Abstract
Purpose
Additional surgery is commonly recommended in gastric cancer patients who have a high risk of lymph node metastasis or a positive resection margin after endoscopic resection. We conducted this study to determine factors related to residual cancer and to determine the appropriate treatment strategy.
Materials and Methods
A total of 28 patients who underwent curative gastrectomy due to non-curative endoscopic resection for early gastric cancer between January 2006 and June 2009 were enrolled in this study. Their clinicopathological findings were reviewed retrospectively and analyzed for residual cancer.
Results
Of the 28 patients, surgical specimens showed residual cancers in eight cases (28.6%) and lymph node metastasis in one case (3.8%). Based on results of the endoscopic resection method, the rate of residual cancer was significantly different between the en-bloc resection group (17.4%) and the piecemeal resection group (80.0%). The rate of residual cancer was significantly different between the diffuse type group (100%) and the intestinal type group (20%). The rate of residual cancer in the positive lateral margin group (25.0%) was significantly lower than that in the positive vertical margin group (33.3%) or in the positive lateral and vertical margin group (66.7%).
Conclusions
We recommended that patients who were lateral and vertical margin positive, had a diffuse type, or underwent piecemeal endoscopic resection, should be treated by surgery. Minimal invasive procedures can be considered for patients who were lateral margin positive and intestinal type through histopathological examination after en-bloc endoscopic resection.
Keywords: Early gastric cancer, Endoscopic resection, Residual cancer, Surgical indication
Introduction
Despite a decrease in the incidence of gastric cancer worldwide, gastric cancer has remained as one of the major causes of death in Korea.(1) Because of regular endoscopic examinations, however, many cases of gastric cancer are diagnosed at an early stage, and the prognosis for cases of early gastric cancer after radical gastrectomy is good: the 5-year survival rate is >90%, and recurrence develops in <5%.(2-4) With regard to lymph node metastasis of early gastric cancers, the incidence of such metastasis was reported to be 3% in mucosal cancers and approximately 20% in submucosal cancers.(5) Due to the development of endoscopic instruments and techniques, for patients with a low risk of lymph node metastasis, endoscopic resection is performed commonly to improve quality of life.(6)
In early gastric cancer, absolute indications for endoscopic resection are cases that are well differentiated, confined to the mucosa, and of type I or IIa, measuring <2 cm or IIc, measuring <1 cm.(7,8) However, these indications have recently been extended to tumors confined to the mucosa without ulceration, regardless of size, or with ulceration, measuring <3 cm, or those invading the superficial submucosal layer (sm1), measuring <3 cm.(9,10) Accordingly, the trend is that numerous endoscopists aggressively perform endoscopic resections.
In endoscopic resection, for incompletely resected cases or for cases with the possibility of lymph node metastasis (such as tumors with invasion into the submucosal layer, with lymphovascular invasion, or with poor differentiation by histopathological examination), it is generally thought that aggressive surgical treatments are required. However, in a recent study, in cases for which the probability of lymph node metastasis was low, and only the lateral margin was positive, the probability of residual cancer is low, and thus follow up observation after re-endoscopic resection is recommended.(11) In addition, in numerous studies, a variable number (14~60%) of patients without residual cancer after surgery have been reported, and thus the absence of residual cancers has been found in many patients who had radical gastrectomy.(11,12)
Therefore, in the current study, analysis of the clinicopathological characteristics of patients who had surgery after non-curative endoscopic resection was done to characterize factors associated with residual cancer in these patients; and appropriate treatments are discussed.
Materials and Methods
The study was conducted at the Busan University Hospital from January 2006 to June 2009. Subjects were 28 patients who had endoscopic resection for early gastric cancer, and for whom it was determined that they had non-curative resection. We thus performed open or laparoscopic gastrectomy. Indications for endoscopic resection at our hospital includes patients who (i) were not suspected to have lymph node metastasis and were diagnosed as having mucosal cancer by endoscopic ultrasound and abdominal computed tomography, (ii) had a well-differentiated and protruded type cancer that was smaller than 2 cm, or a well-differentiated and depressed type smaller than 1 cm without ulceration or a scar. Expanded indications applied only for patients whose surgery risk was high or patients who refused surgery.
