Abstract
Purpose
Most gastric cancer patients undergo operations at large tertiary hospitals in Korea. However, some patients are treated at low volume hospitals. We investigated patient outcomes after gastric surgery at a secondary hospital and compared with outcomes of large volume centers.
Materials and Methods
We included 184 patients who underwent gastric surgery for gastric cancer at our hospital from January 2003 to December 2008. We conducted a retrospective study and evaluated the clinicopathological characteristics, clinical outcomes and survival rate of patients.
Results
Mean age was 61.7 years old. Male to female ratio was 2.2 : 1. Proportion of early gastric cancer was 38.6% and that of advanced gastric cancer was 61.4%. The 5 year overall survival rate of 184 patients was 66.3%. The overall survival rate was significantly lower for people over 62 years old. The morbidity rate and mortality at our hospital were 10.3% and 0.5%, respectively.
Conclusions
The overall survival rate, morbidity and mortality were similar to those of the previous reports from Korea. Treatment of gastric cancer at a secondary hospital is feasible and safe. Standardization of operations and management of gastric cancer patients of the Korean Gastric Cancer Association is the most important factor to achieve these outcomes.
Keywords: Stomach neoplasms, General surgery, Survival, Low volume center, Reference standards
Introduction
Gastric cancer is still the second leading cause of cancer death worldwide.(1) Although the incidence is on the decrease in Korea, according to the annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993~2007) in Korea, in 2007, 25,915 new cases were developed. At 16% of cancers developed during that time, gastric cancer has the highest incidence. It appears to be reduced substantially in comparison with 20.2% in 2002. Nonetheless, 2,700 cases developed in one year. In 2007, 20.3% of male cancers was gastric cancer, which makes it the most frequent. In females gastric cancer was 11.2% of female cancers, which makes it the third most frequent cancer. Thus it is one of the most important cancers in Korea.(2) Since surgical resection is the only treatment that can improve survival rates in gastric cancer, successful surgeries are essential.
Gastric cancer occurs evenly in all geographical areas in Korea. Nonetheless, most patients are treated at large-volume centers in the capital.(3) The outcome of surgeries compared to the number of surgeries performed is of paramount importance to patients and their families. Nevertheless, the size of the hospital and the number of surgery cases performed cannot reflect all technical aspects of treatments for cancer patients. Therefore, we examined the clinicopathological characteristics of patients who underwent surgery for gastric cancer at medium-sized hospitals (about 400 beds) in comparison with large hospitals. Post-surgical morbidity, mortality, and long-term survival rate were examined, and compared with the published treatment outcomes of surgery in large hospitals.
Materials and Methods
The subjects were all patients who were diagnosed with gastric cancer and received surgery in the Department of Surgery, The Catholic University of Korea, School of Medicine, Saint Paul's Hospital from January 2003 to December 2008. There were 184 patients in total, and their medical records were examined retrospectively. The age of the patient, gender, symptoms, and symptomatic period were examined. As clinicopathological factors, the location of gastric cancer, macroscopical classification, histopathological classification, and Lauren's classification were applied. For the TNM disease stage classification, the guidelines of the Union for International Cancer Control (UICC), 6th edition, were used to be able to compare these records with other studies. The