Abstract
Purpose
The incidence of lymph node metastasis has been reported to range from 2.6 to 4.8% in early stage gastric cancer with mucosal invasion (T1a cancer). Lymph node metastasis in early stage gastric cancer is known as an important predictive factor. We analyzed the prediction factors of lymph node metastasis in T1a cancer.
Materials and Methods
A total of 9,912 patients underwent radical gastrectomy due to gastric cancer from October 1994 to July 2006 in the Department Of Surgery at Samsung Medical Center. We did a retrospective analysis of 2,524 patients of these patients, ones for whom the cancer was confined within the mucosa.
Results
Among the 2,524 patients, 57 (2.2%) were diagnosed with lymph node metastasis, and of these, cancer staging was as follows: 41 were N1, 8 were N2, and 8 were N3a. Univariate analysis of clinicopathological factors showed that the following factors were significant predictors of metastasis: tumor size larger than 4 cm, the presence of middle and lower stomach cancer, poorly differentiated adenocarcinoma and signet-ring cell carcinoma, diffuse type cancer (by the Lauren classification), and lymphatic invasion. Multivariate analysis showed that lymphatic invasion and tumor larger than 4 cm were significant factors with P<0.001 and P=0.024, respectively.
Conclusions
The frequency of lymph node metastasis is extremely low in early gastric cancer with mucosal invasion. However, when lymphatic invasion is present or the tumor is larger than 4 cm, there is a greater likelihood of lymph node metastasis. In such cases, surgical treatments should be done to prevent disease recurrence.
Keywords: Stomach neoplasms, Gastric mucosa, Lymphatic metastasis, Predisposing factor
Introduction
The overall annual incidence of gastric cancer in Korea is the highest among all cancers, and it has been reported that gastric cancer after lung cancer is the second major cause of cancer deaths in the country. Due to the directed efforts towards health examination and the development of endoscopic techniques, the incidence of early gastric cancer that was difficult to find in the past has increased.(1) About 52% of gastric cancer surgeries undertaken in our hospital since 2002 were due to early gastric cancer, and according to the other studies carried out recently, approximately 40% of all gastric cancer patients were diagnosed with early gastric cancer.(1,2) In Japan the incidence has been reported as more than 50% of all gastric cancers.(3,4)
Early gastric cancer, regardless of lymph node metastasis, refers to malignant tumors confined to the mucosa or submucosa, and the prognosis of these patients is very good compared to other malignant tumors.(1,5) According to previous studies on the prognostic factors of gastric cancer, the factors that affect the prognosis are patient age, tumor size and location, depth of invasion, grade of histologic differentiation, the degree of lymph node metastasis, distant metastasis, and curative surgery.(5-7) Especially with regards to early gastric cancer, lymph node metastasis as well as depth of invasion have been deemed the most important prognostic factors.(5-7) Typically, lymph node metastases are found in 10 to 15% of early gastric cancer cases, and if early gastric cancer is divided into mucosal cancer and submucosal cancer, it is known to be found in 2.6 to 4.6% of mucosal cancer and in 16.5 to 23.6% of submucosal cancer.(7-9) Due to the advances in endoscopic equipment and techniques, minimally invasive treatments such as endoscopic resection have been actively undertaken. Particularly in the case of early gastric cancer confined to mucosa, it is important to predict lymph node metastasis because the treatment is only completed by endoscopic resection. In case of mucosal cancer, the incidence of lymph node metastasis is very low. However, because it is an important prognostic factor for early gastric cancer, the research on the risk factors, that is, the predictive factors of lymph node metastasis has a significant role in selection of the treatment approach for mucosal cancer and providing the appropriate criteria for the follow-up observations. In this study, we attempted to identify the typical clinicopathological characteristics that appear in early gastric cancer confined to the mucosa, the incidence of lymph node metastasis, and the predictive factors for a target of a large group in the Medical Center.
Materials and Methods
We conducted an analysis of 2,524 patients (25.46%) diagnosed with mucosal cancer upon histological examination among 9,912 patients who underwent radical gastrectomy due to gastric cancer from October 1994 to July 2006 in the Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine. With reference to the medical records and pathology reports, we investigated gender, age, tumor size and location, gross appearance, histologic differentiation, Lauren classification, lymphatic invasion, vascular invasion, perineural invasion and surgical methods, and performed a retrospective analysis about the correlations between each clinicopathological factors and lymph node metastasis.