For endoscopic resection, we used an endoscopic instrument for the upper digestive tract with a single channel (GIF-Q260, Olympus, Tokyo, Japan). The margins of the lesions were accurately distinguished by an endoscopic congo red test. While securing more than a minimum 5 mm safety margin in the vicinity of lesions, the mucosa was dissected with a needle knife (flex knife, Olympus, Tokyo, Japan) turning around 360 degrees, and using an electric knife (IT knife, MTW Company, Wesel, Germany). The tissue containing the tumor was resected completely from the muscle layer along the lower layer of the mucosa.
After endoscopic resection, cases with tumors that had infiltrated the lateral margin or the vertical margin were determined as incomplete resections. Not only incomplete resection also cases with a poorly differentiated carcinoma, cases with lymphovascular invasion, and cases with tumor infiltration to a depth more than 500 µm were classified as non-curative endoscopic resections. Based on the medical record of these patients, gender, age, endoscopic resection methods, surgical methods, tumor size, tumor location, and histological classification of tumors were reviewed retrospectively. These cases were analyzed by dividing them into an en bloc resection group and a piecemeal resection group according to the method of endoscopic resection. The en bloc resection group was divided again into two groups based on a tumor size of <3 cm or ≥3 cm. Histological classifications were analyzed after dividing subjects into (i) a differentiated group including well differentiated and moderately well differentiated carcinomas, and (ii) an undifferentiated group including poorly differentiated and signet ring cell carcinomas. Patients were also classified according to the cause of surgery; they were divided into a group with a high probability of lymph node metastasis, a group positive for the lateral margin only, a group positive for the vertical margin only and a group positive for both lateral and vertical margins.
For statistical analysis, SPSS for Windows (ver. 12.0, SPSS, Chicago, IL, USA) was used. For comparisons between the presence or absence of residual cancers according to clinicopathological characteristics, Fisher's exact test and Linear by linear associations were applied. Differences with a P-value less than 0.05 were considered to be statistically significant.
Results
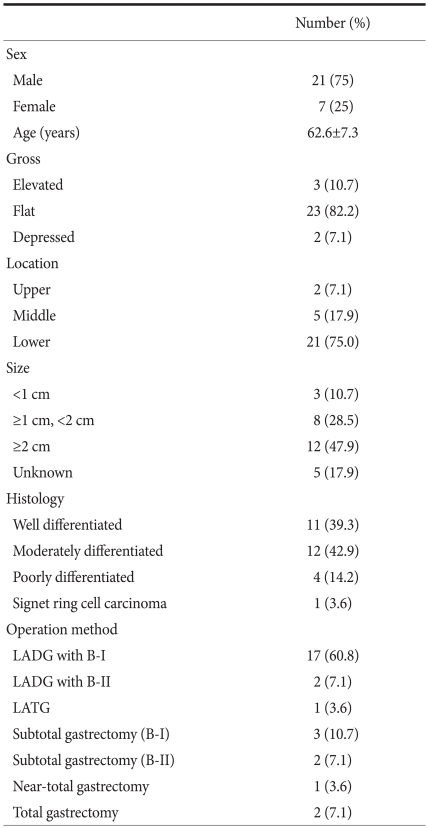
Of the 28 patients, 21 (75%) were male, and 7 (25%) female. The ratio of male to female was 3 : 1. The mean age was 62.6 years (range: 48~74) years. Regarding surgical methods, open gastrectomy was done for 8 patients; subtotal gastrectomy with Billroth I anastomosis for 3 cases, subtotal gastrectomy with Billroth II for 2, near-total gastrectomy with Roux-en-Y gastrojejunostomy for 1, and total gastrectomy with Roux-en-Y esophagojejunostomy for 2. Laparoscopic gastrectomy was done for a total of 20 patients; laparoscopy-assisted distal gastrectomy with Billroth I anastomosis for 17, laparoscopy-assisted distal gastrectomy with Billroth II anastomosis for 2, and laparoscopy-assisted total gastrectomy with Roux-en-Y esophagojejunostomy for 1 case (Table 1). After surgery, significant complications did not develop; the average follow up period was 27 months (range: 13~39 months), and cancer recurrence or death did not occur.
Table 1.
