Abstract
Purpose
The present study was conducted to investigate the low compliance rate of the critical pathway (CP) and whether CP is effective for treatment of gastric cancer in radical gastrectomy.
Methods
The medical records of 631 patients who had undergone radical gastrectomy with D2 lymph node dissection were reviewed. This study compared data from patients in early gastric cancer (EGC) and advanced gastric cancer (AGC) groups, which were further subdivided into general care (non-CP) and CP groups.
Results
The mean length of preoperative hospital stays were significantly different between the EGC and AGC patients (P < 0.05). However, there was no difference in the mean length of postoperative hospital stays between non-CP and CP groups among either EGC patients or AGC patients (P > 0.05). The postoperative and total cost of hospitalization was not statistically different between either of the groups (P > 0.05); however, the mean preoperative costs were significantly different (P < 0.05).
Conclusion
We conclude that use of the CP following gastrectomy is unnecessary. To decrease the length of hospital stay and associated costs, preoperative examination and consultation should be performed before admission.
Keywords: Critical pathways, Fast-track, Stomach neoplasms, Gastrectomy
INTRODUCTION
The critical pathway (CP), also known as the clinical path or pathway, is a set of guidelines for patient care that outlines the objectives of the patient care plan along with the corresponding ideal sequence and timing of physician and staff action for achieving these goals with optimal efficiency [1,2]. The use of the CP is intended to allow for the standardization of patient management, as well as improve the quality and efficiency of patient care. The term 'clinical pathway' was coined in 1985 by Zander et al. at the New England Medical Center. The CP is used for the treatment of many medical conditions across the continuum of care [3-5]. Some studies have found that the critical pathways can lead to a decrease in postoperative length of stay and hospital charges, such as in colorectal [6,7], liver [8], vascular [9,10], obstetric [11], urologic [12,13], and pediatric procedures [14].
The CP was first implemented at Our Hospital in September 2004, and the first use of electric medical record (EMR)-based CP was for the management of cataract surgery patients in the Department of Ophthalmology in August 2005. Since then, six CPs have been developed at the Department of Surgery for use in the treatment of stomach cancer, breast cancer, thyroid diseases and benign gallbladder diseases. The CPs that were developed include procedures such as gastrectomy, routine mastectomy, mastectomy with neoadjuvant chemotherapy, total thyroidectomy, subtotal thyroidectomy and laparoscopic cholecystectomy. Additionally, EMR-based critical pathways were developed and applied in July 2006 to provide care for patients following gastrectomy for gastric cancer. However, compliance with the CP developed for treatment of stomach cancer was lower than for other diseases. Therefore, we investigated the low compliance rate of the CP for stomach cancer and analyzed the effectiveness of the CP for reducing the length of hospital stay and associated care costs.
METHODS
The medical records of 631 consecutive patients who had undergone radical gastrectomy with D2 lymph node dissection between December 1st, 2006 and November 30th, 2007 were reviewed. This included patient characteristics and data from hospital records (sex, age, length of hospital stay and tumor, node, metastasis [TNM] stages), as well as information on the specific operative methods used (resection, combined resection and reconstruction). The patients were divided into early gastric cancer (EGC) and advanced gastric cancer (AGC) groups for data comparison, based on differences between the two groups in the range of lymph node dissection, length of postoperative hospital stay and postoperative complications. Each group was further subdivided into a general care (non-CP) group and a critical pathway (CP) group.
To compare the effects of application of the CP to the management of different cases, four diseases were analyzed: stomach cancer, breast cancer, thyroid disease and benign gallbladder disease. The analysis included 495 cases of mastectomy, 322 cases of thyroidectomy, 371 cases of laparoscopic cholecystectomy, and 380 cases of gastrectomy during the period between May and September of 2007.
Inclusion and exclusion criteria for CP
Patients included in the study were those diagnosed with gastric adenocarcinoma and those with potentially curative diseases. Exclusion criteria were laparoscopic surgery, emergency surgery, concomitant malignancies, palliative surgery, severe comorbidities, and severe malnutrition (American Society of Anesthesiologists grading grades 4).