surgical methods, reconstruction methods, the number of resected lymph nodes, postoperative complications and survival rate were examined, since they are the outcomes pertinent to surgery. In the examination of postoperative complications, wound seromas were included in wound infection. Hemorrhage was defined as bleeding in drainage tubes or the nasogastric tube, vital signs were changed, or postoperative hemoglobin value was reduced by more than 3 g/dl. Intestinal obstruction was defined as abdominal pain or vomiting, and the ileus was detected by plain abdominal imaging. Pulmonary complications were defined as fever and diagnosed by plain radiological images. The leakage from anastomosis and intra-abdominal abscesses were diagnosed by clinical symptoms such as abdominal pain and fever and abdominal computed tomography. Postoperative death was defined as death within 30 days after surgery during hospitalization. In cases where radical resection was performed, stages were assessed. For cases with serosal infiltration or with lymph node metastasis, considering the condition of the patient, chemotherapy based on 5-FU was performed after surgery. For inoperable cases, palliative chemotherapy was performed. Surgeries were performed by surgeons with more than 20 years experience and 2 surgeons with experience in more than 100 cases. Except in cases with distant metastasis or inoperable tumors in which palliative surgery was performed, a lymphadenectomy higher than D2 was the standard surgery. For early gastric cancer, a lymphadenectomy higher than D1+β was performed. The record of surgery and the record of the information of patients were prepared using the common form of the Department of Surgery, The Catholic University of Korea, School of Medicine prepared following the form of the Korean Gastric Cancer Association. For the management of patients after surgery, the common critical pathway of the Department of the Upper Gastrointestinal tract by The Catholic University of Korea, School of Medicine was applied.(4) At our hospital, the ambulatory follow-up observation was performed at a 3 month interval for 3 years after surgery, and subsequently at a 6 month interval for the next 5 years. Tumor marker checks and abdominal computed tomography were performed at a 3 month interval and esophagogastroscopic examination was performed at a 6 month interval. Based on the data, the medical records of patients who visited our outpatient department or were hospitalized between April 2010 and July 2010 were analyzed, patients responded to follow-up telephone interviews. All results were analyzed retrospectively based on medical records. The research protocols were approved by the IRB of our institute (IRB No.: PC10RISI0036).
In our study, statistical analysis was performed using the SPSS ver. 12.0 statistics program for Windows (SPSS Inc., Chicago, IL, USA). the survival rate was obtained by the Kaplan-Meier method. Statistical significance between survival curves was validated by a log rank test, and a P-value less than 0.05 was considered to be significant.
Results
184 patients received surgery for gastric cancer at our hospital from January 2003 to February 2008. They were all enrolled in this study.
1. Gender and age
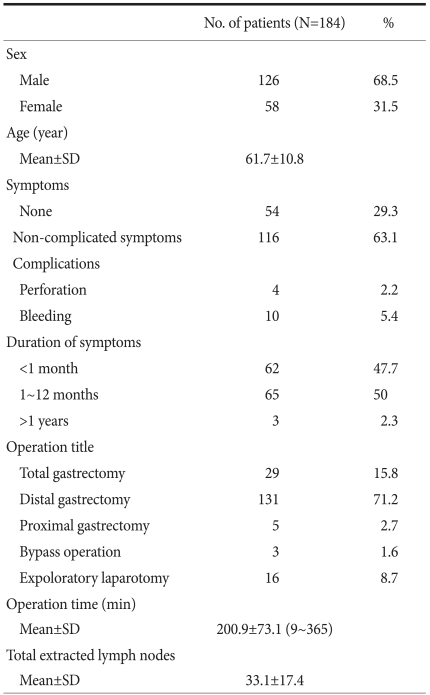
Among the total patients, 126 cases were male (68.5%) and 58 cases were female (31.5%). The ratio of male to female was 2.2 : 1. The mean age was 61.7 years (Table 1).
Table 1.
Characteristics of stomach cancer patients and surgery
SD = standard deviation.
2. Symptoms
Pain and discomfort of the upper abdomen was most prevalent in 100 cases (54.3%). Asymptomatic cases (29.3%) were next, followed by hemorrhage (5.4%). In symptomatic cases, the symptomatic period of less than 6 months was 110 patients, and it accounted for approximately 85% of patients (Table 1).
