Abstract
Purpose
Attempt to identify the beneficial effects associated with surgical procedures on survival outcome of patients with recurrent cholangiocarcinoma.
Methods
921 patients diagnosed with cholangiocarcinoma underwent surgical resection with curative intent in a single institute during the last 15 years. Patients with recurrent disease were divided into two groups according to whether surgical procedures were performed for the treatment of recurrence. Clinicopathologic variables, ranges of survival based on sites of recurrence, and types of treatment were analyzed retrospectively.
Results
The median follow-up period was 21.8 months and 316 (34.3%) patients had recurrence. 27 (group A) patients with recurrent disease were treated surgically and 289 patients (group B) were not treated. Liver resection, metastasectomy, pancreaticoduodenectomy, partial pancreatectomy, and regional lymph node dissection were performed on the patients in group A. The overall survival rate was statistically higher in group A (P = 0.001). Among the surgical procedures, resection of locoregional recurrences (except liver) in abdominal cavity (4.0 to 101.8 months vs. 0.6 to 71.6 months) and metastasectomy of abdominal or chest wall (3.5 to 18.9 months vs. 1.9 to 2.2 months) showed remarkable differences with respect to the range of survival.
Conclusion
Better survival outcomes can be expected by performing surgical resection of locoregional recurrences (except liver) in abdominal cavity and abdominal or chest wall metastatic lesions in recurrent cholangiocarcinoma.
Keywords: Recurrence, Range of survival, Cholangiocarcinoma, Radiofrequency ablation
INTRODUCTION
Cholangiocarcinoma is the second most common primary hepatobiliary malignancy originating from the epithelium of the intrahepatic, perihilar, or extrahepatic bile ducts, with the frequencies of 5 to 30%, 40 to 50%, and 20 to 30%, respectively [1-5]. The incidence of intrahepatic cholangiocarcinoma is increasing worldwide, especially in Asia, while the incidence of extrahepatic cholangiocarcinoma is somewhat stable or declining [6-11]. Surgical resection remains the only curative treatment option for cholangiocarcinoma, but most patients are far advanced at the time of presentation, which leads to a poor survival outcome [12-16]. In addition, spreading widely through bile ductules and extensive invasion to adjacent organs, cholangiocarcinoma has frequent locoregional or distant recurrences via lymphatic chains or hematogenous metastasis [4,5,12,17,18]. Kobayashi et al. [19] reported distant metastases were more common than locoregional recurrence after R0 resection in patients with perihilar cholangiocarcinoma.
The 5-year overall survival rate after surgical resection of cholangiocarinoma is reported to be 5 to 40%, and the median overall survival time and median recurrence-free survival time is 27 to 33 and 9 to 27 months, respectively [6-8,10,13,15,18-20]. Many published series have reported the risk factors for poor survival and recurrence; perineural invasion via lymphatic pathway and the proximity to the peripheral nerve plexus, presence of lymph node metastasis, poor differentiation, higher T stage, positive surgical resection margin and high level of carbohydrate antigen (CA) 19-9 [12,15,17,19-21]. The recurrence sites vary from local or locoregional recurrence in abdominal cavity to distance metastasis including abdominal or chest wall, skeletal muscles, lung, bone or brain [1,6,5,22,23].
Repeated hepatic resection for large, resectable tumors or radiofrequency ablation (RFA) for several small nodules have demonstrated their effectiveness on survival for the treatment of recurrent intrahepatic cholangiocarcinoma in some series [17,18,24].
However, there is no large-scale series to verify beneficial effects in terms of survival through surgical treatments including other types of recurrent cholangiocarcinomas. Thus, we attempted to identify whether or not there is a survival benefit to patients with recurrent cholangiocarcinoma who had undergone surgical resection with curative intent according to the recurrent sites and the types of resection.
METHODS
Study patients
Nine hundred forty-nine patients who underwent surgical treatment for cholangiocarcinoma in a single institute between May 1995 and July 2010 were reviewed retrospectively based on electronic medical records with pathologic reports. They proved to be primary adenocarcinomas pathologically arising from the bile duct epithelium. With the exception of 28 patients who had palliative resection, 921 patients underwent curative-intent surgical resection (R0 = 830, R1 = 91).
