Abstract
Purpose
We did a retrospective study to understand the prognostic effects of preoperative blood transfusions in stomach cancer surgery.
Materials and Methods
Data for 1,360 patients who underwent gastrectomy for stomach cancer between 2001 and 2009 were retrospectively reviewed. We analyzed factors that affect preoperative transfusion and clinicopathologic features. We also analyzed 5-year and overall survival rates of the transfusion and non transfusion subgroups.
Results
Sixty patients (4.4%) required blood transfusion within the preoperative period. The transfused group included patients who took aspirin or clopidogrel (P<0.001), with more advanced T stages (P<0.001), with more advanced nodal metastasis (P=0.00), and with more advanced stages (P=0.00) than the non transfusion group. On multivariate analysis, preoperative transfusion was a statistically significant negative influence on 5-year survival and overall survival rates (58.2% vs 79.9% (P=0.00), 58.2% vs 76.8% (P=0.00)). Applying Cox-regression analyses, blood transfusion did appear to have an effect on prognosis and on 5-year and overall survival rates.
Conclusions
We found a direct negative relation between preoperative transfusion and long term prognosis in patients receiving gastric cancer surgery.
Keywords: Stomach neoplasm, Blood transfusion, Prognosis
Introduction
Transfusion is an important method for saving the life of patients. Nevertheless, transfusion can cause infection, and may also induce a hemolytic transfusion reaction, and cause an iron overload, post-transfusional graft versus host disease, and other side effects, and it may cause polycythemia.(1) Other studies have reported that transfusion not only causes such problems, but also, it elevates the recurrence rate of cancer, the rate of pertinent complications and the mortality rate.(2-4)
Presently, the decision to do a transfusion is decided on by numerous clinicians with different educational backgrounds and points of view. At each medical institution, their own transfusion standard is taught orally and transfusion is decided accordingly. As a means to resolve such problems, guidelines for appropriate transfusion have been suggested not only in developed countries but also in Korea.(5) However, a common standard value for transfusion can not be applied to all patients because factors associated with patients are very diverse.(6,7)
In the treatment of cancer patients, cases requiring transfusion can be broadly divided into three types: (i) cases with low presurgical hemoglobin values, (ii) cases where cancer surgery induces substantial hemorrhage, and (iii) cases in which depressed marrow develops due to chemotherapy or radiation therapy.(8)
Different outcomes of preoperative transfusion on the survival rate of gastric cancer patients have been reported.(9-12) Since postsurgical hemorrhage is a complication caused by erroneous surgical techniques, in cases that have this complication it is a problem to analyze the effect of transfusion itself on the survival rate. In addition, it has been reported that regardless of transfusion, the loss of blood itself increases the recurrence rate of cancer.(13) Therefore, in patients who underwent a gastrectomy for gastric cancer, we examined the effect of transfusion on survival rate by retrospectively analyzing the group who received preoperative transfusion and the group who did not receive preoperative transfusion.
Materials and Methods
Among patients diagnosed with gastric adenocarcinoma initially, and who underwent a gastrectomy in the department of surgery, Chung Nam National University Hospital, between January 2001 and December 2009, retrospective analysis was performed. There were 1,542 patients whose data could be assessed. Gastrectomy was performed by the same surgeon according to standardized techniques. To reduce errors in survival rate, cases where surgery was performed for radical gastrectomy but distant metastasis as well as for peritoneal metastasis were excluded from this study. These were evident macroscopically and thus these patients underwent palliative gastrectomy (N=120). In addition, to reduce errors in survival rate caused by surgical complications, transfusions performed for hemorrhage occurring during surgery and after surgery were excluded (N=62). Even patients who received a transfusion prior to surgery and received a transfusion again during surgery or after surgery were excluded from this study. Therefore, the patients included in this study were 1,360 patients who received transfusion prior to surgery.