Clinicopathological characteristics of the 28 patients who underwent surgical resection after endoscopic resection for early gastric cancer
LADG = laparoscopy assisted distal gastrectomy; B-I = billroth I anastomosis; B-II = billroth II anastomosis; LATG = laparoscopy assisted total gastrectomy.
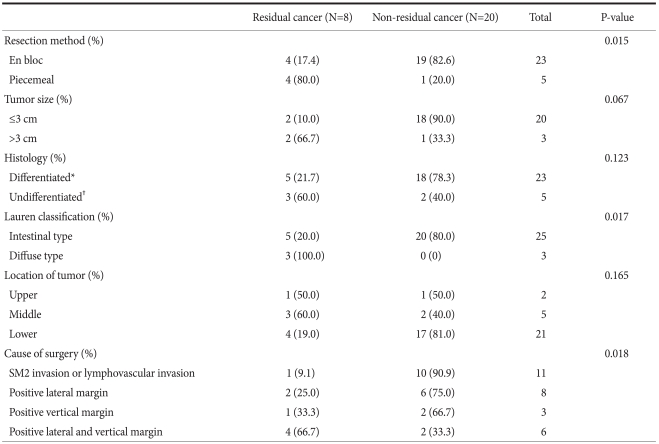
After surgery, cases showing residual cancer by histopathological examination included 8 patients (28.6%), and lymph node metastasis was detected in 1 patient (3.6%). According to the endoscopic resection methods, among 23 patients who had en block resection, residual cancer was detected in 4 patients (17.4%). On the other hand, in the group who had piecemeal resection, residual cancer was found in 4 of 5 patients (80.0%), and thus residual cancer was significantly higher in the piecemeal resection group (P=0.015). In cases where the tumor was resected en bloc, the incidence of residual cancer depended on the size of the endoscopically resected cancer: 2 of 20 patients (10.0%) had residual cancer in cases where the tumor was smaller than 3 cm; for tumors larger than 3 cm, 2 of 3 patients (66.7%) showed residual cancer; However, this difference was not statistically significant. According to Lauren's classification, in the intestinal type of gastric cancer, 5 of 25 patients (20.0%) had residual cancer; in the diffuse type, 3 of 3 (100.0%) showed residual cancer; and the difference was statistically significant (P=0.017). In the group in which the lateral margin and the vertical margin were negative, but the cancer had invaded the submucosal layer or lymphovascular duct, 1 of 11 patients (9.1%) had residual cancer. In the group positive for the lateral margin only, 2 of 8 patients (25.0%) had residual cancer. In the group positive for the vertical margin only, 1 of 3 patients (33.3%) showed residual cancer. In the group positive for both the lateral margin and the vertical margin, residual cancer was detected in 4 of 6 patients (66.7%), and the difference was statistically significant (P=0.018) (Table 2).
Table 2.
Relationship between residual cancer and non-residual cancer according to the pathological characteristics
SM = submucosa. *Well differentiated and moderately differentiated; †Poorly differentiated and signet ring cell type.
Discussion
The worldwide incidence of early gastric cancer is variable. In Japan, it accounts for 50% of all cases of gastric cancer. In Korea, according to the Korean Gastric Cancer Society, early gastric cancer accounts for approximately 33% of cases of gastric cancer, and the trend is towards a gradual increase in the proportion.(13,14) Therefore, recently, treatment methods have changed greatly. The standard treatment for gastric cancer was radical gastrectomy. For early gastric cancer with a low risk of lymph node metastasis, endoscopic resection has been done in many cases to achieve a better quality of life. Nevertheless, in cases of early gastric cancer that have invaded into the submucosal layer, lymph node metastasis has been reported to be present in up to 20%. Thus, it is accepted that after endoscopic resection, for the group for which the possibility of lymph node metastasis is relatively high, radical gastrectomy should be performed.(8,15-17) In our study, similarly, in 27 patients with invasion to SM2, where the possibility of lymph node metastasis is high, 1 of 27 patients (3.7%) showed lymph node metastasis, and we found that this cannot be ignored.