3. The location of tumors and macroscopic classification
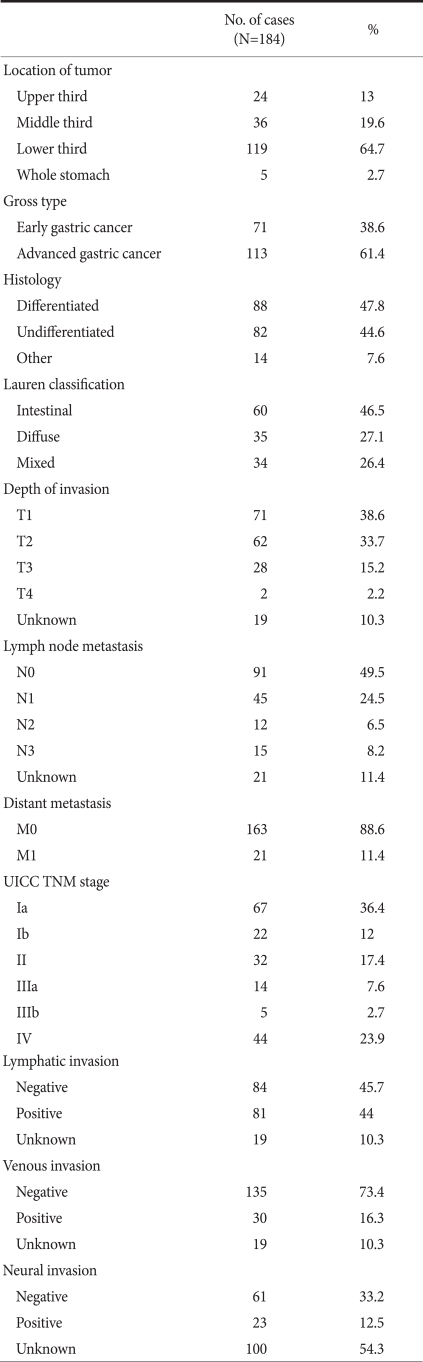
In 24 cases (13%), the tumor was located in the upper area of the stomach. In 36 cases (19.6%) it was in the middle area, and in 119 cases (64.7%) it was the lower area. In five cases the tumor had invaded the entire stomach (Table 2). In the macroscopic classification, 71 cases (38.6%) had early gastric cancer 61.4% of cases had advanced gastric cancer. Among early gastric cancer cases, type IIc was 62%, which was the most prevalent. Type III was 2.8%, which was the least frequent. In advanced gastric cancer cases, Borrmann type 1 was 3 cases (2.7%), which was least frequent. Type 3 was 86 cases (76.1%), which was most prevalent.
Table 2.
Five-year survival rate according to clinicopathologic features
UICC = Union for International Cancer Control.
4. The results and surgical methods
There were 29 total gastrectomies, 131 distal gastrectomies, and 5 proximal gastrectomies. Gastroduodenostomy was performed in 96 cases (73.3% of the entire cases. Gastrojejunostomy was performed in 35 cases (26.7%). Two cases were inoperable and thus palliative resection was performed. In resection cases, the operation time was an average of 201 minutes, with a maximum of 365 minutes and a minimum of 95 minutes. 19 cases were unable to have a resection, 3 cases were terminated by bypass, and 16 cases were terminated by diagnostic laparatomy. There were on average 33.1 resected lymph nodes (±17.4). The post-surgical hospitalization period was 12.3 days (Table 1).
5. Histological results of tumors
In the T classification, 71 cases (38.6%) were T1, 62 cases (33.7%) were T2, 28 cases (15.2%) were T3, 4 cases (2.2%) were T4 and 19 cases (10.3%) were unknown. In the N classification, 91 cases (49.5%) were N0, 45 cases (24.5%) were N1, 12 cases (6.5%) were N2, 15 cases (8.5%) were N3, and 21 cases (11.4%) were unknown.
In the M classification, 163 cases (88.6%) were M0, and 21 cases (11.4%) were M1. In regard to the disease stage, 67 cases (36.4%) were stage Ia, 22 cases (12.0%) were stage Ib, 32 cases (17.4%) were stage II, 14 cases (7.6%) were stage IIIa, 5 cases (2.7%) were stage IIIb, and 44 cases (23.9%) were stage IV (Table 3).
Table 3.
Characteristics of tumors
UICC = Union for International Cancer Control.