Perioperative management of radical gastrectomy for gastric cancer
Non-CP groups
Patients were admitted three to four days before surgery. Preoperative laboratory tests and diagnostic modalities (endoscopy and computed tomography [CT] scan) were performed in the outpatient clinic. After the patients were admitted, the presence of concomitant diseases was assessed and consultation for operative safety was obtained. The patients underwent bowel preparation at preoperative day one, and antibiotics (intravenous Cefazolin and Gentamicin) were administered before surgery and on the first postoperative day. The nasogastric tube was removed when the patient passed flatus and began taking sips of water. In the absence of complications after the ingestion of water, the patients were started on a semi-fluid diet. The patients were maintained on a semi-fluid diet for a minimum of two days, after which time they were placed on a soft brand diet. The closed suction drain was removed after water was ingested without complications. After confirmation in the pathology report, the patients were discharged from the hospital.
CP groups
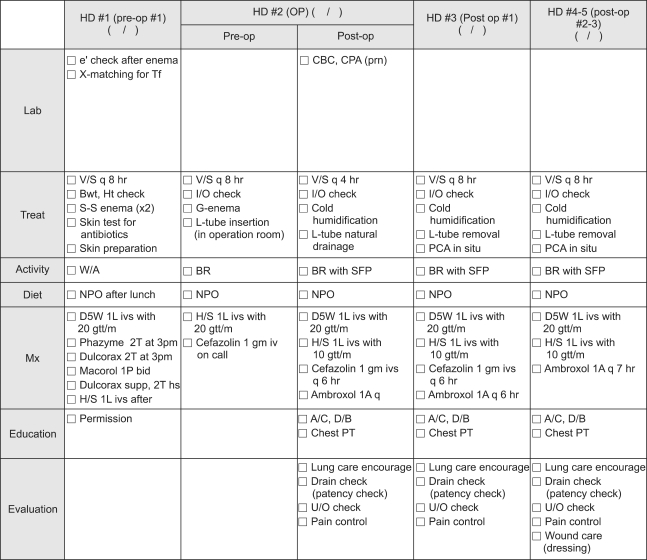
Patients were assessed using preoperative laboratory tests and diagnostic modalities (endoscopy and CT scan); concomitant diseases were also investigated and consultation for operative safety was obtained at the outpatient clinic before admission. The patients were admitted two days before surgery, at which time the operative safety was checked again. Following surgery, the nasogastric tube was removed on the first postoperative day, and sips of water were administered to the patients on the third postoperative day irrespective of passing flatus. If water was ingested without complication, the patients were started early on a semi-fluid diet on the fourth postoperative day, and the closed suction drain was removed. In the absence of specific complications or complaints, the patients were discharged from the hospital on the seventh to eighth postoperative day (Figs. 1, 2).
Fig. 1.
Critical pathway for hospital staff. HD, hospital day; Pre-op, preoperative; OP, operation; e', electrolyte; X matching for Tf, cross matching for transfusion; CBC, complete blood cell count; CPA, Chest PA; v/s, vital sign; Bwt, body weight ; Ht, height; I/O, input and output; L-tube, levin tube; PCA, patient controlled analgesia; W/A, ward ambulation; BR, bed rest; SFP, semi-Fowlers position; A/C, active cough; D/B, deep breathing.
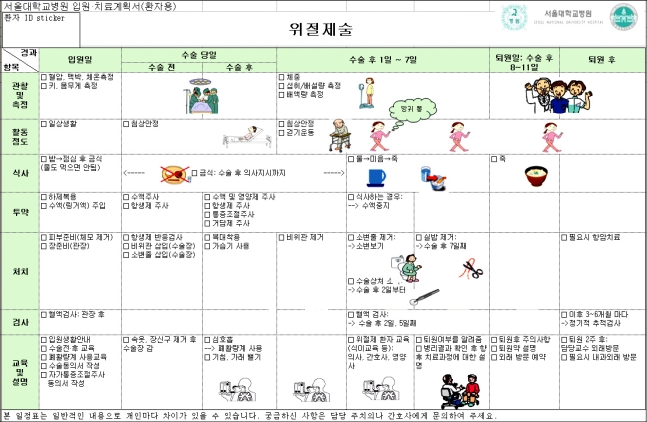
Fig. 2.