The transfusion group was defined as cases who received a transfusion of concentrated red blood cells, during the transfusion period. This period was defined as cases who received transfusion within the 30 days prior to surgery. The standard for transfusion was that transfusion was performed for cases with hemoglobin values lower than 8 g/dl, where the vital signs were stable, and where there were no anemic symptoms, and where transfusion was decided by re-evaluating hemoglobins. For cases with hemoglobin values of 8~10 g/dl, transfusion was performed considering risk factors associated with inappropriate oxygenation (rate of blood loss, cardiopulmonary capacity, oxygen consumption, coronary artery diseases, etc.). For patients who found it difficult to adjust to anemia (those older than 65 years, cases associated with cardiovascular or respiratory diseases), cases with hemoglobin lower than 10 g/dl received transfusions.
Clinicopathological factors of the transfusion group and the non-transfusion group were analyzed. Age, gender, body mass index, and comorbid diseases were included in the analysis. For recurrent cases, a history of aspirin or Clopidogrel intake, cancer stage, the status of gastric outlet obstruction, and transfusion volume were included.
To determine the stage of gastric cancer, we used the AJCC classification (2002, 6th edition). For the evaluation of clinicopathological factors, chi-square tests were performed. For the analysis of the survival rate of patients, the Kaplan-Meier method was applied. For the validation of significance, the log-rank test was applied. Multivariate analysis was performed applying a Cox proportional regression model. We examined whether preoperative transfusion is an independent prognostic factor that affects survival rate.
For statistical analysis, we used SPSS for Windows (SPSS Inc., Chicago, IL, USA).
Results
1. Patient characteristics
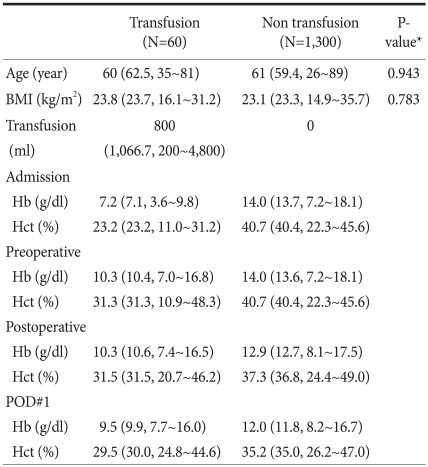
The study was conducted on 1,360 patients. The characteristics of the patients are described in Table 1. There were 975 males (71.7%) and 385 females (28.3%). In the transfusion group, there were 43 males (71.7%) and 17 females (28.3%). In the non-transfusion group, there were 931 males (71.7%) and 368 females (28.3%). The median age of the transfusion group and the non-transfusion groups was, respectively, 60 years (range: 35~81 years) and 61 years, (range: 26~89 years).The median body mass index of the two groups was 23.8 kg/m2 (16.1~31.2) and 23.1 kg/m2 (14.9~35.7), respectively (Table 1).
Table 1.
Patient characteristics (N=1,360)
BMI = body mass index; Hb = hemoglobin; Hct = hematocrit; POD = postoperative day; Data were median (mean, range). *Chi-square test.
There were 60 patients (4.4%) who received a transfusion prior to surgery. The median transfusion volume was 800 ml (range: 200~4,800 ml). The median Hb values of the transfusion group (i) at the time of admission, (ii) prior to surgery, (iii) after surgery, and (iv) 1 day after surgery were 7.2 g/dl (3.5~9.8 g/dl), 10.3 g/ dl (7.0~16.8 g/dl), 10.3 g/dl (7.4~16.5 g/dl), and 9.4 g/dl (7.7~16.0 g/dl), respectively. The analogous median Hb values of the non-transfusion group were 14.0 g/dl (7.2~18.1 g/dl), 14.0 g/dl (7.2~18.1 g/dl), 12.9 g/dl (8.1~17.5 g/dl), and 12.0 g/dl (8.2~16.7 g/dl), respectively (Table 1).