Among all of 2,524 patients, 1,616 (64.0%) were men, 908 (36.0%) were women, and their median age was 53.6 (21~87 years old). According to the tumor size, they were divided into four groups, i.e., less than 1 cm, 1~2 cm, 2~4 cm, 4 cm or more. Depending on the location of the primary tumor, they were divided into the upper stomach (upper third), the middle stomach (middle third), or the lower stomach (lower third). We used the classification of TNM staging published in 2010 by American Joint Committee for Cancer Stage, 7th edition, and lymph node dissection ranges were classified based on the agreement published by the Japanese Gastric Cancer Association.(10) The statistical analyses were carried out using SPSS for Windows (Ver. 17.0, SPSS Inc., Chicago, IL, USA). Clinicopathological prognostic factors were compared using Chi-square test and multivariate analysis was performed using Cox regression hazards model. The statistical significance was set at a value of P<0.05.
Results
1. Univariate analysis of clinicopathological factors associated with lymph node metastasis
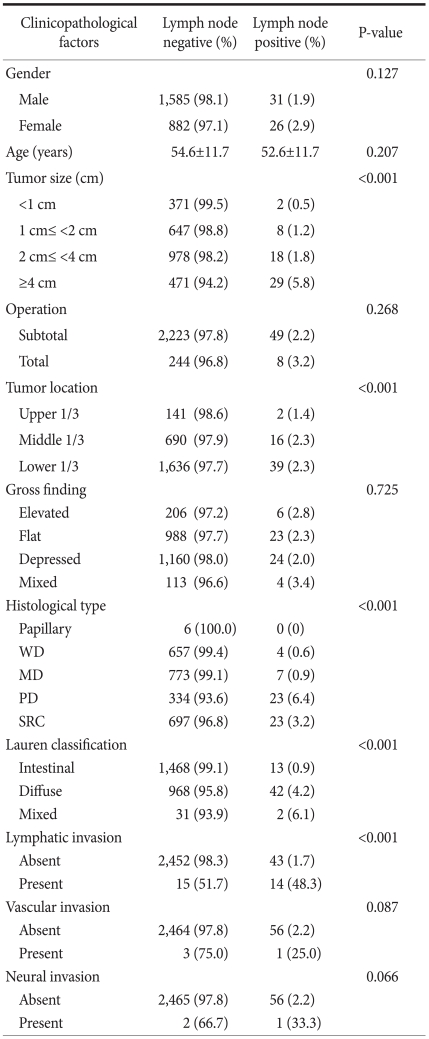
Among total 2,524 patients, lymph node metastasis was found in 57 patients (2.2%), and out of these 57 people, 41 were diagnosed with N1, 8 with N2 and 8 with N3a. Patient gender (male 54.4% vs. female 45.6%, P=0.127) and age (54.6 vs. 52.6 years old, P=0.207) were not related with lymph node metastasis. Lymph node metastases were found in 8 (3.17%) of 252 patients underwent radical total gastrectomy and 49 (2.16%) of the 2,272 patients underwent radical subtotal gastrectomy, but the result was not statistically significant (P=0.268).
The result of analysis depending on the size of the tumor revealed that lymph node metastasis was found in 2 (0.5%) of 360 patients with less than 1 cm, 8 (1.2%) of 645 patients with 1~2 cm, 18 (1.8%) of 993 patients with 2~4 cm, and 29 (5.8%) of 498 patients with 4 cm or more. As the size of the tumor increased, the number of metastatic lymph nodes increased, and the differences were of statistical significance (P<0.001) (Table 1). Classified according to the location of tumor, the upper stomach cancer was found in 144 cases, the middle stomach cancer in 706 cases, the lower stomach cancer in 1,675 cases, and lymph node metastasis was found in 2 cases (1.4%), 16 cases (2.3%), and 39 cases (2.3%), respectively. In the middle and lower stomach, there was a higher incidence of lymph node metastasis which was statistically significant (P<0.001).
Table 1.
Relationship between clinicopathological factors and lymph node metastasis
WD = well differentiated; MD = moderately differentiated; PD = poorly differentiated; SRC = signet-ring cell.
According to the gross appearance of tumors, they were classified as 212 cases of elevated type, 1,011 cases of flat type, 1,184 cases of depressed type, and mixed type totaled 117 cases. Lymph node metastasis was found in 6 cases (2.8%), 23 cases (2.3%), 24 cases (2.0%), and 4 cases (3.4%), respectively, but were not statistically significant (P=0.725). According to the analysis of histological classification, among all of mucosa cancers, no lymph node metastasis was shown in 6 cases of papillary adenocarcinoma. It was found in 4 (0.6%) out of 661 cases of all well differentiated tubular adenocarcinoma, 7 (0.9%) out of 780 cases of all moderately differentiated tubular adenocarcinoma, 23 (6.4%) out of 357 cases of all poorly differentiated adenocarcinoma and 23 (3.2%) of 720 cases of all signet-ring cell carcinoma. It showed that the lymph node metastases in poorly differentiated adenocarcinoma and signet-ring cell carcinoma have significantly higher incidence (P<0.001). In 57 cases of lymph node positive patients, there were 0 (0%) cases in papillary adenocarcinoma, 4 (7%) cases in well differentiated tubular adenocarcinoma, 7 (12.1%) cases in moderately differentiated tubular adenocarcinoma, 23 (40.4%) cases in poorly differentiated tubular adenocarcinoma, and 23 (40.4%) cases in signet-ring cell carcinoma.