Critical pathway for patient.
Surgical procedure
All cases underwent either a radical subtotal gastrectomy with gastroduodenostomy (Billroth I) or a gastrojejunostomy (Billroth II). Roux-en-Y esophagojejunostomy with or without a Paulino pouch was performed after total gastrectomy. D2 lymph node dissection with curative intent was performed on all patients [15]. For tumors located in the lower third of the stomach, the double-stapling Billroth I method was used [16]. However, if the tumor extended to the upper part of the stomach and was located close to the pylorus, or if there was a scar at the duodenal bulb, Billroth II gastrojejunostomy was performed. Billroth II anastomosis was generally preferred when tumor invasion extended beyond the subserosa.
Application and termination of CP
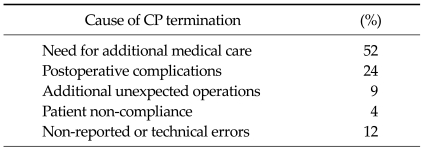
The designation of cases to be managed by the CP and termination of the CP were determined by the residents in charge of the patients. The causes for termination of the CP were classified according to four criteria: 1) additional medical care (laboratory tests) or workup needed due to medical problems (unexpected abdominal pain or discomfort, fever, etc.), 2) postoperative complications (anastomotic leakage, fluid collection, wound problems, intestinal obstruction, etc.), 3) non-compliance of the patients (delayed discharge, etc.), and 4) additional operations performed during gastrectomy (hysterectomy, etc.).
Statistical analyses
Statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA). The data are presented as means ± SD. For validation of significance, the paired t-test and the χ2 test were applied. Data were considered to be statistically significant if the probability was less than 5%.
RESULTS
Patient characteristics
The mean age of the patients was 59.2 ± 12.1, and the male to female ratio was 2.1:1. The most frequently performed surgical procedure was radical subtotal gastrectomy (476, 75.4%), followed by radical total gastrectomy (121, 19.2%), proximal gastrectomy (22, 3.5%), and pylorus preserving gastrectomy. A combined multi-organ resection was performed on 19% of the patients. TNM staging classified 65.3% of the cancers at stage I, followed by 16.6% at stage II, 14.4% at stage III, and 4% at stage IV.
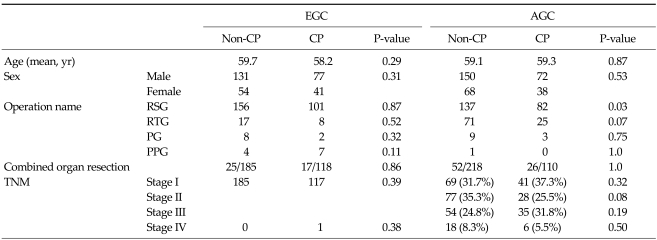
Comparison of non-CP and CP groups in EGC and AGC patients showed no differences between these two groups, except for a higher number of subtotal gastrectomies in the CP group of AGC patients (Table 1).
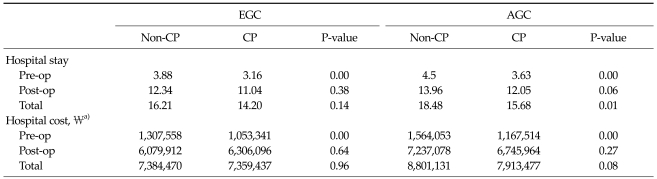
Table 1.
Patient characteristics
EGC, early gastric cancer group; AGC, advance gastric cancer group; CP, critical pathway; RSG, radical subtotal gastrectomy; RTG, radical total gastrectomy; PG, proximal gastrectomy; PPG, pylorus preserving gastrectomy; TNM, tumor, node, metastasis.