Korenaga et al.(18) reported that among 11 patients who had gastrectomy after incomplete endoscopic mucosal resection, residual cancer was detected in 4 of 5 patients with invasion of tumors into the margin of the resection area; residual cancer was detected in 3 of 6 patients with invasion into the submucosal layer. Nagano et al.(11) reported that in cases of submucosal cancer, or with a positive vertical margin after endoscopic mucosal resection, the possibility of the presence of residual cancer is high, and thus radical gastrectomy is required. In cases limited to the mucosa and invasion into the lateral margin, residual cancer was detected in 5.8% (18/309). On the other hand, in cases with invasion into the submucosal layer and invasion of tumors into the vertical margin, the ratio of residual cancer was high - it was 15.4% (6/39). In a recent study, in lesions that invaded the lateral margin, the possibility of the presence of residual cancer was low, and it has been proposed that re-endoscopic resection should be done.(19) Actually, in re-endoscopic resection, there are problems due to the scar of the previous resection, it is difficult to separate the submucosal layer, and the location of residual cancer is difficult to assess accurately. Hence, if the entire scar area is resected, the possibility of consequent complications such as hemorrhage is high. In our study, even in the group with negative resection margins, residual cancer was detected in 1 patient, and thus it is thought that even if the resection margin after en bloc resection is negative, a comprehensive follow-up observation is required. In addition, the rate of residual cancer in the group whose lateral margin was positive was significantly lower than in the group whose vertical margin was positive, and the difference was statistically significant. Nonetheless, in cases whose lateral margin was positive, residual cancer was detected in 25% of patients, which is not a small proportion, and thus it is difficult to determine treatment protocols based on these results alone.
The principle of endoscopic resection is to use en bloc resection because of the possibility of residual cancer cells remaining if piecemeal resection is performed. Oda et al.(20) reported that among 714 patients who had endoscopic resection, endoscopic mucosal resection was done on 411 patients, and endoscopic submucosal dissection was done on 303 patients. The en bloc resection rate of endoscopic submucosal dissections (97.2%) was higher than that of endoscopic mucosal resections (56.0%), and the ratio of curative resection was significantly higher in the group who had endoscopic submucosal dissection (73.6%). The 3-year disease-free survival rate was also higher in the group who had endoscopic submucosal dissection. In our study, similarly, in comparison with the endoscopic en bloc resection group (17.4%), the ratio of residual cancer in the piecemeal resection group was higher, and thus it is thought that for the piecemeal resection group, aggressive treatments are required.
In our study, in cases with undifferentiated cancers as determined by histological classification, residual cancer was detected in 60% of cases, and thus it was higher than the proportion in the differentiated cancer group; but the difference was not statistically significant. However, using Lauren's classification, residual cancer was present in all diffuse types, which suggests that after endoscopic resection, if the tumor is of the diffuse type, comprehensive observation and treatments are required.
In regard to the site of tumors, it is thought that in the upper and middle third of the stomach, where endoscopic resection is relatively difficult technically, residual cancer would be abundant, and residual cancers were detected in 50% in the upper third, in 60% in the middle third, and in 19% in the lower third. In comparison with the upper and middle third, the ratio of residual cancer in the lower third was low, but, since the number of cases was small, the difference was not statistically significant.
After endoscopic resection, in cases of non-curative resection, surgical treatments for the patients could not only completely resect lesions, but also, a complete pathological evaluation could be performed through lymphadenectomy. On the other hand, recurrent lesions are mucosal lesions in most cases. Hence, some experts have expressed the concern that it may lead to excessive treatments.(19) Therefore, it is thought if factors associated with a low possibility of residual cancer could be found, minimal invasive procedures for such patients, such as follow-up observation after re-endoscopic resection may be adequate and radical gastrectomy not warranted. In our study, in the en bloc resection group, the intestinal type group, and the group in which only the lateral resection margin was positive, the ratio of residual cancer was significantly lower, and thus it is thought that radical gastrectomy for such patients should be reconsidered. However, in the piecemeal resection group, the diffuse type group, or the group with lateral as well as vertical margins being positive, residual cancer is abundant and surgery must be done.
In our study, although the number of subjects was small, we conclude that patients who underwent piecemeal endoscopic resections, patients who have diffuse type tumors, or patients whose lateral margin and vertical margin are positive, should be treated by surgery because the incidence of residual cancer is significantly high. On the other hand, a minimally invasive procedure such as re-endoscopic resection and careful follow-up can be considered for patients who have a positive lateral margin and the intestinal type of cancer (after en block endoscopic resection) because the possibility of the presence of residual cancer is low. However, to establish appropriate treatment principles, more studies are required in the future.