2. Transfusion
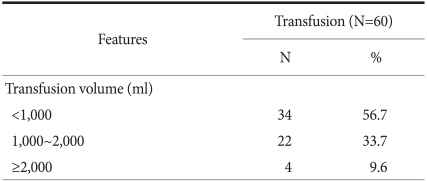
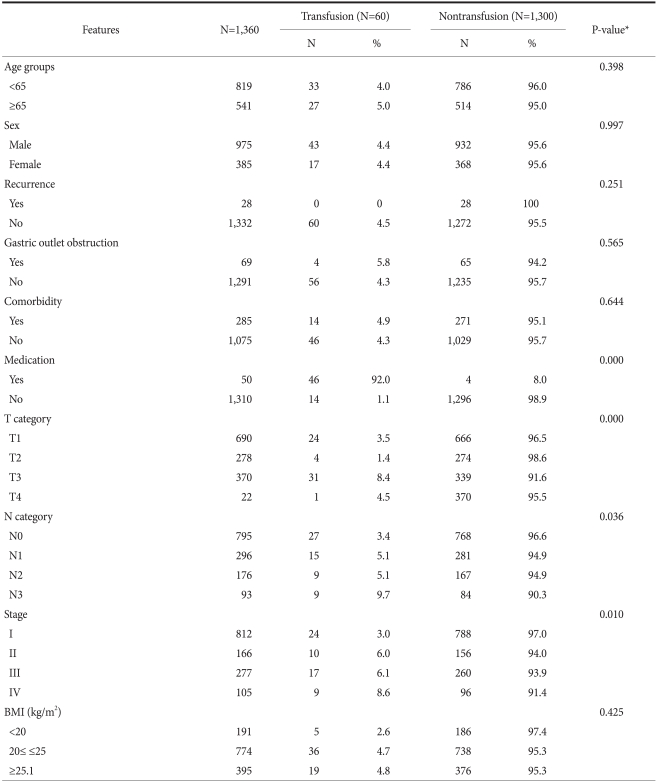
Of the 1,360 patients who had a gastrectomy, 60 patients received a preperative transfusion (4.4%). 34 patients (56.7%) received a transfusion volume less than 1,000 ml, 22 patients (33.7%) received 1,000~2,000 ml, and 4 patients (9.6%) received more than 2,000 ml (Table 2). We used a Chi-square test to evaluate clinicopathological factors of patients, especially factors associated with transfusion. For patients taking aspirin or Clopidogrel (P=0.00), cases in an advanced T stage (P=0.05), cases with more lymph node metastasis (P=0.00), and cases whose disease stage of gastric cancer was higher, more transfusions were performed (P=0.00) (Table 3).
Table 2.
Transfusion group according to the transfusion volume
Table 3.
Patients' clinicopathologic characteristics according to the subgroups
BMI = body mass index. *Chi-square test.
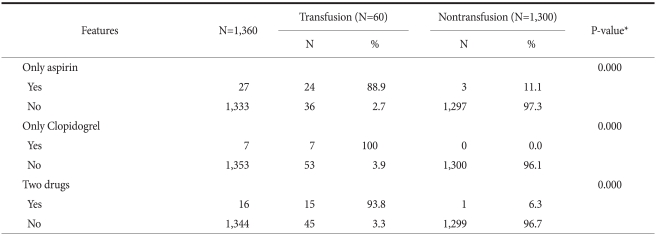
We did a subgroup analysis for patients that had used aspirin or Clopidogrel (Table 4). It was found that cases who had taken aspirin only (P=0.00), Clopidogrel only (P=0.00), and both (P=0.00) received significantly more transfusions.
Table 4.
Medications
*Chi-square test.
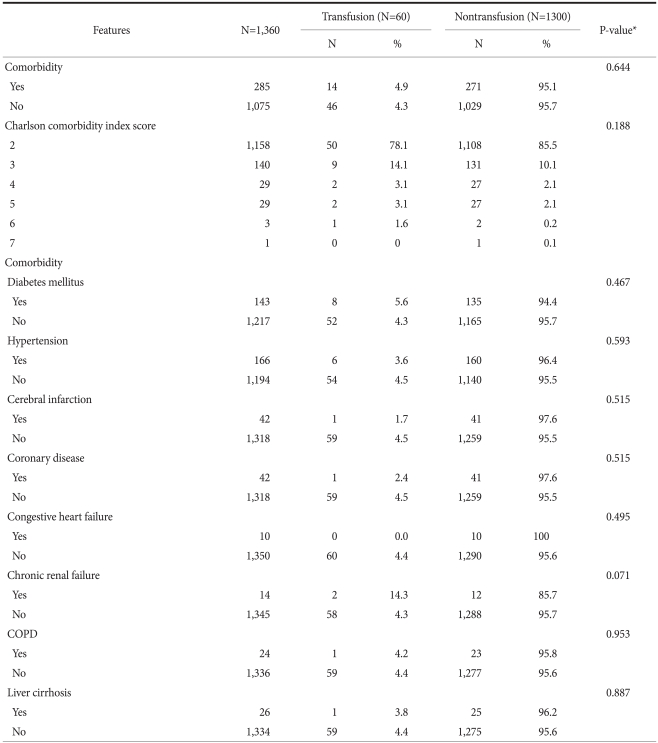
The number of comorbid diseases at the time of surgery (P=0.264) and the status of those comorbid diseases (P=0.644) did not receive significantly more transfusions (Table 5).
