Abstract
Purpose
The purpose of this study was to investigate the reliability and the clinical applicability of the adenosine-triphosphate-based chemotherapy response assay (ATP-CRA) as a method of determining in vitro chemosensitivity in patients with gastric cancer.
Materials and Methods
A total of 243 gastric cancer tissue samples were obtained from gastrectomies performed between February 2007 and January 2010. We evaluated the effectiveness of the ATP-CRA assay in determining the chemosensitivity of gastric cancer specimens using eleven chemotherapeutic agents - etoposide, doxorubicin, epirubicin, mytomicin, 5-fluorouracil, oxaliplatin, irinotecan, docetaxel, paclitaxel, methotraxate, and cisplatin - for chemosensitivity studies using ATP-CRA. We assessed the failure rate, the cell death rate, and the chemosensitivity index.
Results
The failure rate of ATP-CRA was 1.6% (4/243). The mean coefficient of variation for triplicate ATP measurements was 6.5%. Etoposide showed the highest cell death rate (35.9%) while methotrexate showed the lowest (16.6%). The most active chemotherapeutic agent was etoposide, which most frequently ranked highest in the chemosensitivity test: 31.9% (51/160). Oxaliplatin was more active against early gastric cancers than advanced gastric cancers, whereas docetaxel was more active against advanced cancers. The lymph node negative group showed a significantly higher cell death rate than the lymph node positive group when treated with doxorubicin, epirubicin, and mitomycin.
Conclusions
ATP-CRA is a stable and clinically applicable in vitro chemosensitivity test with a low failure rate. The clinical usefulness of ATP-CRA should be evaluated by prospective studies comparing the regimen guided by ATP-CRA with an empirical regimen.
Keywords: Stomach neoplasms, Chemosensitivity assay, ATP based chemoresponse
Introduction
Gastric cancer is the fourth leading cause of cancer death worldwide.(1) Gastric cancer is the most prevalent cancer in Korea and the treatment of gastric cancer patients accounts for the highest percentage of the national health expenditure, 19.1% of all cancer-related health insurance payments.(2) Even after curative resection, advanced gastric cancer patients are given postoperative chemotherapy due to the high risk of recurrence because by adding chemotherapy we may expect better patient survival than with surgery alone.(3-6)
For gastric cancer, many chemotherapeutic agents are used. However, the chemotherapeutic effect of these drugs on gastric cancer is variable. Furthermore, neither a uniformly effective nor a standard chemotherapeutic regimen for gastric cancer has been clearly established. In efforts to improve the response rate to chemotherapy, in vitro chemosensitivity tests have been employed to select the optimal chemotherapeutic agents for individual patients. Recently, adenosine-triphosphate based chemotherapy response assay (ATP-CRA) has demonstrated promising results in various types of cancers, such as melanoma, lung cancer, breast cancer, colorectal cancer and ovarian cancer.(7-11) Yet, little is known about the applicability and reliability of ATP-CRA as an in vitro chemosensitivity test in gastric cancer. The purpose of this study was to investigate the reliability and the clinical applicability of ATP-CRA as a method of in vitro chemosensitivity in patients with gastric cancer.
Materials and Methods
From February 2007 to January 2010, 243 patients who were preoperatively or intraoperatively diagnosed with advanced gastric cancer, stage II or greater, including one remnant gastric cancer at the Yonsei University College of Medicine, Severance Hospital were enrolled for the study. All patients agreed to the chemosensitivity test of their resected tumors and gave informed consent. We excluded patients who had received neo-adjuvant chemotherapy or were concurrently diagnosed with malignancies of another site. All the data on patients' characteristics and pathologic features of the resected tumors were collected by retrospective review of medical records.