6. Early complications after surgery and mortality
Among the 184 cases, 19 cases developed complications (10.3%). Among them, 6 cases were wound complications, 2 cases were hemorrhage, 3 cases were intestinal obstruction, 2 cases were anastomosis leakage, and 3 cases were respiratory complications. Patients with second operations did so for one case of hemorrhaging, one case of anastomosis leakage, and the remaining patients were improved by symptomatic treatments. One patient died of sepsis following an intra-peritoneal abscess due to duodenal stump leakage after a distal gastrectomy, making the mortality rate 0.5%.
7. Postsurgical adjuvant? chemotherapy
Among all patients, 82 patients (45.3%) received adjuvant chemotherapy after radical resection. For the 21 patients who could not get a radical resection, palliative chemotherapy was performed (11.6%). 81 patients (44%) did not receive chemotherapy.
8. 5-year survival rate
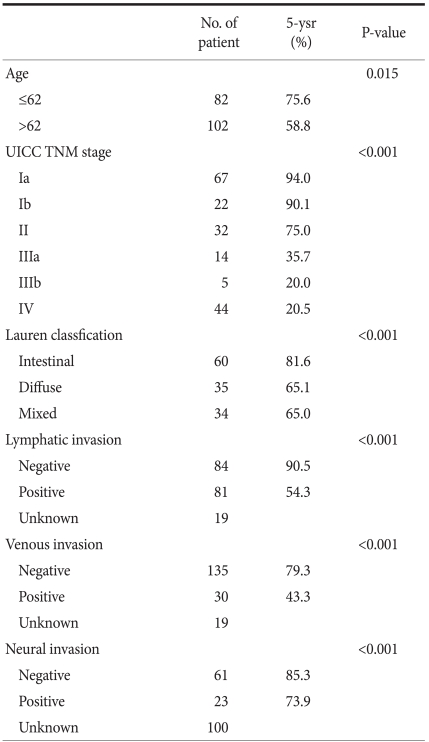
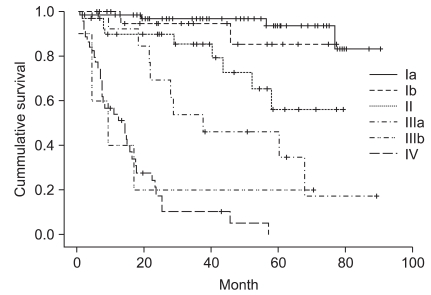
All 184 patients were able to be followed up on without any dropouts in the middle of the study. The average follow-up period was 4.3 years (±25.3 months, minimal 1 month, maximal 90 months). The 5-year survival rate of all patients who underwent surgery was 66.3%. The 5-year survival rate broken down by disease stages looks like this: stage Ia was 94.0%, Ib was 90.1%, II was 75.0%, IIIa was 35.7%, IIIb was 20.0%, and IV was shown to be 20.5% (Fig. 1). Based on the mean age of 62 years, the survival rate of the group younger than 62 years was 75.6% and the group older than 62 years was 58.8%, which was significantly different (P=0.015). In Log lank test, P-value was 0.001, and thus in univariate analysis, it was significant. However, the number of all patients was small, and particularly stage IIIb was only 5 cases. Thus it was too small to assess statistical significance (Table 3). Among the 163 patients who experienced radical resection, 18 patients (11%) had confirmed recurrence. The recurrence area was in the order of the peritoneum (9 patients), lymph nodes (6 patients), lung (2 patients), colon (2 patients), liver (2 patients), anastomosis area (2 patients), and ovary (2 patients). Among them, 5 patients had cancer recur in more than 2 sites. For one case recurred in the anastomosis area, radical resection was usable and was performed. The remaining cases were treated with second chemotherapy without surgery.
Fig. 1.
Kaplan-Meier survival curves according to tumor stages.
Discussion
In Korea, particularly surgery for gastric cancer patients, the tendency is to receive operation at large tertiary hospitals in the capital area. The phenomenon becomes more serious as large hospitals built cancer centers. However, in reality, more than 60% of all patients are treated at small-sized hospitals. Nevertheless, in gastric cancer patients, studies on the association of the number of surgery cases with surgery outcomes and prognosis are not abundant.