Table 5.
Comorbidity
COPD = Chronic obstructive pulmonary disease. *Chi-square test.
When multivariate analysis was performed on the association between clinicopathological factors and transfusions, no significant factors were detected.
3. Survival rate
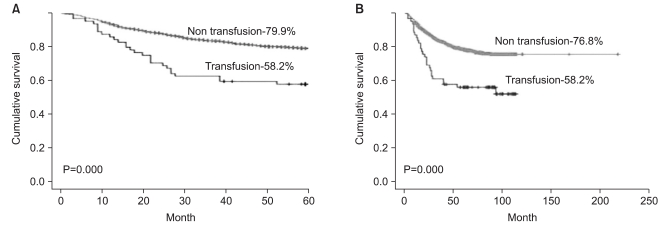
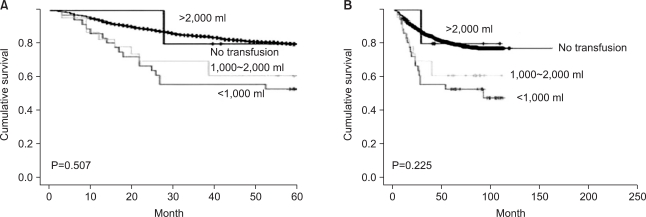
The 5-year survival rate of the transfusion group was 58.2% for the non-transfusion group it was 79.9%, and this difference was significant (P=0.00) (Fig. 1A). Regarding the overall survival rate, similarly, the transfusion group had a rate of 58.2%, the non-transfusion group 76.8%, and the difference was significant (P=0.00) (Fig. 1B). The patient group that received transfusions of 0~1,000 ml transfusion or 1,000~2,000 ml had lower 5-year survival and overall survival rates than the non-transfusion group; this difference was not significant (Fig. 2).
Fig. 1.
Preoperative blood transfusion and survival rate according to subgroup analysis. (A) 5-year survival. (B) Overall survival .
Fig. 2.
Survival rate according to the volume of transfusion. (A) 5-year survival. (B) Overall survival.
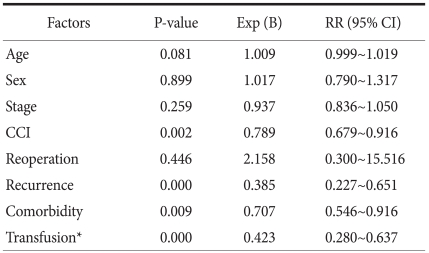
In univariate analysis, factors exerting effects on survival rate were transfusion prior to surgery (P=0.00), high Charlson comorbidity index score (P=0.001), patients with comorbidity (P=0.005), recurrent gastric cancer (P=0.00), large transfusion volumes (P=0.00), cases taking aspirin or clopidogrel (P=0.00), and disease stage (P=0.03). To determine whether transfusions had an independent effect on prognosis, we did multivariate analysis. Factors that may mediate adverse effects on prognosis were selected, and analysis was done using a Cox-regression model. Significant factors included disease recurrence (P=0.00), cases with a high Charlson comorbidity index score (P=0.002), and cases who received transfusion prior to surgery (P=0.00) (Table 6).
Table 6.
Multivariate analysis
RR = relatvie risk; CI = confidence interval; CCI = Charlson comorbidity index score. *Preoperative transfusion.