ATP-CRA was performed as described elsewhere.(8,10) All tissue specimens were obtained after surgical resection. Immediately after the surgical resection of a tumor, the specimen was sent to a pathology laboratory and a pathologist confirmed the tumor tissue. Then, a 0.5 cubic centimeter piece of the cancer tissue was collected. The tissue specimens were stored in HBSS (GIBCO BRL, Rockville, MD, USA), containing 100 IU/ml penicillin (Sigma, St Louis, MO, USA), 100 µg/ml streptomycin (Sigma, St Louis, Mo, USA), 100 µg/ml gentamicin (GIBCO BRL, Rockville, MD, USA), 2.5 µg/ml amphotericin B (GIBCO BRL, Rockville, MD, USA) and 5% fetal bovine serum (FBS; GIBCO BRL, Rockville, MD, USA) and promptly transported to the laboratory. These tissue specimens underwent initial washing with 70% ethanol before being quantified and minced to a size less than 1 mm mechanical disaggregation. Then, for enzymatic disaggregation, they were incubated at 37℃ with 5% CO2 for 12 to 16 hours with extracellular matrix degrading enzymes such as dispase (Sigma, St Louis, Mo, USA), pronase (Sigma, St Louis, Mo, USA) and DNase (Sigma, St Louis, Mo, USA). Cells were harvested using a cell strainer (BD Falcon, Bedford, MA, USA). To remove red blood cells, normal cells, and excess debris, the cell suspensions were subjected to Ficoll-Hypaque (1077-1, Sigma, St Louis, Mo, USA) gradient centrifugation at 400 g for 15 min and anti-CD45 antibody conjugated magnetic beads (Miltenyi Biotech, Auburn, CA, USA). Trypan blue exclusion test was used to determine the viability of isolated cells.
After dilution of the separated tumor cells to 2,000~20,000 viable cells/100 µl using IMDM (GIBCO BRL, Rockville, MD, USA), including 10% FBS, they were seeded in triplicate to a 96-well ultra low attachment microplate (Costar, Cambridge, MA, USA), which restricts the growth of normal cells. In the treated groups, 100 µl of chemotherapeutic agents were added to the seeded cells; while in the untreated control groups, 100 µl of IMDM without chemotherapeutic agents was added to 3~6 wells of the microplate. The test drug concentrations were determined based on the peak plasma concentrations according to previous reports and preliminary training set experiments: etoposide (3.57 µg/ml), doxorubicin (1.5 µg/ml), epirubicin (1.2 µg/ml), mitomycin (0.2 µg/ml), 5-FU (10 µg/ml), oxaliplatin (2.9 µg/ml), irinotecan (4.7 µg/ml), docetaxel (3.7 µg/ml), paclitaxel (8.5 µg/ml), MTX (0.37 µg/ml) and cisplatin (2.5 µg/ml).(8,12,13) Three dilutions (0.2-, 1-, and 5-fold) of the test drug concentration were used in triplicate whenever sufficient number of cancer cell were available. For the purpose of quality control, a negative control group of 3~6 wells of seeding medium without cells and two positive control groups of 3 wells that contained the minimal (105 pg ATP) and the median (280 pg ATP) amounts of ATP, as measured in 1,000 harvested tumor cells were included in the culture plate, respectively. The microplate was cultured for 48 hours at 37℃ in 5% CO2 with concomitant exposure to drugs. Then, the cells were lysed and the ATP content of each well were measured using the luciferin-luciferase system (Roche, Mannheim, Germany), followed by flash type luminescence measurements on a Victor 3 multi-label counter (PerkinElmer Boston, MA, USA).
Each of the cancer cell death rate (CDR) with luminscence values were calculated by the following formula.
A chemosensitivity index (CI) is calculated as the sum of the percentage inhibition at each concentration tested (CI=300-sum%Inhibition at 0.2-, 1-, and 5-fold of test drug concentration). The higher the value of CI, the greater the resistance to an anti-cancer drug. For every experiment, we calculated the intra assay mean coefficient of variation for quality control. For the calculation of coefficient of variation value, the luminescence values of each specimen were measured 3 times.
The chemosensitivity test of the ATP-CRA was considered a failure when the intra assay mean coefficient of variation for triplicate ATP measurements resulted in any value of over 30 or those of the untreated control group which had a measurement less than 105 pg ATP that of the positive control group. When inadequate numbers of cells were harvested or cell culture failed due to microorganism contamination, the test was also regarded as failure.
1. Statistical analysis
All statistical analyses were performed using the "Statistical Package for Social Science (SPSS)" version 18.0 for windows (SPSS Inc., Chicago, IL, USA). The difference of the chemosensitivity index between the early and the advanced gastric cancer groups, the serosa involved and the serosa non-involved groups, and the lymph node negative and the lymph node positive groups were compared using the Student t-test. A P-value <0.05 was considered statistically significant.