Comparison of morbidity rate and readmission rate between non-CP and CP groups in EGC and AGC patients
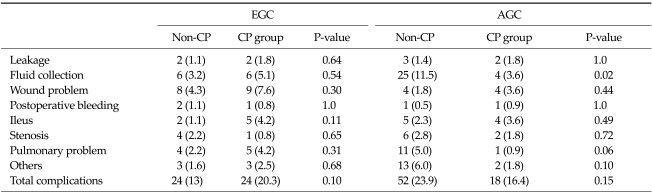
The overall morbidity rate was 18.7%, and there were no significant differences in the morbidity rate between the non-CP and CP groups in the EGC and AGC patients (P = 0.10 and P = 0.15). In surgical complication assessment, fluid collection was more frequently noted in the non-CP group than in the CP group in AGC patients (P = 0.02), but there were no significant differences in other complications. Additionally, four patients (1.8%) in the non-CP group and one patient (0.9%) in the CP group in the AGC group required readmission 30 days after surgery, but the difference was not statistically significant (P = 0.66). There were no mortality cases in either group (Table 2).
Table 2.
Operative morbidities between the non-CP and CP groups
Values are presented as number (%).
EGC, early gastric cancer group; AGC, advance gastric cancer group; CP, critical pathway.
Length of hospital stay and cost comparison between non-CP and CP groups in EGC and AGC patients
The mean length of preoperative hospital stays were significantly different between the EGC and AGC patients (P < 0.05). The mean length of total hospital stays was 18.4 days in the non-CP group and 15.6 days in the CP group in AGC patients, and this difference was statistically significant (P = 0.01). However, there was no difference in the mean length of postoperative hospital stays between non-CP and CP groups in either EGC patients or AGC patients (P > 0.05).
The postoperative and total cost of hospitalization was not statistically different between either of the groups (P > 0.05); however, the mean preoperative costs were significantly different (P < 0.05, Table 3).
Table 3.
Comparison of the mean length of hospital stay between the non-CP, CP groups
EGC, early gastric cancer group; AGC, advance gastric cancer group; CP, critical pathway; Pre-op, preoperative; Post-op, postoperative.
a)Korean monetary unit.
Termination causes of gastrectomy CP
The most common cause of the termination of CP in gastrectomy was the need for additional medical care (52%), followed by the incidence of postoperative complications (24%), performance of additional unexpected operations (9%), patient non-compliance (4%), and non-reported or technical errors (12%) (Table 4).
Table 4.
Termination causes of gastrectomy critical pathway (CP)
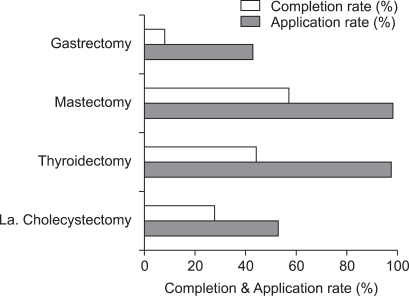
Comparison of CP application and completion rate
The application rate of the CP in gastrectomy was 43% and the completion rate was 19%. The CP was applied in cases of mastectomy, thyroidectomy and laparoscopic cholecystectomy at rates of 99%, 98% and 53%, respectively. The completion rates for CP in cases of mastectomy, thyroidectomy and laparoscopic cholecystectomy were 58%, 45% and 53%, respectively (Fig. 3).
Fig. 3.
Comparison of application rate, completion rates in four disease groups. La. Cholecystectomy, laparoscopic cholecystectomy.
DISCUSSION
Hospitals are under pressure to reduce costs while maintaining or increasing the quality of care. Development of the CP began with the introduction of diagnosis-related groups and the prospective payment system in the United States, which was implemented to improve hospital management through measures such as shortening the length of hospital stay, reducing the costs of hospitalization, and increasing the efficiency of nursing care [17].