The above factors that exerted effects on survival rate were compared with the survival rate of patients who received a preoperative transfusion. Examining the 5-year survival rate of patients with comorbid diseases according to the status of transfusion, the transfusion group and the non-transfusion group were 42.9% and 73.8%, respectively, and there was the statistically significant difference (P=0.02). Similarly, overall survival rates were 42.9% and 69.4%, respectively, and a significant difference was found (P=0.004). Among those without comorbid diseases, the transfusion and non-transfusion groups had 5-year survival rates of 62.9% and 73.9%, respectively (P=0.0023), overall survival rates were 62.9% and 78.6%, respectively, both differences were significant.
In cases of disease recurrence, none of the patients received a transfusion prior to surgery. Examining the nonrecurrent group, the 5-year survival rates of the transfusion and non-transfusion groups were 58.2% and 80.5%, respectively (P=0.00), overall survival rates were 77.6% and 68.2%, respectively (P=0.00). Both differences were statistically significant.
Discussion
Among studies that have analyzed factors associated with red blood cell transfusion in surgery, and reviewing the results of studies pertinent to surgery for cancer, Benoist reported that in rectal cancer surgery, in cases older than 65 years, cases with heavier body weight, and cases with presurgical hemoglobin values lower than 12.5 g/dl, red blood cell transfusion was performed more frequently.(14) Vamvak as and Carven(15) reported that in colorectal cancer surgery, in cases with cardiovascular diseases or congestive heart failure, cases with diabetes, cases who were anemic prior to surgery, cases older than 75 years and cases that were female, red blood cell transfusion was performed more frequently. Matsumata et al.(16) reported that in liver cancer surgery, low body weight was a factor causing red blood cell transfusion.
In our study, which was on gastric cancer surgery, factors associated with red blood cell transfusion were analyzed. The results of Chi-square tests show that significant factors were cases who had taken aspirin or Clopidogrel (P=0.000), cases with an advanced T-stage category (P=0.000), those with advanced lymph node metastasis (P=0.036), and cases with advanced disease stage (P=0.010) (Table 2). The frequency of transfusion was higher in T1 and the frequency of transfusion was lower in T4, which is thought to be due to the fact that the number of T4 patients was very small, only 22 patients. Multivariate analysis was performed on the above statistically significant factors by binary logistic regression, and no factors statistically significant. Our interpretation is that the factors were not independent factors.
Transfusion has been reported to raise the survival rate of patients with a transplanted kidney.(17) But that study was done prior to the development of immune suppressors, and transfusion was performed routinely to suppress immune reactions. In addition, based on the results of studies in which transfusion reduces disease recurrence in autoimmune diseases, transfusion could be considered to work through immune suppression. The effect of transfusion on the regulation of immunity has caused great problems in surgery outcomes. According to a study reported by Taylor et al.(18) which followed 1,717 trauma patients in the intensive care unit, it was shown that nosocomial infections were 6 times higher in the transfusion group than in the non-transfusion group, and mortality was 2 times higher. Other studies showed similar results that transfusion lowers survival rates for gastric cancer, colon cancer, rectal cancer, breast cancer, lung cancer, and renal cancer.(19-22) We examined the mechanism for the alteration of immune function after transfusion. Hematopoiesis function in vivo was decreased, the functioning of natural killer cells and phagocytic cells was impaired, the activity of T-helper cells was reduced, the activity of T-suppressor cells were increased, and interleukin-2 production was reduced. Therefore, patients who receive a transfusion develop serious problems in resistance to infection and in cancer metastasis.(23)
In our study, similarly, the survival rate of the transfusion group was shown to be significantly lower than of the non-transfusion group. The transfusion volume did not correlate with the survival rate. Hence, it is thought that the transfusion itself mediated its effects on the survival rate rather than the transfusion volume. When the survival rate was re-examined, and we excluded recurrent cases and cases with comorbid diseases that have been determined to be factors exerting effects on survival rates by multivariate analysis, the survival rate was significantly lower in the transfusion group than in the non-transfusion group. In our study, since recurrent patients did not receive a transfusion, their survival rate could not be compared. However, as shown by multivariate analysis, it can be concluded that preoperative transfusion lowers the survival rate of gastric cancer patients (Table 6).