Reviewing the studies showed that the number of surgeries exert effects on post-surgical mortality. Finlayson et al.(5) report in a study conducted on patients treated at veterans hospitals that the mortality of large volume centers was 6.9%, middle volume centers was 7.4%, and low volume centers was 8.7%. Although differences were shown, it was not statistically significant. In a study conducted in Sweden, Hansson et al.(6) report that comparing the mortality at 2 months after surgery, the mortality at hospitals corresponding to university hospitals performing a large number of operations was lower than the mortality at local hospitals. Smith et al.(1) report that the data of the entire US was examined, and it was found that post-surgical mortality was lower in the hospital group with a large number of annual surgery cases. In contrast, it has been also reported that the number of surgery cases was not associated with post-surgical mortality. Damhuis et al.(7) examine the data of the Rotterdam cancer registration center, and report that post-surgical mortality was not associated with the number of surgeries performed. In addition, Reavis et al.(8) report that hospitals performing more than 13 surgeries annually were classified as the large volume group, hospitals performing between 6 and 12 cases as the medium volume group, and hospitals performing less than 5 cases as the low volume group, out of 121 hospitals total and 2,169 cases examined. Postoperative complications, re-hospitalization rates and mortality were compared. Differences among the groups were not observed. Reviewing studies conducted in Korea on gastric cancer patients regarding the mortality and morbidity after surgery, Park et al.(9) report that in 719 patients, it was 17.4% and 0.6%, respectively. Cho et al.(10) report that in 1,028 patients, it was 5.9% and 0.1%, respectively. Kim et al.(3) report that in 201 patients, it was 10.4% and 0.5%, respectively. In the results of our study, after gastric resection, complications were developed in 19 cases (10.3%), and there was one death by sepsis. However, since the definition of complications after surgery is vague sometimes, it may be influenced by associated diseases and many other factors, and in retrospective studies it is unavoidably affected by the presence or absence or records. Therefore, Lee and Yang(11) report that it is required to standardize the definition of complications after a gastrectomy, recoding forms and treatment protocols.
Some studies examine the association of the number of surgeries for gastric cancer performed in a hospital and the post-surgical long-term survival rate, Nomura et al.(12) examine the relationship of 5-year survival rate with the number of surgeries performed at a hospital by analyzing the database registered at the Osaka cancer registration center. When the surgeries performed by hospitals from 1975 to 1979 were divided into 4 groups from hospitals performing many surgeries to hospitals performing few surgeries and compared, it was observed that the odds ratio of the long-term survival rate of patients was significantly different. However, when the difference during the 5 years from 1990 to 1994 of the three groups excluding hospitals performing the least number of surgeries was examined, the differences disappeared. The authors said that it might be due to the standardization of surgery and advanced surgical techniques were applied even in small-sized hospitals. In a study conducted in Germany, the 5-year survival rate of the hospital group performing more than 20 cases of surgery annually was 10.6%, which was significantly higher than 3.4% of hospitals performing 1~4 cases of surgery annually.(13) Reviewing the above studies, it appears that the long-term survival rate of large-volume centers is advantageous. Nevertheless, studies performed in western countries have limitations because they were studies conducted in areas where the incidence of gastric cancer is low, and the standard cases of a large volume center is no more than 10~20 cases per year. Thus it is not appropriate to apply directly to Korean cases. Even in studies conducted in Japan where the incidence is as high as Korea, the minimum surgery group showing differences was less than 20 cases annually, and differences were not detected in the group performed more than 20 cases of gastrectomy surgery annually.(12) In another study conducted in Korea, the long-term survival rate of stage Ia was 92.7%, stage Ib was 89.1%, stage II was 82.4%, stage IIIa was 67.5%, stage IIIb was 41.4%, stage IV was 24.2%, and the overall 5-year survival rate was reported to be 69.6%.(14) In another study examining the overall 5-year survival rate, Noh et al.(15) reports 65%, and Park et al.(16) reports 66.8%. In our study, the 5-year survival rate of all patients after surgery for gastric cancer was 66.3%, which was not greatly different from other recent reports. The 5-year survival rate according to disease stage was that stage Ia was 94.0%, stage Ib was 90.1%, stage II was 75.0%, stage IIIa was 35.7%, stage IIIb was 20.0%, and stage IV was shown to be 20.5% (Fig. 1).