Footnotes
This work was supported by a Pusan National University Hospital Clinical Research Grant.
References
- 1.Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Analyses of prognostic factors and gastric cancer specific survival rate in early gastric cancer patients and Its clinical implication. J Korean Surg Soc. 2003;65:309–315. [Google Scholar]
- 3.Shin DW, Hyung WJ, Noh SH, Min JS. Risk factors for recurrence after curative surgery for early gastric cancer. J Korean Gastric Cancer Assoc. 2001;1:106–112. [Google Scholar]
- 4.Shin JK, Shin YD, Yoon C, Joo HZ. Risk factors affecting lymph node metastasis and recurrence in early gastric cancer. J Korean Gastric Cancer Assoc. 2001;1:119–123. [Google Scholar]
- 5.Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992;79:241–244. doi: 10.1002/bjs.1800790319. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH. Endoscopic resection of early gastric cancer in Korea: recent results and future directions. J Korean Gastric Cancer Assoc. 2009;9:39–45. [Google Scholar]
- 7.Kida M, Tanabe S, Saigenji K. Enodscopic mucosal resection for gastric cancer: necessity of 'Incision and Stripping Method' and present status. Dig Endosc. 2003;15(Suppl 1):S15–S18. [Google Scholar]
- 8.Takeshita K, Tani M, Inoue H, Saeki I, Hayashi S, Honda T, et al. Endoscopic treatment of early oesophageal or gastric cancer. Gut. 1997;40:123–127. doi: 10.1136/gut.40.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association. Treatment Guideline for Gastric Cancer in Japan. 2nd ed. Tokyo: Kanehara; 2004. [Google Scholar]
- 11.Nagano H, Ohyama S, Fukunaga T, Seto Y, Fujisaki J, Yamaguchi T, et al. Indications for gastrectomy after incomplete EMR for early gastric cancer. Gastric Cancer. 2005;8:149–154. doi: 10.1007/s10120-005-0328-5. [DOI] [PubMed] [Google Scholar]
- 12.Chung YS, Park DJ, Lee HJ, Kim SG, Jung HC, Song IS, et al. The role of surgery after incomplete endoscopic mucosal resection for early gastric cancer. Surg Today. 2007;37:114–117. doi: 10.1007/s00595-006-3328-0. [DOI] [PubMed] [Google Scholar]
- 13.Maehara Y, Kakeji Y, Oda S, Takahashi I, Akazawa K, Sugimachi K. Time trends of surgical treatment and the prognosis for Japanese patients with gastric cancer. Br J Cancer. 2000;83:986–991. doi: 10.1054/bjoc.2000.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korean Gastric Cancer Association. Nationwide gastric cancer report in Korea. J Korean Gastric Cancer Assoc. 2002;2:105–114. [Google Scholar]
- 15.Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, et al. Treatment strategy after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2008;95:1495–1500. doi: 10.1002/bjs.6305. [DOI] [PubMed] [Google Scholar]
- 16.Ryu KW, Choi IJ, Doh YW, Kook MC, Kim CG, Park HJ, et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann Surg Oncol. 2007;14:3428–3434. doi: 10.1245/s10434-007-9536-z. [DOI] [PubMed] [Google Scholar]
- 17.Song KY, Hyung WJ, Kim HH, Han SU, Cho GS, Ryu SW, et al. Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Is gastrectomy mandatory for all residual or recurrent gastric cancer following endoscopic resection? A large-scale Korean multi-center study. J Surg Oncol. 2008;98:6–10. doi: 10.1002/jso.21074. [DOI] [PubMed] [Google Scholar]
- 18.Korenaga D, Orita H, Maekawa S, Maruoka A, Sakai K, Ikeda T, et al. Pathological appearance of the stomach after endoscopic mucosal resection for early gastric cancer. Br J Surg. 1997;84:1563–1566. [PubMed] [Google Scholar]
- 19.Chang JH, Lee IS, You CR, Nam KW, Kwon JH, Suh JP, et al. Re-endoscopic mucosal resection for a residual or locally recurrent gastric lesion after endoscopic mucosal resection. Korean J Gastrointest Endosc. 2007;35:6–13. [Google Scholar]
- 20.Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]