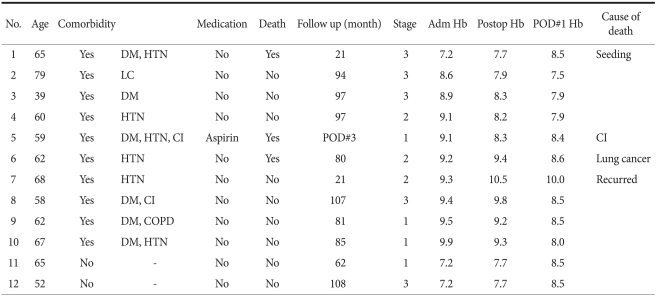
Among patients with comorbid diseases, and where the presurgical hemoglobin value was lower than 10.0 g/dl, 10 patients did not receive a transfusion. Among patients without comorbid diseases and where the presurgical hemoglobin value was lower than 8.0 g/dl, 2 patients did not receive a transfusion (Table 7). Among them, there were 3 cases of patients who died within 5 years. The causes of death were: complications caused by peritoneal metastasis 5 months after surgery, deterioration of a cerebral infarction, and complications caused by disease recurrence that developed 1 year after surgery. In the patient who died due to deterioration of a cerebral infarction, hemoglobin values prior to surgery, after surgery, and one day after surgery were 9.1 g/dl, 8.3 g/dl, and 8.4 g/dl, respectively. Nonetheless, since the patient did not show abnormal vital signs or other abnormalities, transfusion was not performed. Three days after surgery, the patient suddenly showed cerebral infarction symptoms and was transferred to the department of neurology. During treatment in the intensive care unit, he died on the day of transfer to the neurology department. The patient was taking aspirin for cerebral infarction and discontinued it for 7 days prior to surgery, but problems did not develop during surgery. Aspirin might affect the deterioration of a cerebral infarction. Nonetheless, his low hemoglobin value might have had adverse effects. After reviewing the case of this patient, we think that the case definitely required an appropriate transfusion.
Table 7.
Characteristics of patients who did not received preoperative transfusion in spite of indication cases
Adm = admission; Hb = hemoglobin; Postop = postoperative; POD = postoperative day; DM = diabetes mellitus; HTN = hypertension; LC = liver cirrhosis; CI = cerebral infarction; COPD = chronic obstructive pulmonary disease.
In the late 1980s, as attention came to be paid to the problems of the spread of acquired immune deficiency syndrome, hepatitis, etc., studies in animals or humans increased.(24,25) Among such studies, was a report about patients who did not receive a transfusion because of religious reasons. Even if their hemoglobin value was not within the normal range, if the intravascular volume was maintained, they could survive.(26) Therefore, to decide on whether to give a transfusion, factors other than hemoglobin values should be considered together.
An absolute standard of the appropriate time for preoperative transfusion has not been established yet. According to a recently published guideline, the lowest limit in healthy individuals has been suggested to be a hemoglobin value lower than 6 g/dl.(27) Nonetheless, according to the 1988 World Health Organization guideline for preoperative transfusion, a transfusion should not be decided on based on one factor only. They suggested 7 factors for the determination of whether to do a transfusion:(28) (1) the severity of chronic anemia, (2) the presence or absence of comorbid diseases, (3) the volume of persistent hemorrhage, (4) clinical syndrome, (5) anaerobic metabolism (lactic acidosis), (6) perfusion index, and (7) the physiologic index-oxygen extraction ratio.
When patients with cardiovascular diseases, lung diseases, renal diseases, sepsis, or cerebral diseases were compared with patients without these diseases. The former could not withstand anemia as easily.(29) Similar to studies reporting that low hemoglobin values cause the deterioration of cardiovascular diseases and cerebral infarction, and raise mortality,(30) in our study, although only 1 patient (8.3%), it was found that for cases with comorbid diseases difficult to withstand anemia, blood transfusion could be of help to improve survival rate.
In cancer patients with comorbidities, there are numerous previous studies that analyze the effect on the long-term prognosis or the decision of treatment strategies. Among the standardized markers or tools with which to evaluate the overall prognosis of patients, a representative one is the Charlson comorbidity index (CCI).(31) In our study, cases with comorbid diseases were scored by the CCI, and applying the score, the effect on survival rate was analyzed. The results from multivariate analysis showed that the CCI is a factor that exerts effects on the overall survival rate of gastric cancer patients. Nevertheless, in a study reported by Lübke et al.(32) it was reported that in gastric cancer patients, CCI was not a factor predicting a worse prognosis after surgery. Therefore, to see if CCI exerts effects on overall prognosis in gastric cancer patients, further studies may be required.