Cholangiocarcinoma was classified according to the tumor location according to preoperative radiologic and final pathologic findings, which included intrahepatic (n = 226), perihilar (n = 222), and extrahepatic (n = 474). Cholangiocarcinoma of gallbladder was not included in this series. 74, 78 and 164 patients had recurrent diseases during the study period, respectively. These 316 patients were divided into the following 2 groups: patients who underwent surgical resection for recurrence (group A) and patients who were not treated (group B).
Initial procedures and managements
To the patients with intrahepatic cholangiocarcinomas, anatomic liver resection, including segmentectomy, hemihepatectomy, extended hemi-hepatectomy, or tri-sectionectomy (with or without caudate lobectomy) were primarily considered. Wedge resection was also considered for small tumors located at the liver dome. Variable procedures, such as major hepatectomy with bile duct resection, hepatopancreaticoduodenectomy, pancreaticoduodenectomy, segmental bile duct resection, or liver transplantation were performed for perihilar cholangiocarcinoma according to Bismuth-Corlette type, tumor location and degree of infiltration. Finally, segmental bile duct resection or pancreaticoduodenectomy was primarily considered for extrahepatic cholangiocarcinoma based on the location of the tumor (upper-, middle-, and lower-third). Hepatopancreaticoduodenectomy was applied to the patient with widespread cholangiocarcinoma and suspected invasion to the hepatoduodenal ligaments considering the medical condition of the patient.
Routine frozen-sectional biopsy of ductal resectional margin was carried out to confirm microscopic extension during surgery. When the proximal or distal margin was proved to be tumor positive, additional resection of bile or hepatic ducts was performed. The regional lymph nodes in the hepatoduodenal ligament, peri-pancreatic, celiac, and along the common hepatic artery were dissected, but the degree of lymph node dissection was determined by the attending surgeon. If the pathologic evaluation revealed lymph node metastasis or positive resection margins, the patient was recommended to receive adjuvant treatment. 5-fluorouracil-based chemotherapy and radiotherapy (44-54 Gy) were generally applied, although there were differences in the dose and treatment period according to the individual.
Types of treatment for recurrence
Patients were routinely followed in the outpatient department with CA 19-9 levels and abdominal contrast-enhanced computed tomography (CT) every 3 to 6 months. Recurrence was highly suspected when the level of serum CA 19-9 increased, and confirmed with CT with or without 18F-fluorodeoxyglucose positron emission tomography by experienced radiologists. It was classified as one of followings; intrahepatic recurrence, locoregional recurrence except liver, distant metastasis.
The inclusion criteria for RFA generally followed the indications for hepatocellular carcinoma; <3 tumor lesions with a maximum diameter <3 cm, single nodular lesions with a maximum diameter <5 cm, no major vessel or bile duct involvement, and no uncorrectable coagulopathy [25]. The technical effectiveness was verified with a CT after 1 month with no residual unablated tumors.
Further hepatic resection was considered according to the anatomic location, size, and number of lesions when RFA could not be performed. Metastasectomy was considered for metastatic lesions of the abdominal wall, chest wall, peritoneum, and solitary pulmonary nodules. Pylorus-preserving pancreaticoduodenectomy or Whipple's procedure was performed to treat locoregional recurrence after segmental bile duct resection for extrahepatic cholangiocarcinoma.
Statistical analysis
A Student's t test (two-tailed) and χ2 test or Fisher's exact test were used to analyze continuous or categorical variables, respectively. Overall survival was calculated using the Kaplan-Meier method and differences in survival were determined using the log-rank test. Prognostic factors on overall survival were indentified using multivariate Cox proportional hazards models. Statistical calculations were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA). P-values < 0.05 were considered statistically significant.
RESULTS
Demographics
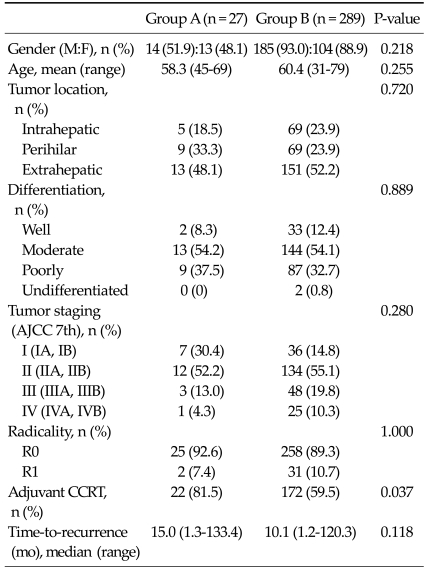
The median follow-up period was 21.8 months (range, 1.8 to 153.0 months) and the number of patients was 27 and 289 in group A and B, respectively. There were no statistical differences in gender, age and pre-operative carcinoembryonic antigen and CA 19-9 levels. In addition, tumor size, location, differentiation, radicality, the ratio of lymph node metastasis, tumor staging and time-to-recurrence did not have significant differences, except the rate of patients receiving adjuvant concurrent chemoradiotherapy, which was shown to be statistically higher in group A (P = 0.037, Table 1).