Introduction of the CP helped streamline the postoperative care of surgical patients and reduced the cost and length of hospital stay for patients undergoing general surgical procedures [6,17,18]. However, studies have drawn conflicting conclusions on the efficacy of the CP. In some studies, the success of critical pathways may reflect patient selection bias, and may be the result of clinical 'cherry picking' rather than the effect of pathway management [19]. A study of 32 Connecticut hospitals [20], as well as other studies of medical and surgical pathways [1,21], have reported that critical pathways do not reduce the length of hospital stay more than the pre-pathway secular trends in comparable local hospitals with similarly managed care pressures. The results of the present study show that, while the mean preoperative hospital stay decreased in EGC and AGC patients, there were no differences in the length of postoperative hospital stay and hospital cost. The study also showed that a preoperative workup at an outpatient clinic contributed significantly to shortening the length of preoperative hospital stay and reducing the hospital cost. Thus, utilizing preoperative examination and consultation in outpatient clinics would decrease the length of hospital stay and cost without the necessity of implementing the CP for the treatment of gastric cancer.
The completion rate of the CP for gastrectomy patients was only 19%, but the rates for patients undergoing mastectomy, thyroidectomy and laparoscopic cholecystectomy were 45 to 58%. We conclude that CP is more effective in patients with benign disease or undergoing non-bowel surgery than in those undergoing gastrectomy. This is possibly due to gastrectomy being a major operation often associated with postoperative hemodynamic changes. In the present study, the most common causes of the termination of CP in gastrectomy patients were the need for additional medical care and the development of postoperative complications. Most upper gastrointestinal surgeries often require additional medical care and incur more postoperative complications compared to benign disease or non-bowel surgery. Therefore, it is possible to encourage the introduction of the CP in upper gastrointestinal surgery with the acceptance of an additional repeat orders and the allowance for frequent additional orders (ex, complete blood cell count, electrolyte, Chest X-ray) in limited periods. Another cause of no differences of hospital stay is that it is same hospital course between CP group and non-CP group after diet. In the absence of complications after the ingestion of water, patients were started on a semi-fluid diet in both groups. The patients were discharged from the hospital after two days of semi-fluid diet and one day of a soft brand diet. Therefore, our results indicate that the introduction of the CP is not beneficial for the treatment of conditions that require upper gastrointestinal surgery.
We conclude that the introduction of the CP in cases of gastrectomy is unnecessary. We recommend increased utilization of preoperative examination and operative surgery consultations for decreasing the length of hospital stay and the associated costs of hospitalization.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Pearson SD, Goulart-Fisher D, Lee TH. Critical pathways as a strategy for improving care: problems and potential. Ann Intern Med. 1995;123:941–948. doi: 10.7326/0003-4819-123-12-199512150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Coffey RJ, Richards JS, Remmert CS, LeRoy SS, Schoville RR, Baldwin PJ. An introduction to critical paths. Qual Manag Health Care. 1992;1:45–54. [PubMed] [Google Scholar]
- 3.Hindle D, Yazbeck AM. Clinical pathways in 17 European Union countries: a purposive survey. Aust Health Rev. 2005;29:94–104. doi: 10.1071/ah050094. [DOI] [PubMed] [Google Scholar]
- 4.Sermeus W, Depreitere R, De Waele K, Vanhaecht K, Vlayen J. An intruduction to clinical pathways. Brussel: Federaal Kenniscentrum voor de Gezondheidszorg (KCE); 2005. [Google Scholar]
- 5.Zander KS, Bower KA, Etheredge MR. Nursing case management: blueprints for transformation. Boston: New England Medical Center Hospital; 1987. [Google Scholar]