In this study, by excluding postsurgical transfusion or transfusion during surgery and thus ruling out their potential effects on survival rate, the effect of preoperative transfusion could be analyzed. In addition, to rule out the effect of technical errors that may occur during surgery for gastric cancer, cases that developed hemorrhage during surgery or after surgery were also excluded. To examine the effect of transfusion during surgery or after surgery, additional studies are now required.
In some past studies, it was shown that transfusion was not an independent factor affecting survival rates of gastric cancer patients. Such a discrepancy was thought to be due to the fact that they all were retrospective studies, the size of the recruited groups was different, the selection of subject patients was not consistent, and statistical methods were not identical. Therefore, prospective studies and continuous studies applying meta-analysis are required.
In our study of gastric cancer patients, using Chi-square tests, we found that factors associated with an increase in the frequency of preoperative red blood cell transfusions were aspirin or Clopidogrel, an advanced T or N category, and advanced disease stage. These were not determined to be independent factors by binary logistic regression multivariate analysis. In Kaplan-Meier survival curves, the 5 year survival rate and overall survival rate of the transfusion group was significantly lower than the non-transfusion group. From Cox regression survival analysis, preoperative transfusion was an independent factor mediating adverse effects on the survival of gastric cancer patients. Therefore, to achieve a better prognosis of gastric cancer patients, preoperative transfusion should not be performed if possible. Nevertheless, for patients who can not withstand anemia well, selective, appropriate transfusion may be required.
References
- 1.Ministry of Health in Japan. About the use of blood products. 3rd ed 2007. [Google Scholar]
- 2.Hyung WJ, Noh SH, Shin DW, Huh J, Huh BJ, Choi SH, et al. Adverse effects of perioperative transfusion on patients with stage III and IV gastric cancer. Ann Surg Oncol. 2002;9:5–12. doi: 10.1245/aso.2002.9.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033. doi: 10.1002/14651858.CD005033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson KR, Berenholtz SM, Dorman T, Garrett E, Lipsett P, Kaufman HS, et al. Preoperative predictors of blood transfusion in colorectal cancer surgery. J Gastrointest Surg. 2002;6:753–762. doi: 10.1016/s1091-255x(02)00043-4. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control of Health and Welfare in Korea; Institute of Transfusion in Korea. Guidelines for transfusion. 2009. [Google Scholar]
- 6.Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, et al. British Committee for Standards in Haematology; Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 7.British Committee for Standards in Haematology. Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 8.Wilkerson DK, Rosen AL, Sehgal LR, Gould SA, Sehgal HL, Moss GS. Limits of cardiac compensation in anemic baboons. Surgery. 1988;103:665–670. [PubMed] [Google Scholar]
- 9.Kaneda M, Horimi T, Ninomiya M, Nagae S, Mukai K, Takeda I, et al. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion. 1987;27:375–377. doi: 10.1046/j.1537-2995.1987.27587320526.x. [DOI] [PubMed] [Google Scholar]
- 10.Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989;13:637–643. doi: 10.1007/BF01658891. [DOI] [PubMed] [Google Scholar]
- 11.Moriguchi S, Maehara Y, Akazawa K, Sugimachi K, Nose Y. Lack of relationship between perioperative blood transfusion and survival time after curative resection for gastric cancer. Cancer. 1990;66:2331–2335. doi: 10.1002/1097-0142(19901201)66:11<2331::aid-cncr2820661113>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg. 1994;167:256–260. doi: 10.1016/0002-9610(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 13.Singh SK, Marquet RL, de Bruin RW, Hop WC, Westbroek DL, Jeekel J. Consequences of blood loss on growth of artificial metastases. Br J Surg. 1988;75:377–379. doi: 10.1002/bjs.1800750427. [DOI] [PubMed] [Google Scholar]