Table 1.
Demographics and clinicopathologic variables
AJCC, American Joint Committee on Cancer; CCRT, concurrent chemoradiotherapy.
Recurrence after surgical resection
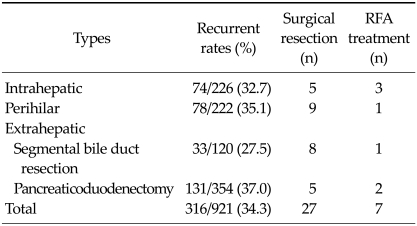
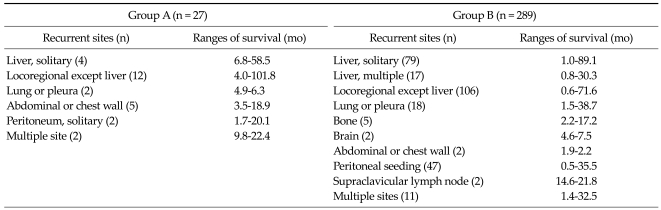
5, 9 and 13 patients underwent surgical treatment for recurrent disease in the case of intrahepatic, perihilar, and extrahepatic cholangiocarcinoma, respectively (Table 2). The recurrent rate was highest after pancreaticoduodenectomy (37.0%) for extrahepatic cholangiocarcinoma. The recurrent sites were as follows; solitary (n = 83) or multiple (n = 17) hepatic recurrence, locoregional recurrence except liver (n = 118), peritoneal seeding (n = 49), lung or pleura (n = 20), abdominal or chest wall (n = 7), bone (n = 5), brain (n = 2), supraclavicular lymph node (n = 2) and multiple site recurrence (n = 13).
Table 2.
Recurrent rates according to the tumor types after R0, R1 resection
RFA, Radiofrequency ablation.
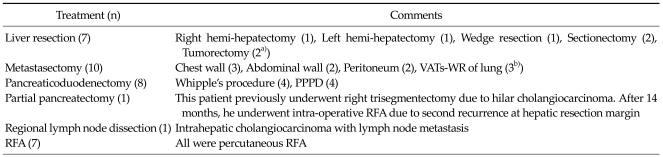
Hepatic resection or RFA was performed for liver metastasis. Pancreaticoduodenectomy, partial pancreatectomy, and regional lymph node dissection were performed for locoregional recurrent lesions. Metastasectomy was performed for recurrent masses in the abdominal or chest wall, lung or pleura, and peritoneal solitary metastasis. Partial pancreatectomy was performed on one patient who had a locoregional recurrence in the pancreas after right tri-segmentectomy for type IIIA hilar cholangiocarcinoma. One patient had a recurrence in regional lymph nodes 17.4 months after left lateral sectionectomy for intrahepatic cholangiocarcinoma located at segments II and III (Table 3). In the current study, we did not perform surgical resection or RFA for multiple hepatic metastatic lesions, and surgical procedures for peritoneal seeding, supraclavicular lymph node and bone or brain metastasis.
Table 3.
Treatment types for recurrence
VATs-WR, video-assisted thoracic surgery-wedge resection, PPPD, pylorus-preserving pancreaticoduodenectomy, RFA, radiofrequency ablation.
a)One patient underwent RFA after 2 months due to second hepatic recurrence. b)One patient underwent hepatic tumorectomy after 1 month due to second hepatic recurrence.
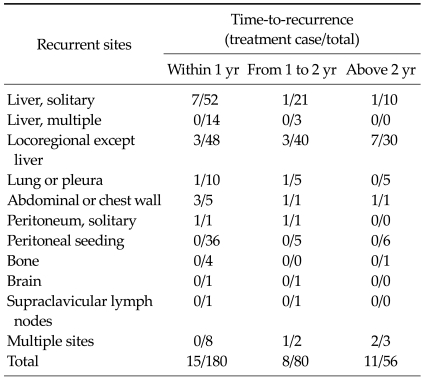
180, 80 and 56 patients had recurrence within 1 year, from 1 to 2 years, and above 2 years, respectively. During these periods, various treatments for recurrence were performed to 9.3%, 10.0% and 19.6% of the recurrent patients, respectively (Table 4).