According to Lauren's histological classification, there were 1,481 cases of intestinal type, 1,010 cases of diffuse type and 33 cases of mixed type. Lymph node metastasis in each case was found in 13 cases (0.9%), 42 cases (4.2%), and 2 cases (6.1%), respectively. There was a significantly higher incidence of lymph node metastasis in diffuse type (P<0.001). Lymph node metastasis was found in 43 cases among 2,495 patients without lymphatic invasion and in 14 cases among 29 patients with lymphatic invasion. This demonstrated that lymphatic invasion is a factor influencing lymph node metastasis in mucosal cancer (P<0.001). Lymph node metastasis was found in 56 of 2,520 cases without vascular invasion and in one of 4 cases with vascular invasion (P=0.087), and it was found in 56 of 2,521 cases without perineural invasion, and in one of 3 cases with perineural invasion (P=0.066). Based on these results, vascular invasion and perineural invasion were not statistically significant predictive factors for lymph node metastasis in mucosal cancer.
2. Multivariate analysis of clinicopathological factors associated with lymph node metastasis
The multivariate analysis on the five factors found to be statistically significant in the univariate analysis, namely the size and location of tumor, histologic differentiation, Lauren classification, and lymphatic invasion showed that cases of the tumor larger than 4cm in size (odds ratio 4.465, 95% confidence interval; 1.027~19.414, P=0.024) and cases with lymphatic invasion (odds ratio 49.662, 95% confidence interval; 20.045~123.039, P<0.001) were correlated independently with lymph node metastasis (Table 2). In the univariate analysis, the larger tumor size showed the higher incidence of lymph node metastasis, but in multivariate analysis, it showed that it is significant as an independent predictive factor of lymph node metastasis only in the cases of tumor size greater than 4 cm. The location of the tumor, histologic differentiation (classified with differentiated and undifferentiated types) and Lauren classification showed no independent correlation with lymph node metastasis.
Table 2.
Multivariate analysis of potential risk factors for lymph node metastasis
95% CI = 95% confidence interval.
3. Analysis of the frequency of lymph node metastasis according to the tumor size in the patient with lymphatic invasion
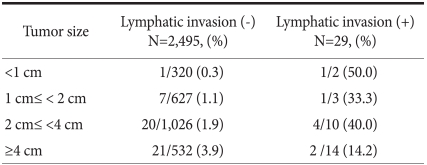
Among 29 patients with lymphatic invasion, 2 patients had tumors with diameter less than 1 cm and lymph node metastasis was shown in one (50%) of them. One (33.3%) of 3 patients had tumor size of 1~2 cm, and with tumor size of 2~4 cm, 4 (40.0%) out of 10 patients presented with positive lymphatic invasion. Two (14.2%) of 14 patients had tumor larger than 4 cm in size and also presented with lymphatic invasion (Table 3).
Table 3.