Results
1. Clinicopathologic characteristics
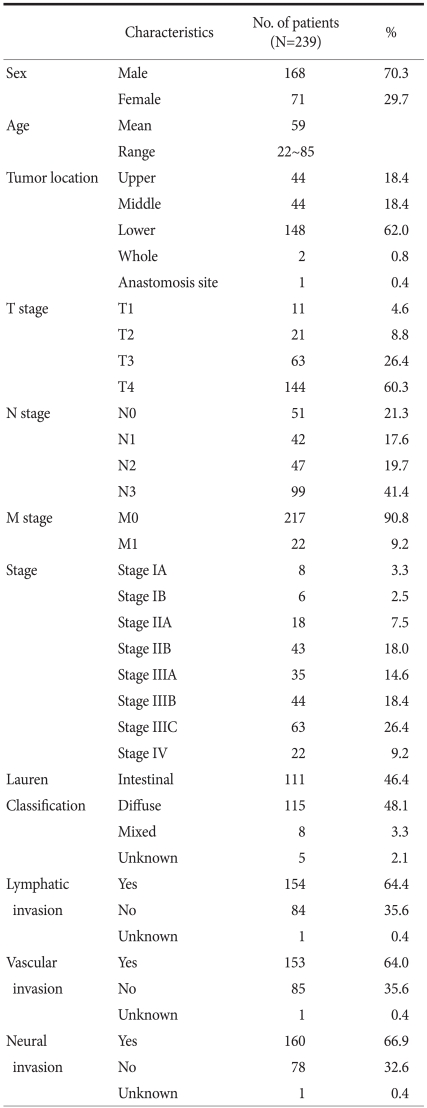
Of the 243 patients, 4 of the patients were excluded from the study due to failure in the chemosensitivity test. The clinicopathologic features those 239 patients are presented in Table 1. Of the 239 patients, 168 were men and 71 were women. The mean age of 239 patients was 59.0 years (range, 22~85 years). The distribution of the TNM stage according to the 7th AJCC classification included 8 Stage IA (3.3%), 6 Stage IB (2.5%), 18 Stage IIA (7.5%), 43 Stage IIB (18.0%), 35 Stage IIIA (14.6%), 44 Stage IIIB (18.4% ), 63 Stage IIIC (26.4%), and 22 Stage IV (9.2%).
Table 1.
Clinicopathologic features of the patients
2. In vitro chemosensitivity test results
Of the 243 patients who underwent gastrectomy, 4 cases failed the chemosensitivity test. Test failure rate was 1.6 % (4/243) and the reasons of the failure were as follows. Two tumor specimens showed lower measured values of the untreated control lower than those of the positive control group (105 pg ATP). These results were due to insufficient amount of viable cells or unacceptable viabilities of tumor cells. A microorganism contamination was observed in one specimen, and the other specimen did not yield any viable tumor cells. The mean coefficient of variation for triplicate ATP assay was 6.5±2.0% (range, 1.9~17.9%). Although ATP-CRA results were obtained in 239 specimens, not all ATP-CRA produced complete results for all chemotherapeutic agents. Moreover, CIs for all the chemotherapeutic drugs were calculated in 160 specimens (66.9%).
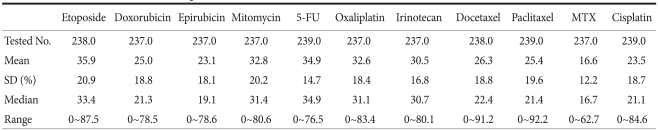
The cytotoxic effect for test drug concentrations of the chemotherapeutic agents on cell death rate ranged from 0 to 92.2% (Table 2). The highest cancer cell death rate was demonstrated in cells treated with etoposide (35.9%), followed by 5-FU (34.9%), and mitomycin (32.8%). The agents which resulted in a wide range of cell death rates were paclitaxel (0~92.2%), docetaxel (0~91.2%), etoposide (0~87.5%). MTX showed the least cytotoxic effect (16.6%) and the narrowest range (0~62.7%).
Table 2.
Cell death rate at 1- fold test drug concentration
5-FU = 5-fluorouracil; MTX = methotrexate; SD = standard deviation.