Table 4.
Patterns of recurrence according to the recurrent sites and time-to-recurrence
Overall survival and range of survival after recurrence
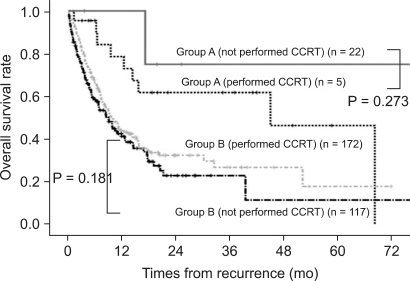
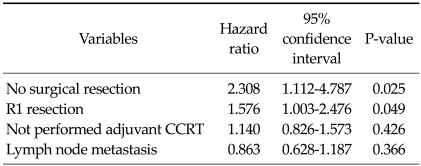
The median times to recurrence were 15.0 and 10.1 months in group A and B, respectively (P = 0.118). The median times from recurrence to surgical treatment for recurrent disease in group A was 27 days (range, 14 to 182 days). The median survival times after recurrence were 18.9 months (range, 1.7 to 101.8 months) and 7.7 months (range, 0.5 to 89.1 months) in group A and B, respectively. The cumulative overall survival rate after recurrence was significantly higher in group A than group B (P = 0.001, Fig. 1). The additional chemoradiotherapy did not show significant survival benefits in either group. The beneficial effect of surgical treatment for recurrent disease with respect to the ranges of survival was remarkable in the case of locoregional recurrence except liver and abdominal or chest wall metastasis (Table 5). Surgical resection for recurrent cholangiocarcinoma was identified as an independent prognostic factor on overall survival according to multivariate analysis (HR = 2.308, P = 0.025) (Table 6).
Fig. 1.
Overall survival rate after recurrence according to surgical treatment and concurrent chemoradiotherapy (CCRT) for recurrent cholangiocarcinoma (group A; patients treated surgically for recurrence, group B; patients not treated, P-value between the two groups = 0.001).
Table 5.
Recurrent sites and ranges of survival after recurrence
Table 6.
Multivariate analysis of prognostic factors on overall survival after recurrence
CCRT, concurrent chemoradiotherapy.
DISCUSSION
The long-term survival outcomes of patients who undergo surgical treatment for cholangiocarcinoma is still disappointing, although there have been recent advances in imaging modalities, peri-operative treatment, and surgical techniques. Additionally, because of the advanced stage at presentation, inadequate identification of anatomic location, and/or poor medical conditions, surgical resection is often non-curative [10]. To this end, therapeutic strategies for advanced or metastatic cholangiocarcinoma have been developed, such as systemic chemotherapy, brachytherapy, photodynamic therapy and hepatic-arterial embolization or cryoablation [7,10,19,26]. However, most have limited roles in representing satisfactory outcomes on survival.
It is reported that liver and abdominal lymph nodes around the hepatoduodenal ligament, periportal, common hepatic artery, and posterior pancreatoduodenal areas are common sites of recurrence in patients with cholangiocarcinoma [5,19,27,28]. Occasionally, distant lymph nodes in the posterior mediastinum or supraclavicular area can be involved. These findings have commonly been accepted to reflect disease progression and are associated with a poor prognosis.
Repeated liver resection in intrahepatic cholangiocarcinoma, repeated RFA, or a combination for locoregional recurrent tumor have been reported to be effective with respect to survival in highly selected patients [9,17,18,25]. However, our study extended the concept of repeated resection beyond the liver to extrahepatic intra-abdominal locoregional recurrences, abdominal or chest wall and lung or pleura. In addition to performing repeated hepatectomy or RFA for recurrent hepatic lesions, resection of recurrences in the abdominal cavity and abdominal or chest wall were attempted. In our experience, there were two of five cases showing positive resection margins after metastasectomy of the abdominal wall. Second recurrences occurred in these two patients, so they underwent repeated resection around the previous area of resection. Thus, wider excision must be considered during metastasectomy of the abdominal or chest wall, and mesh could be useful for covering large fascial defects in these cases.