- 14.Benoist S, Panis Y, Pannegeon V, Alves A, Valleur P. Predictive factors for perioperative blood transfusions in rectal resection for cancer: A multivariate analysis of a group of 212 patients. Surgery. 2001;129:433–439. doi: 10.1067/msy.2001.112068. [DOI] [PubMed] [Google Scholar]
- 15.Vamvakas EC, Carven JH. Allogeneic blood transfusion, hospital charges, and length of hospitalization: a study of 487 consecutive patients undergoing colorectal cancer resection. Arch Pathol Lab Med. 1998;122:145–151. [PubMed] [Google Scholar]
- 16.Matsumata T, Itasaka H, Shirabe K, Shimada M, Yanaga K, Sugimachi K. Strategies for reducing blood transfusions in hepatic resection. HPB Surg. 1994;8:1–6. doi: 10.1155/1994/98027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Opelz G, Terasaki PI. Poor kidney-transplant survival in recipients with frozen-blood transfusions or no transfusions. Lancet. 1974;2:696–698. doi: 10.1016/s0140-6736(74)93268-1. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RW, Manganaro L, O'Brien J, Trottier SJ, Parkar N, Veremakis C. Impact of allogenic packed red blood cell transfusion on nosocomial infection rates in the critically ill patient. Crit Care Med. 2002;30:2249–2254. doi: 10.1097/00003246-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Nowak MM, Ponsky JL. Blood transfusion and disease-free survival in carcinoma of the breast. J Surg Oncol. 1984;27:124–130. doi: 10.1002/jso.2930270214. [DOI] [PubMed] [Google Scholar]
- 20.Hyman NH, Foster RS, Jr, DeMeules JE, Costanza MC. Blood transfusions and survival after lung cancer resection. Am J Surg. 1985;149:502–507. doi: 10.1016/s0002-9610(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 21.Manyonda IT, Shaw DE, Foulkes A, Osborn DE. Renal cell carcinoma: blood transfusion and survival. Br Med J (Clin Res Ed) 1986;293:537–538. doi: 10.1136/bmj.293.6546.537-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JT, Taylor FH, Thearle PB. Blood transfusion and outcome in stage III head and neck carcinoma. Arch Otolaryngol Head Neck Surg. 1987;113:307–310. doi: 10.1001/archotol.1987.01860030083014. [DOI] [PubMed] [Google Scholar]
- 23.Grzelak I, Zaleska M, Olszewski WL. Blood transfusions downregulate hematopoiesis and subsequently downregulate the immune response. Transfusion. 1998;38:1104–1114. doi: 10.1046/j.1537-2995.1998.38111299056323.x. [DOI] [PubMed] [Google Scholar]
- 24.Carson JL, Chen AY. In search of the transfusion trigger. Clin Orthop Relat Res. 1998;357:30–35. doi: 10.1097/00003086-199812000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Chen AY, Carson JL. Perioperative management of anaemia. Br J Anaesth. 1998;81(Suppl 1):20–24. [PubMed] [Google Scholar]
- 26.Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 27.Practice Guidelines for blood component therapy: a report by the American society of anesthesiologists task force on blood component therapy. Anesthesiology. 1996;84:732–747. [PubMed] [Google Scholar]
- 28.Consensus Conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703. [PubMed] [Google Scholar]
- 29.van de Watering LM, Hermans J, Houbiers JG, van den Broek PJ, Bouter H, Boer F, et al. Beneficial effects of leukocyte depletion of transfused blood on postoperative complications in patients undergoing cardiac surgery: a randomized clinical trial. Circulation. 1998;97:562–568. doi: 10.1161/01.cir.97.6.562. [DOI] [PubMed] [Google Scholar]
- 30.Naidech AM, Drescher J, Ault ML, Shaibani A, Batjer HH, Alberts MJ. Higher hemoglobin is associated with less cerebral infarction, poor outcome, and death after subarachnoid hemorrhage. Neurosurgery. 2006;59:775–779. doi: 10.1227/01.NEU.0000232662.86771.A9. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 32.Lübke T, Mönig SP, Schneider PM, Hölscher AH, Bollschweiler E. Does Charlson-comorbidity index correlate with short-term outcome in patients with gastric cancer? Zentralbl Chir. 2003;128:970–976. doi: 10.1055/s-2003-44805. [DOI] [PubMed] [Google Scholar]