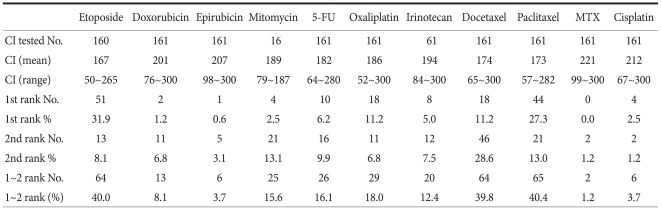
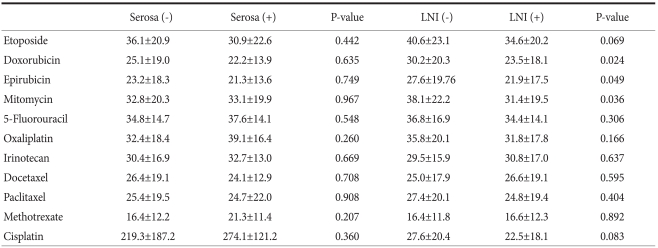
Table 3 shows each CI which indicates chemosensitivity of anticancer drug calculated by the formula described in the Method section. The values of CI were heterogeneous among specimens. The most active chemotherapeutic agent was etoposide which most frequently had the highest (top-ranked) chemosensitivity, 31.9% (51/160) for the tested specimens. When we compared the cell death rates according to the pathologic characteristics, there was a significant difference in cell death rates according to lymph node metastasis. The lymph node negative group showed significantly higher cell death rates than the lymph node positive group in doxorubicin, epirubicin, and mitomycin (P<0.05, respectively) (Table 4).
Table 3.
Chemosensitivity index (CI)*
5-FU = 5-fluorouracil; MTX = methotrexate. *Chemosensitivity index (CI) = 300-sum%Inhibition at 0.2-, 1-, and 5-fold of test drug concentration).
Table 4.
Comparison of cell death rate at 1- fold test drug concentration
LNI = lymph node involvement.
Discussion
From the early 1980s, many different types of in vivo and in vitro chemosensitivity tests were developed. Many tests such as subrenal capsule assay, human tumor clonogenic assay (HTCA), thymidine incorporation assay (TIA), succinic dehydrogenase inhibition assay (SDI), methylthiazoletetrazolium (MTT) assay, histoculture drug response assay (HDRA) were investigated.(14-19) There were, however, limitations in their clinical use due to various problems; lengthy assay period, difficulty in primary culture, a requirement of large specimen for the assay, contamination of fibroblasts, the different action mechanism of anti-cancer drugs, and the use of extremely high concentration of anti-cancer agents.(19-23) On the contrary, the ATP-CRA has demonstrated many advantages over above mentioned chemosensitivity tests. The ATP-CRA can be performed with a very small amount of cancer tissue, effectively eliminates or suppresses normal cells from the tissue specimens, has a higher sensitivity for evaluating viable cells, and is more accurate than previous chemosensitivity tests.(16) ATP-CRA has been explored in many types of cancer as a method of selecting chemotherapy regimens based on individual difference in a variety of anti-cancer drugs.(7-13)
The concept of in vitro chemosensitivity test is that it may help to differentiate the response of individual cancer patients to chemotherapeutic agents. ATP-CRA showed clinical benefit for assay-guided chemotherapy in breast cancer and ovarian cancer.(9,11) However, no studies have conclusively shown that an in vitro chemosensitivity test can predict the chemotherapy response of an individual patient. So far, the use of an in vitro chemosensitivity assay for clinical practice is not recommended except in clinical trials. (23) Unlike breast or ovarian cancer, the benefits of chemotherapy after gastrectomy for gastric cancer are not fully established; and even though, some phase III randomized prospective clinical trials have shown survival benefits of chemotherapy,(24-26) no standard chemotherapeutic regimen for gastric cancer can be recommended. Therefore, provided that an in vitro chemosensitivity assay could accurately predict the in vivo chemo-responsiveness of the patients, its application may be an ideal method of identifying the most effective patient specific chemotherapy agent.
The study results using different methods of in vitro chemosensitivity assay in gastric cancer inconsistent.(27,28) These contradictory results may come from differences in the nature of each method, differences in chemotherapeutic agents, and the study design. In this study, the cell death rate for each chemotherapeutic drug showed wide ranges. This may represent heterogeneous response of each cancer tissues to various anti-cancer agents. Thus, selecting proper drugs which can show effective chemotherapeutic effect is important.