- 6.Pritts TA, Nussbaum MS, Flesch LV, Fegelman EJ, Parikh AA, Fischer JE. Implementation of a clinical pathway decreases length of stay and cost for bowel resection. Ann Surg. 1999;230:728–733. doi: 10.1097/00000658-199911000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephen AE, Berger DL. Shortened length of stay and hospital cost reduction with implementation of an accelerated clinical care pathway after elective colon resection. Surgery. 2003;133:277–282. doi: 10.1067/msy.2003.19. [DOI] [PubMed] [Google Scholar]
- 8.Lin DX, Li X, Ye QW, Lin F, Li LL, Zhang QY. Implementation of a Fast-Track clinical pathway decreases postoperative length of stay and hospital charges for liver resection. Cell Biochem Biophys. 2011 May 10; doi: 10.1007/s12013-011-9203-7. [Epub]. DOI: 10.1007/s12013-011-9203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber TS, Carlton LM, Harward TR, Russin MM, Phillips PT, Nalli BJ, et al. Impact of a clinical pathway for elective infrarenal aortic reconstructions. Ann Surg. 1998;227:691–699. doi: 10.1097/00000658-199805000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niino T, Hata M, Sezai A, Yoshitake I, Unosawa S, Shimura K, et al. Optimal clinical pathway for the patient with type B acute aortic dissection. Circ J. 2009;73:264–268. doi: 10.1253/circj.cj-08-0319. [DOI] [PubMed] [Google Scholar]
- 11.Santillan A, Govan L, Zahurak ML, Diaz-Montes TP, Giuntoli RL, 2nd, Bristow RE. Feasibility and economic impact of a clinical pathway for pap test utilization in Gynecologic Oncology practice. Gynecol Oncol. 2008;109:388–393. doi: 10.1016/j.ygyno.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Koch MO, Smith JA., Jr Influence of patient age and co-morbidity on outcome of a collaborative care pathway after radical prostatectomy and cystoprostatectomy. J Urol. 1996;155:1681–1684. [PubMed] [Google Scholar]
- 13.Leibman BD, Dillioglugil O, Abbas F, Tanli S, Kattan MW, Scardino PT. Impact of a clinical pathway for radical retropubic prostatectomy. Urology. 1998;52:94–99. doi: 10.1016/s0090-4295(98)00130-7. [DOI] [PubMed] [Google Scholar]
- 14.Zand DJ, Brown KM, Lichter-Konecki U, Campbell JK, Salehi V, Chamberlain JM. Effectiveness of a clinical pathway for the emergency treatment of patients with inborn errors of metabolism. Pediatrics. 2008;122:1191–1195. doi: 10.1542/peds.2008-0205. [DOI] [PubMed] [Google Scholar]
- 15.Park DJ, Lee HJ, Kim HH, Yang HK, Lee KU, Choe KJ. Predictors of operative morbidity and mortality in gastric cancer surgery. Br J Surg. 2005;92:1099–1102. doi: 10.1002/bjs.4952. [DOI] [PubMed] [Google Scholar]
- 16.Yang HK, Lee HJ, Ahn HS, Yoo MW, Lee IK, Lee KU. Safety of modified double-stapling end-to-end gastroduodenostomy in distal subtotal gastrectomy. J Surg Oncol. 2007;96:624–629. doi: 10.1002/jso.20883. [DOI] [PubMed] [Google Scholar]
- 17.Hirasaki S, Tanimizu M, Moriwaki T, Hyodo I, Shinji T, Koide N, et al. Efficacy of clinical pathway for the management of mucosal gastric carcinoma treated with endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2004;43:1120–1125. doi: 10.2169/internalmedicine.43.1120. [DOI] [PubMed] [Google Scholar]
- 18.Dy SM, Garg PP, Nyberg D, Dawson PB, Pronovost PJ, Morlock L, et al. Are critical pathways effective for reducing postoperative length of stay? Med Care. 2003;41:637–648. doi: 10.1097/01.MLR.0000062552.92534.BE. [DOI] [PubMed] [Google Scholar]
- 19.Wright CD, Wain JC, Grillo HC, Moncure AC, Macaluso SM, Mathisen DJ. Pulmonary lobectomy patient care pathway: a model to control cost and maintain quality. Ann Thorac Surg. 1997;64:299–302. doi: 10.1016/S0003-4975(97)00548-1. [DOI] [PubMed] [Google Scholar]
- 20.Holmboe ES, Meehan TP, Radford MJ, Wang Y, Marciniak TA, Krumholz HM. Use of critical pathways to improve the care of patients with acute myocardial infarction. Am J Med. 1999;107:324–331. doi: 10.1016/s0002-9343(99)00239-9. [DOI] [PubMed] [Google Scholar]
- 21.Pearson SD, Kleefield SF, Soukop JR, Cook EF, Lee TH. Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001;110:175–180. doi: 10.1016/s0002-9343(00)00705-1. [DOI] [PubMed] [Google Scholar]