig. 3.
The numbers of operations per month.
Discussion
Less pain, faster recovery, and cosmetic superiority of laparoscopic surgery in comparison with laparotomy have been verified, thus laparoscopic surgery has been widely applied to diverse gastrointestinal diseases, including EGC. EGC is on the rise due to improvements in diagnostic methods and changes in the concept of routine health examinations.(5) The 5-year survival rate for EGC patients who undergo surgical treatment has been reported to be >90%.(6-8) Based on the fact that most EGC patients have low rates of lymph node metastasis as a means to improve the quality of life after surgery, LAG is on the rise, and it has become a standardized procedure. Thus, LAG is performed not only at large institutions, but most institutions which treat patients with gastric cancer. However, LAG is a difficult and complex procedure for technical aspects, and thus most surgeons cannot perform the procedure
Because LAG can be performed only after the accumulation of surgical experience in laparoscopic surgery, a long time is required to master the learning curve for LAG. In order to perform complex procedures, such as LAG, it is necessary to select an appropriate patient group. In addition, surgeons should understand the anatomy, adapt to the laparoscopic view rapidly, and be able to manage laparoscopic surgical instruments accurately and safely. In addition, the preparation of personnel (an experienced surgical team) and equipment is required to master the learning curve for LAG through many cases.
In LAG a sufficient learning curve is required to maintain good surgical outcomes post-operatively. Kim et al.(9) reported that operative time was improved from the 50th case in patients undergoing laparoscopy-assisted distal gastrectomy. Zhang and Tanigawa(10) reported on the learning curve of laparoscopic surgery for gastric cancer and concluded that 60~90 cases of experience were required to complete the learning curve. Jin et al.(11) suggested the learning curve for LAG involving complex and difficult procedures, such as extended lymphadenectomy (higher than D1+β lymphadenectomy), combined surgical procedures, and total gastrectomy or the extension of the selection of patients should be attempted after completing the learning curve. Based on multivariate analysis of risk factors for complications of LAG, we have reported that co-morbidities and the experience of the surgeon are risk factors for the development of post-operative complications.(12) Nevertheless, the above studies were reports of large institutions, and studies conducted in small institutions do not exist. Our study was conducted on a low-volume institution performing <50 cases for gastric cancer annually. As shown in Fig. 3, because an average of one surgery was performed per month, it is difficult to master the learning curve in a short time. In most studies examined the learning curve for LAG, 40~90 cases have been considered to be the point at which the learning curve was mastered. In low-volume institutions, as in the current study, it takes 5~10 years to accumulate the recommended number of cases. As shown Fig. 1, the operative time shows that the learning curve is not mastered completely and is still ongoing. Because the absolute number of surgery cases is small or the lack of continuity of surgery, the time required to master the learning curve becomes longer, and an experienced surgical team and the preparation of equipment is unsatisfactory. In addition, the selection of patients may be extended inappropriately, such as to elderly patients and patients with an ASA score >3, resulting in the induction of surgical as well as non-surgical complications.
However, despite disadvantageous conditions pertinent to mastering the learning curve at low-volume institutions, the results of our study show relatively good outcomes with respect to the incidence of complications. It is thought that various experiences in conventional open gastrectomy of the surgeon, participation in numerous education programs pertinent to laparoscopic surgery, and the adaptation to laparoscopic surgery of the first assistant are of great help. Furthermore, it was determined that not only gastric cancer, but the application of laparoscopy to diverse gastrointestinal diseases was helpful.
LAG can be performed safely at low-volume institutions. Nevertheless, LAG is limited by mastering the learning curve in a short time. In order to overcome this, more experienced surgical teams should be assembled and equipment is required. Surgeons should have abundant experience in laparotomy, and together with the selection of appropriate patients, systematic education and experience as assistant should be preceded in order to adapt to laparoscopic views.
References
- 1.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azagra JS, Goergen M, De Simone P, Ibanez-Aguirre J. The current role of laparoscopic surgery in the treatment of benign gastroduodenal diseases. Hepatogastroenterology. 1999;46:1522–1526. [PubMed] [Google Scholar]
- 3.Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg. 1999;229:49–54. doi: 10.1097/00000658-199901000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keats AS. The ASA classification of physical status--a recapitulation. Anesthesiology. 1978;49:233–236. doi: 10.1097/00000542-197810000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Korea Gastric Cancer Association. Nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2002;2:105–114. [Google Scholar]
- 6.Lee CH, Lee SI, Ryu KW, Mok YJ. Chronological changes in the clinical features of gastric cancer. J Korean Gastric Cancer Assoc. 2001;1:161–167. [Google Scholar]
- 7.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Analysis of prognostic factors and gastric cancer specific survival rate in early gastric cancer patients and its clinical implication. J Korean Surg Soc. 2003;65:309–315. [Google Scholar]
- 8.Japanese Gastric Cancer Association Registration Committee. Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 9.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Tanigawa N. Learning curve of laparoscopic surgery for gastric cancer, a laparoscopic distal gastrectomy-based analysis. Surg Endosc. 2009;23:1259–1264. doi: 10.1007/s00464-008-0142-3. [DOI] [PubMed] [Google Scholar]
- 11.Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28–33. doi: 10.1007/s00464-005-0634-3. [DOI] [PubMed] [Google Scholar]
- 12.Park JM, Jin SH, Lee SR, Kim H, Jung IH, Cho YK, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc. 2008;22:2133–2139. doi: 10.1007/s00464-008-9962-4. [DOI] [PubMed] [Google Scholar]