There were reports regarding ATP-CRA for gastric cancer patients with a small study sample size.(29,30) One is a study of the effectiveness of ATP-CRA guided chemotherapy for unresectable gastric cancer patients. Although they suggested potential benefits of ATP-CRA guided chemotherapy by demonstrating complete remission and long-term survival, they could not evaluate the methodological stability of ATP-CRA.(29) The other is a study similar to our study. However, Lee(30) showed only cell death rate at a specific concentration of chemotherapeutic agent. In his study, he did not show the chemosensitivity index which can be a baseline data for the clinical application of ATP-CRA. In our study, ATP-CRA showed a low failure rate (1.6%) and a low intra assay mean coefficient of variation (6.5%) in a large study population. Thus, our study confirms that ATP-CRA is a stable and clinically applicable in vitro chemosensitivity assay suited for validation studies of assay-guided chemotherapy for gastric cancer patients. This high success rate may be related to the rapid and exact sampling of the cancer tissue by the pathologist performed immediately after a surgical resection and the effective elimination of normal cells processing of the specimen with the ficoll gradient centrifugation and anti-CD45 immunomagnetic separation.(8) Moreover, the chemo-response to certain anti-cancer drugs was related to the characteristics of the tumor. This further supports the pursuit of an individualized chemotherapeutic approach in gastric cancer.
Our study has several limitations. A few of the anti-cancer agents which showed high cell death rates may be difficult to evaluate in practice since they are not included in clinically used regimens. In addition, since we studied the ATP-CRA only for single agents, the effect of combining anti-cancer drugs could not be investigated. Thus, we cannot estimate the interaction between the drugs and different pharmacokinetic effects of individual patients. Nevertheless, our study provided the possibility of an in vitro detection of the chemotherapeutic agents with a potential for a high success rate. From these results, studies on survival benefits of various anti-cancer agents for gastric cancer based on ATP-CRA results and a prospective study comparing in vitro chemosensitivity assay-guided chemotherapy with empiric chemotherapy are warranted.
In conclusion, ATP-CRA is a stable and clinically applicable in vitro chemosensitivity test with a low failure rate. The clinical usefulness of ATP-CRA should be evaluated by prospective studies comparing regimen guided by ATP-CRA with the empirical regimen.
References
- 1.Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia C. Cancer mortality in the European Union, 1970-2003, with a joinpoint analysis. Ann Oncol. 2008;19:631–640. doi: 10.1093/annonc/mdm597. [DOI] [PubMed] [Google Scholar]
- 2. [Accessed January 4, 2010]. http://www.nhic.or.kr/
- 3.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS, Fleming TR, Peterson RF, Berenberg JL, Mc-Clure S, Chapman RA, et al. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg Oncol. 1995;2:488–494. doi: 10.1007/BF02307081. [DOI] [PubMed] [Google Scholar]
- 6.Höhler T, Möhler M. New chemotherapeutic options in advanced gastric cancer. Onkologie. 2003;26(Suppl 7):54–59. doi: 10.1159/000076176. [DOI] [PubMed] [Google Scholar]
- 7.Neale MH, Myatt NE, Khoury GG, Weaver P, Lamont A, Hungerford JL, et al. Comparison of the ex vivo chemosensitivity of uveal and cutaneous melanoma. Melanoma Res. 2001;11:601–609. doi: 10.1097/00008390-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin DH, et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005;37:223–227. doi: 10.4143/crt.2005.37.4.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cree IA, Kurbacher CM, Untch M, Sutherland LA, Hunter EM, Subedi AM, et al. Correlation of the clinical response to chemotherapy in breast cancer with ex vivo chemosensitivity. Anticancer Drugs. 1996;7:630–635. doi: 10.1097/00001813-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Huh JW, Park YA, Lee KY, Sohn SK. Heterogeneity of adenosine triphosphate-based chemotherapy response assay in colorectal cancer-secondary publication. Yonsei Med J. 2009;50:697–703. doi: 10.3349/ymj.2009.50.5.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000;77:258–263. doi: 10.1006/gyno.2000.5728. [DOI] [PubMed] [Google Scholar]