In the result of our study, the distribution of patients by stage was that stage Ia was 36.4%, stage Ib was 12.0%, stage II was 17.4%, stage IIIa was 7.6%, stage IIIb was 2.7%, and stage IV was 23.9%. Comparing the result with the report of the 2004 nationwide gastric cancer report in Korea, hospitals performing less than 100 cases annually, which was group 1, and in this group stage Ia was 39.3%, stage Ib was 13.0%, stage II was 12.4%, stage IIIa was 9.7%, stage IIIb was 4.9%, and stage IV was 20.2%. Hospitals performing more than 500 cases of surgery annually and thus classified as group 4 had similar values, since stage Ia was 41.0%, stage Ib was 16.0%, stage II was 13.8%, stage IIIa was 10.1%, stage IIIb was 4.3%, and stage IV was 14.6%. Although the tendency was that stage IV was slightly more abundant at our study, differences were not detected. An average of 33 lymph nodes were resected during surgery, which was not different from the result of the report average 34 lymph nodes.(17) Nonetheless, in regard to pathological findings, the record of lymph node invasion and perineural invasion was omitted in many cases. This is due to the absence of pathologists in charge of only gastric cancer because of the size of the hospital.
Our hospital performs approximately 30~40 gastrectomies for gastric cancer annually, and the number of operations are smaller than large hospitals. Nevertheless, surgeons specializing in gastric cancer perform surgery and manage surgery almost from the beginning to the end because the absolute number of residents is small, the level of participation in surgery is high, and it is thought that the care of patients prior to and after surgery should be more comprehensive. For the management of the admission of patients for surgery, the standardized critical pathway of the division of gastrointestinal surgery, Department of General Surgery, The Catholic University of Korea, School of Medicine has been applied, and patients are managed by the identical gastric cancer record form, and thus it is considered that the quality of the management of patients is not different from large centers.(4) In addition, since the number of cases is not large, it has an advantage that the surgeons in charge could directly conduct and manage telephone interviews.
In Korea, after the establishment of the Korean Gastric Cancer Association, efforts have been made to prepare comprehensive medical records for the standardization of surgery and the management of the data of gastric cancer patients.(18) Consequently, surgical outcomes have been improved continuously, and similar results were obtained from most hospitals. Nonetheless, in Korea, there was no report that the post-operative outcomes for gastric cancer performed after the classification of hospitals according to the number of gastric cancer surgeries as well as long-term survival rates.
This study has limitations in that it may contain errors of retrospective studies, the number of total patients was small, and the follow-up period was not sufficient. In addition, this study is not based on the one to one comparison with the data of large volume centers but based on the comparison of the literature. Moreover, sufficient data and standard for large hospitals were not secured. However, starting with this, if data management practices improve to the level of Japan or other countries so that the overall data management is more standardized and the content of the current data management by Korean Central Cancer Registry becomes more comprehensive, more objective indexes could be provided. Furthermore, surgical treatments for gastric cancer performed by each hospital could be evaluated objectively.