Lymph node metastasis rate assessed by combining lymphatic invasion and tumor size in mucosal cancer
Discussion
Recently worldwide incidence and mortality of gastric cancer has been decreasing, but it is remains the fourth most common malignant tumor and the second leading cause of cancer deaths. (11,12) According to the report of cancer incidence from 2003 to 2005 in Korea, announced by Korea Central Cancer Registry, Department of Health and Welfare, gastric cancer is most common type with 18.3% of the total cancer incidence in Korea. It is ranked first among men and is ranked third with 13.7% following breast cancer and thyroid cancer among women. In the past, laparotomy was often suggested for radical treatment of gastric cancer, but recently minimally invasive treatments by cancer resection have been widely conducted with the development of endoscopic surgery techniques and equipments as early diagnosis of gastric cancer rates are increasing due to the activation of medical examination. Unlike radical laparotomy surgery, in particular, by the advantage of organ preservation after surgery and the better quality of life in patients, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are frequently conducted in the large medical centers. In early gastric cancer, EMR indications are known for elevated lesions of less than 2 cm in size confined to the mucosa with mucosal invasion and cancer cell differentiation and depressed lesions of less than 1 cm in size without ulcer.(13) With the introduction of ESD, treatments by endoscopic resection have been also conducted for early stage gastric cancer of less than 3 cm in size with mucosal invasion of upper third of stomach,(14) and in Korea, the complete resection rate in endoscopic resection for early gastric cancer is an estimated 75~87%.(15,16) In this study, the incidence of lymph node metastasis in mucosal cancer was 2.2%, and it showed the same or lower result compared with other existing studies showing about 3~5%.(12,17-20) Haruta et al.(20) reported that 14 cases (1.9%) among 718 cases of mucosa cancer showed lymph node metastasis and ulcers were associated with all these cases and mucosal cancer without ulceration showed no lymph node metastasis regardless of the size of tumor and histologic differentiation. According to previous studies of lymph node metastasis, the analysis of various clinicopathological factors (age, gender, tumor size and location, gross appearance, histologic differentiation, presence of ulceration, lymphatic invasion, etc.) showed that age, depressed type, tumor size, undifferentiated type, the case associated with ulcer, and the case with lymphatic invasion have significant correlations with lymph node metastasis. Among these, lymphatic invasion, the case associated with ulcer, and the tumor size larger than 3cm were reported as the risk factors in predicting lymph node metastasis.(21,22)
The univariate analysis of our research data showed that the factors associated with lymph node metastasis are tumor size, the middle and lower third areas of the stomach, poorly differentiated adenocarcinoma and signet-ring cell carcinoma, diffuse type by Lauren classification, and lymphatic invasion, showing similar results consistent with other existing studies.(23,24) The multivariate analysis on these factors showed that the independent predictive factors associated with lymph node metastasis in mucosal cancer are the tumor size (4 cm or more) and lymphatic invasion. Also, metastatic frequency analysis according to the tumor size in patients with lymphatic invasion showed that a higher frequency of lymph node metastasis appears in the cases of lymphatic invasion in mucosal cancer with tumor less than 4 cm. A 41 year-old man who showed lymphatic invasion in mucosal cancer less than 1cm in size was treated with subtotal gastrectomy for type IIc lesions at gastric angle. According to the pathological examination results, it was a poorly differentiated adenocarcinoma 0.5 cm in size and intestinal type according to Lauren classification. He showed no vascular and perineural invasion, and no metastasis of cancer among total 59 retrieved lymph nodes. In the same group a 58 year-old women underwent subtotal gastrectomy for mucosal cancer type IIb lesions at the low third of stomach, and showed poorly differentiated adenocarcinoma of 0.4 cm in size and diffuse type by histologic examination. She showed lymphatic invasion and metastasis of cancer was found in one of 48 resected lymph nodes. This indicates that even if the tumor size was less than 1 cm, in case of lymphatic invasion, there was an increase in the incidence of lymph node metastasis. This implies that lymphatic invasion in early gastric cancer confined to mucosa is the most strong predictive factor associated with lymph node metastasis in mucosa cancer.
Recently, based on the results of surgical data analysis that lesions confined to mucosa without ulceration did not show LN metastasis regardless of their sizes, there are moves to expand the indication for ESD.(9) However, according to the results of this study, even lesions confined to mucosa should be treated with surgery because the possibility of lymph node metastasis increases if their sizes of lesions are larger than 4 cm.
According to Saka et al.,(25) the overall recurrence rate of early gastric cancer is relatively low, between 1.4~2.8%. The recurrence rate of early gastric cancer without lymph node metastasis was 0.6~0.7%, whereas the recurrence rate with lymph node metastasis was 7~20%, showing a relatively increased result. Also in the study of Han et al., early gastric cancer recurrence in all patients was 2.4%, but it tended to have a higher rate of 7.3% in patients with lymph node metastasis. This is due to the pathologic characteristics of gastric cancer. Even in early gastric cancer, lymph node metastasis is a very important prognostic factor, and we presume it will be very helpful in determining the treatment strategy of early gastric cancer to predict lymph node metastasis.
Recently, the rate of patients with early gastric cancer among the total gastric cancer patients has been increasing. Especially in mucosal cancer, endoscopic resection is a widely performed procedure, but requires cautious patient selection even in early stage gastric cancer since there is a possibility of cancer recurrence in the cases with lymph node metastasis. Recently, there are moves to expand the target range of conducting ESD, but even for early gastric cancer confined to mucosa, ESD should not be conducted and it should be treated with surgery because the frequency of lymph node metastasis increases when lymphatic invasion is present or the tumor size is larger than 4 cm.