- 12.Weisenthal LM, Dill PL, Finklestein JZ, Duarte TE, Baker JA, Moran EM. Laboratory detection of primary and acquired drug resistance in human lymphatic neoplasms. Cancer Treat Rep. 1986;70:1283–1295. [PubMed] [Google Scholar]
- 13.Bird MC, Bosanquet AG, Gilby ED. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985;3:1–10. doi: 10.1002/hon.2900030102. [DOI] [PubMed] [Google Scholar]
- 14.Bogden AE, Griffin W, Reich SD, Costanza ME, Cobb WR. Predictive testing with the subrenal capsule assay. Cancer Treat Rev. 1984;11(Suppl A):113–124. doi: 10.1016/0305-7372(84)90050-1. [DOI] [PubMed] [Google Scholar]
- 15.Rozencweig M, Hofmann V, Sanders C, Rombaut W, Früh U, Martz G. In vitro growth of human malignancies in a cloning assay. Recent Results Cancer Res. 1984;94:1–7. doi: 10.1007/978-3-642-82295-7_1. [DOI] [PubMed] [Google Scholar]
- 16.Tanigawa N, Kern DH, Hikasa Y, Morton DL. Rapid assay for evaluating the chemosensitivity of human tumors in soft agar culture. Cancer Res. 1982;42:2159–2164. [PubMed] [Google Scholar]
- 17.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- 18.Vescio RA, Redfern CH, Nelson TJ, Ugoretz S, Stern PH, Hoffman RM. In vivo-like drug responses of human tumors growing in three-dimensional gel-supported primary culture. Proc Natl Acad Sci USA. 1987;84:5029–5033. doi: 10.1073/pnas.84.14.5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman AE, Hoffman RM. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci USA. 1986;83:2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Von Hoff DD, Clark GM, Stogdill BJ, Sarosdy MF, O'Brien MT, Casper JT, et al. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983;43:1926–1931. [PubMed] [Google Scholar]
- 21.Von Hoff DD, Kronmal R, Salmon SE, Turner J, Green JB, Bonorris JS, et al. A Southwest Oncology Group study on the use of a human tumor cloning assay for predicting response in patients with ovarian cancer. Cancer. 1991;67:20–27. doi: 10.1002/1097-0142(19910101)67:1<20::aid-cncr2820670105>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Xu JM, Song ST, Tang ZM, Jiang ZF, Liu XQ, Zhou L, et al. Predictive chemotherapy of advanced breast cancer directed by MTT assay in vitro. Breast Cancer Res Treat. 1999;53:77–85. doi: 10.1023/a:1006122912146. [DOI] [PubMed] [Google Scholar]
- 23.Schrag D, Garewal HS, Burstein HJ, Samson DJ, Von Hoff DD, Somerfield MR. American Society of Clinical Oncology Technology Assessment: chemotherapy sensitivity and resistance assays. J Clin Oncol. 2004;22:3631–3638. doi: 10.1200/JCO.2004.05.065. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 25.Neri B, Cini G, Andreoli F, Boffi B, Francesconi D, Mazzanti R, et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br J Cancer. 2001;84:878–880. doi: 10.1054/bjoc.2000.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 27.Kodera Y, Ito S, Fujiwara M, Mochizuki Y, Ohashi N, Ito Y, et al. In vitro chemosensitivity test to predict chemosensitivity for paclitaxel, using human gastric carcinoma tissues. Int J Clin Oncol. 2006;11:449–453. doi: 10.1007/s10147-006-0618-x. [DOI] [PubMed] [Google Scholar]
- 28.Iwahashi M, Nakamori M, Nakamura M, Noguchi K, Ueda K, Nakatani Y, et al. Individualized adjuvant chemotherapy guided by chemosensitivity test sequential to extended surgery for advanced gastric cancer. Anticancer Res. 2005;25:3453–3459. [PubMed] [Google Scholar]
- 29.Park JY, Kim YS, Bang S, Hyung WJ, Noh SH, Choi SH, et al. ATP-based chemotherapy response assay in patients with unresectable gastric cancer. Oncology. 2007;73:439–440. doi: 10.1159/000136802. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH. The results of the ATP Based chemotherapy response assay in gastric cancer tissues. J Korean Gastric Cancer Assoc. 2007;7:160–166. [Google Scholar]