In this series, the recurrence rate of cholangiocarcinoma showed 34.3%, and about 57% of recurrence occurred within 1 year. However, only 8.3% of patients with recurrence were treated surgically in this period, while 10.0% and 19.6% of patients were treated from 1 to 2 years and above 2 years, respectively. Thus, early surgical resection should be considered to acquire greater survival benefits.
There are some drawbacks in this retrospective study. First, differences in the clinical course and pathogenesis among the three types of cholangiocarcinoma may exist. But it was reported that there were no significant differences in clinical presentation, disease stage and survival between intra- and extra-hepatic cholangiocarcinoma [6]. Second, a relatively smaller incidence of recurrence than in previous studies was identified. It might be influenced not by the development of the palliative treatment options, but also missed patients who were previously treated surgically. In fact, patients with cholangiocarcinoma have been increasing recently who were treated surgically or with RFA. Therefore, further follow-up studies for these patients are warranted to understand tumor biology and diverse patterns of tumor recurrence to make up for these limitations. Francis et al. [9] and Zhang et al. [4] introduced regulation of cholangiocarcinoma from a biologic point of view. Their reviews suggested that genetic hypermethylation, interleukin-6, growth factors, gastrointestinal hormones, neuroregulatory molecules, angiogenesis, or E-cadherin-mediated cell-to-cell adhesion expressed by the Slug gene are related to growth or invasiveness of the tumor. Third, although most of the clinicopathologic characteristics were similar in our two groups, the rate of patients who underwent adjuvant CCRT was higher in group A. This selection bias could be an interference factor in drawing our conclusion. Finally, overall survival has room for modification because the recurrence patterns between the two groups were not similar. Thus, further prospective controlled study is needed to overcome this limitation.
In conclusion, according to this large-series study in a single center, we have to consider surgical procedures to resectable recurrent lesions to prolong survival in patients with recurrent cholangiocarcinoma. Moreover, adjuvant chemoradiotherapy alone could not acquire a meaningful beneficial outcome on survival without resection of recurrent lesions. Although this series did not confirm any beneficial effect of surgical procedures to recurrent masses in the liver, lung, pleura, or multiple sites on survival, surgical resection of locoregional recurrence in the abdominal cavity and of the abdominal or chest wall metastatic lesions showed remarkable benefits in terms of range of survival. This consideration might help to determine patterns of recurrence and adequate therapeutic options for recurrent cholangiocarcinoma.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kwon OS, Jun DW, Kim SH, Chung MY, Kim NI, Song MH, et al. Distant skeletal muscle metastasis from intrahepatic cholangiocarcinoma presenting as Budd-Chiari syndrome. World J Gastroenterol. 2007;13:3141–3143. doi: 10.3748/wjg.v13.i22.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tao LY, He XD, Qu Q, Cai L, Liu W, Zhou L, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int. 2010;30:215–221. doi: 10.1111/j.1478-3231.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 3.Park SK, Kim YS, Kim SG, Jang JY, Moon JH, Lee MS, et al. Detection of distant metastasis to skeletal muscle by 18F-FDG-PET in a case of intrahepatic cholangiocarcinoma. Korean J Hepatol. 2010;16:325–328. doi: 10.3350/kjhep.2010.16.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang KJ, Wang DS, Zhang SY, Jiao XL, Li CW, Wang XS, et al. The E-cadherin repressor slug and progression of human extrahepatic hilar cholangiocarcinoma. J Exp Clin Cancer Res. 2010;29:88. doi: 10.1186/1756-9966-29-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito Y, Tajima Y, Fujita F, Tsutsumi R, Kuroki T, Kanematsu T. Solitary recurrence of hilar cholangiocarcinoma in a mediastinal lymph node two years after curative resection. World J Gastroenterol. 2007;13:2243–2246. doi: 10.3748/wjg.v13.i15.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singal AG, Rakoski MO, Salgia R, Pelletier S, Welling TH, Fontana RJ, et al. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther. 2010;31:625–633. doi: 10.1111/j.1365-2036.2009.04218.x. [DOI] [PubMed] [Google Scholar]