References
- 1.Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 years' experience at a single institute in Korea. Eur J Surg Oncol. 2008;34:36–41. doi: 10.1016/j.ejso.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim JJ, Song KY, Hur H, Hur JI, Park SM, Park CH. Lymph node micrometastasis in node negative early gastric cancer. Eur J Surg Oncol. 2009;35:409–414. doi: 10.1016/j.ejso.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Higashi H, Natsugoe S, Ishigami S, Uenosono Y, Matsumoto M, Nakajo A, et al. Distribution of lymph node metastasis including micrometastasis in gastric cancer with submucosal invasion. World J Surg. 2003;27:455–459. doi: 10.1007/s00268-002-6601-4. [DOI] [PubMed] [Google Scholar]
- 4.Sano T, Hollowood A. Early gastric cancer: diagnosis and less invasive treatments. Scand J Surg. 2006;95:249–255. doi: 10.1177/145749690609500407. [DOI] [PubMed] [Google Scholar]
- 5.Seo WH, Seo BJ, Yu HJ, Lee HK, Lee WY. Analysis of prognostic factors in 1,435 surgically treated patients with gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:143–151. [Google Scholar]
- 6.Kim CH, Jang SW, Kang SH, Kim SW, Song SK. The significance of lymphatic, venous, and neural invasion as prognostic factors in patients with gastric cancer. J Korean Gastric Cancer Assoc. 2005;5:113–119. [Google Scholar]
- 7.An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749–753. doi: 10.1097/SLA.0b013e31811f3fb7. [DOI] [PubMed] [Google Scholar]
- 8.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]
- 9.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer Association, editors. Japanese Classification of Gastric Carcinoma. 13th ed. Tokyo: Kanehara; 1998. pp. 10–24. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 12.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 14.Adachi Y, Shiraishi N, Kitano S. Modern treatment of early gastric cancer: review of the Japanese experience. Dig Surg. 2002;19:333–339. doi: 10.1159/000065829. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Kim JJ. Endoscopic mucosal resection of early gastric cancer: Experiences in Korea. World J Gastroenterol. 2007;13:3657–3661. doi: 10.3748/wjg.v13.i27.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH. Endoscopic resection of early gastric cancer in Korea: Recent results and future directions. J Korean Gastric Cancer Assoc. 2009;9:39–45. [Google Scholar]
- 17.Han KB, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, et al. Factors affecting prognosis in early gastric cancer. J Korean Gastric Cancer Assoc. 2009;9:238–245. [Google Scholar]
- 18.Shimada S, Yagi Y, Shiomori K, Honmyo U, Hayashi N, Matsuo A, et al. Characterization of early gastric cancer and proposal of the optimal therapeutic strategy. Surgery. 2001;129:714–719. doi: 10.1067/msy.2001.114217. [DOI] [PubMed] [Google Scholar]
- 19.Torii A, Sakai M, Inoue K, Yamabe H, Ueda S, Okuma M. A clinicopathological analysis of early gastric cancer: retrospective study with special reference to lymph node metastasis. Cancer Detect Prev. 1994;18:437–441. [PubMed] [Google Scholar]
- 20.Haruta H, Hosoya Y, Sakuma K, Shibusawa H, Satoh K, Yamamoto H, et al. Clinicopathological study of lymphnode metastasis in 1,389 patients with early gastric cancer: assessment of indications for endoscopic resection. J Dig Dis. 2008;9:213–218. doi: 10.1111/j.1751-2980.2008.00349.x. [DOI] [PubMed] [Google Scholar]
- 21.Shen L, Huang Y, Sun M, Xu H, Wei W, Wu W. Clinicopathological features associated with lymph node metastasis in early gastric cancer: analysis of a single-institution experience in China. Can J Gastroenterol. 2009;23:353–356. doi: 10.1155/2009/462678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamao T, Shirao K, Ono H, Kondo H, Saito D, Yamaguchi H, et al. Risk factors for lymph node metastasis from intramucosal gastric carcinoma. Cancer. 1996;77:602–606. doi: 10.1002/(SICI)1097-0142(19960215)77:4<602::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 23.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, et al. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 24.Kwak CS, Lee HK, Cho SJ, Yang HK, Lee KU, Choe KJ, et al. Analysis of clinicopathological factors associated with lymph node metastasis in early gastric cancer; Review of 2,137 cases. J Korean Cancer Assoc. 2000;32:674–681. [Google Scholar]
- 25.Saka M, Katai H, Fukagawa T, Nijjar R, Sano T. Recurrence in early gastric cancer with lymph node metastasis. Gastric Cancer. 2008;11:214–218. doi: 10.1007/s10120-008-0485-4. [DOI] [PubMed] [Google Scholar]