Although surgery for gastric cancer patients is performed at large hospitals in the capital area in many cases, a large number of surgeries are performed in small sized hospitals where numerous surgeons make efforts to treat gastric cancer patients. Our hospital is one such hospital, and the mortality and morbidity after surgery were not greatly different from large hospitals. This is thought to be due to the continuous standardization of surgery and the application of systemic lymphadenectomy as well as the compliance of the standardization of the Korean Gastric Cancer Association for the management of patients after surgery, the application of post-surgical adjuvant chemotherapy and the follow-up observation. The number of gastric cancer patients was small in the secondary hospitals, so the follow-up could be performed on all 184 subject patients without dropouts, and thus secondary hospitals have advantages such as thorough management of patients. Here, of course, the prerequisite is the presence of clinicians specifically studying gastric cancer. Ultimately, for better treatment outcomes of all gastric patients, the standardization of treatments for gastric cancer should be continued, and furthermore, efforts should be made to achieve the improvement of treatments equally.
References
- 1.Smith JK, McPhee JT, Hill JS, Whalen GF, Sullivan ME, Litwin DE, et al. National outcomes after gastric resection for neoplasm. Arch Surg. 2007;142:387–393. doi: 10.1001/archsurg.142.4.387. [DOI] [PubMed] [Google Scholar]
- 2.Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. 2009. [Google Scholar]
- 3.Kim KY, Yoo MW, Han HS, Yun IJ, Lee KY. The results of gastric cancer surgery during the early stage of a training hospital. J Korean Gastric Cancer Assoc. 2008;8:244–249. [Google Scholar]
- 4.Song KY, Kim SN, Park CH. Critical pathway for operable gastric cancer. J Korean Gastric Cancer Assoc. 2005;5:95–100. [Google Scholar]
- 5.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 6.Hansson LE, Sparén P, Nyrén O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162–169. doi: 10.1097/00000658-199908000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damhuis RA, Meurs CJ, Dijkhuis CM, Stassen LP, Wiggers T. Hospital volume and post-operative mortality after resection for gastric cancer. Eur J Surg Oncol. 2002;28:401–405. doi: 10.1053/ejso.2001.1246. [DOI] [PubMed] [Google Scholar]
- 8.Reavis KM, Hinojosa MW, Smith BR, Wooldridge JB, Krishnan S, Nguyen NT. Hospital volume is not a predictor of outcomes after gastrectomy for neoplasm. Am Surg. 2009;75:932–936. [PubMed] [Google Scholar]
- 9.Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099–1102. doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Kim BS, Kim YH, Lee CH, Yook JH, Oh ST, et al. Clinical analysis of post-operative complications in gastric tumors. J Korean Surg Soc. 2001;61:498–503. [Google Scholar]
- 11.Lee HJ, Yang HK. Quality assurance of gastric cancer surgery. J Korean Gastric Cancer Assoc. 2005;5:79–88. [Google Scholar]
- 12.Nomura E, Tsukuma H, Ajiki W, Oshima A. Population-based study of relationship between hospital surgical volume and 5-year survival of stomach cancer patients in Osaka, Japan. Cancer Sci. 2003;94:998–1002. doi: 10.1111/j.1349-7006.2003.tb01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enzinger PC, Benedetti JK, Meyerhardt JA, McCoy S, Hundahl SA, Macdonald JS, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg. 2007;245:426–434. doi: 10.1097/01.sla.0000245469.35088.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo WH, Seo BJ, Yu HJ, Lee WY, Lee HK. Analysis of prognostic factors in 1,435 surgically treated patients with gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:143–151. [Google Scholar]
- 15.Noh SH, Yoo CH, Kim YI, Kim CB, Min JS, Lee KS. Results after a gastrectomy of 2,603 patients with gastric cancer: analysis of survival rate and prognostic factor. J Korean Surg Soc. 1998;55:206–213. [Google Scholar]
- 16.Park JI, Jin SH, Bang HY, Paik NS, Moon NM, Lee JI. Survival rates after operation for gastric cancer: fifteen-year experience at a Korea cancer center hospital. J Korean Gastric Cancer Assoc. 2008;8:9–19. [Google Scholar]
- 17.The Information Committee of the Korean Gastric Cancer Association. 2004 nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2007;7:47–54. [Google Scholar]
- 18.Korean Gastric Cancer Association. Korean classification of gastric cancer. J Korean Gastric Cancer Assoc. 2002;2:33–42. [Google Scholar]