- 7.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D'Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675–681. doi: 10.1097/SLA.0b013e3181d3d2b2. [DOI] [PubMed] [Google Scholar]
- 9.Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1–G9. doi: 10.1152/ajpgi.00114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamandl D, Herberger B, Gruenberger B, Puhalla H, Klinger M, Gruenberger T. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2008;15:2787–2794. doi: 10.1245/s10434-008-0081-1. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Shimada K, Sakamoto Y, Esaki M, Nara S, Ban D, et al. Clinicopathological characteristics of intrahepatic cholangiocellular carcinoma presenting intrahepatic bile duct growth. J Surg Oncol. 2009;99:161–165. doi: 10.1002/jso.21214. [DOI] [PubMed] [Google Scholar]
- 12.Saxena A, Chua TC, Sarkar A, Chu F, Morris DL. Clinicopathologic and treatment-related factors influencing recurrence and survival after hepatic resection of intrahepatic cholangiocarcinoma: a 19-year experience from an established Australian hepatobiliary unit. J Gastrointest Surg. 2010;14:1128–1138. doi: 10.1007/s11605-010-1203-1. [DOI] [PubMed] [Google Scholar]
- 13.Shen FZ, Zhang BY, Feng YJ, Jia ZX, An B, Liu CC, et al. Current research in perineural invasion of cholangiocarcinoma. J Exp Clin Cancer Res. 2010;29:24. doi: 10.1186/1756-9966-29-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 16.Nagahashi M, Shirai Y, Wakai T, Sakata J, Ajioka Y, Nomura T, et al. Depth of invasion determines the postresectional prognosis for patients with T1 extrahepatic cholangiocarcinoma. Cancer. 2010;116:400–405. doi: 10.1002/cncr.24766. [DOI] [PubMed] [Google Scholar]
- 17.Kurosaki I, Hatakeyama K. Repeated hepatectomy for recurrent intrahepatic cholangiocarcinoma: report of two cases. Eur J Gastroenterol Hepatol. 2005;17:125–130. doi: 10.1097/00042737-200501000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Kamphues C, Seehofer D, Eisele RM, Denecke T, Pratschke J, Neumann UP, et al. Recurrent intrahepatic cholangiocarcinoma: single-center experience using repeated hepatectomy and radiofrequency ablation. J Hepatobiliary Pancreat Sci. 2010;17:509–515. doi: 10.1007/s00534-009-0256-6. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi A, Miwa S, Nakata T, Miyagawa S. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg. 2010;97:56–64. doi: 10.1002/bjs.6788. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto Y, Shimada K, Nara S, Esaki M, Ojima H, Sano T, et al. Surgical management of infrahilar/suprapancreatic cholangiocarcinoma: an analysis of the surgical procedures, surgical margins, and survivals of 77 patients. J Gastrointest Surg. 2010;14:335–343. doi: 10.1007/s11605-009-1072-7. [DOI] [PubMed] [Google Scholar]
- 21.Kim SJ, Lee CY, Kim DG. Analysis of the clinical outcome and prognostic factors of patients with hilar cholangiocarcinoma. J Korean Surg Soc. 2007;73:156–164. [Google Scholar]
- 22.Takahashi Y, Nagino M, Nishio H, Ebata T, Igami T, Nimura Y. Percutaneous transhepatic biliary drainage catheter tract recurrence in cholangiocarcinoma. Br J Surg. 2010;97:1860–1866. doi: 10.1002/bjs.7228. [DOI] [PubMed] [Google Scholar]
- 23.Stergiopoulos C, Kountouras J, Kapetanakis N, Katsinelos P, Kokkali S, Tsapournas G, et al. Distant cutaneous metastasis preceding the diagnosis of ductal cholangiocarcinoma. J Eur Acad Dermatol Venereol. 2009;23:242–243. doi: 10.1111/j.1468-3083.2008.02845.x. [DOI] [PubMed] [Google Scholar]
- 24.Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Significance of repeated resection for recurrent intrahepatic cholangiocarcinoma. Hepatogastroenterology. 2009;56:1–5. [PubMed] [Google Scholar]
- 25.Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, et al. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319–2329. doi: 10.1245/s10434-006-9220-8. [DOI] [PubMed] [Google Scholar]
- 26.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 27.Buell JF, Rosen S, Yoshida A, Labow D, Limsrichamrern S, Cronin DC, et al. Hepatic resection: effective treatment for primary and secondary tumors. Surgery. 2000;128:686–693. doi: 10.1067/msy.2000.108220. [DOI] [PubMed] [Google Scholar]
- 28.Konishi M, Iwasaki M, Ochiai A, Hasebe T, Ojima H, Yanagisawa A. Clinical impact of intraoperative histological examination of the ductal resection margin in extrahepatic cholangiocarcinoma. Br J Surg. 2010;97:1363–1368. doi: 10.1002/bjs.7122. [DOI] [PubMed] [Google Scholar]