Abstract
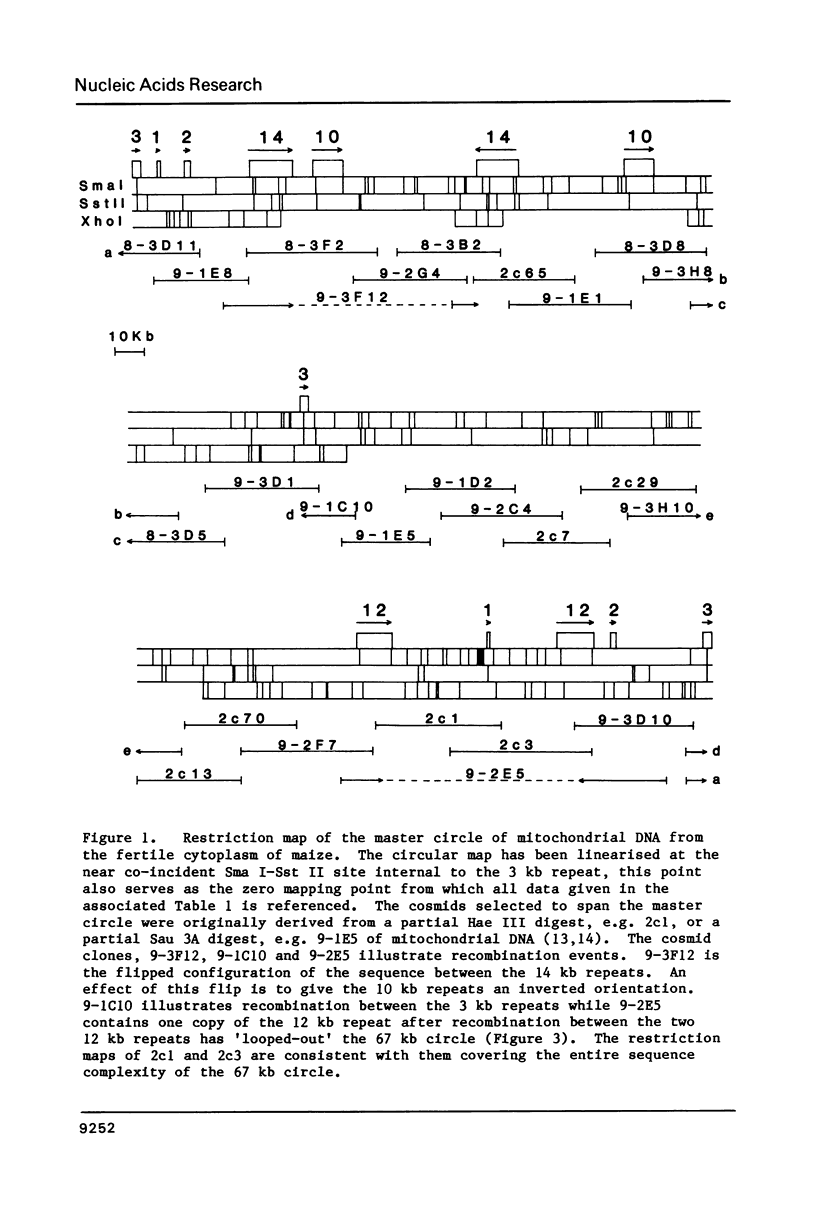
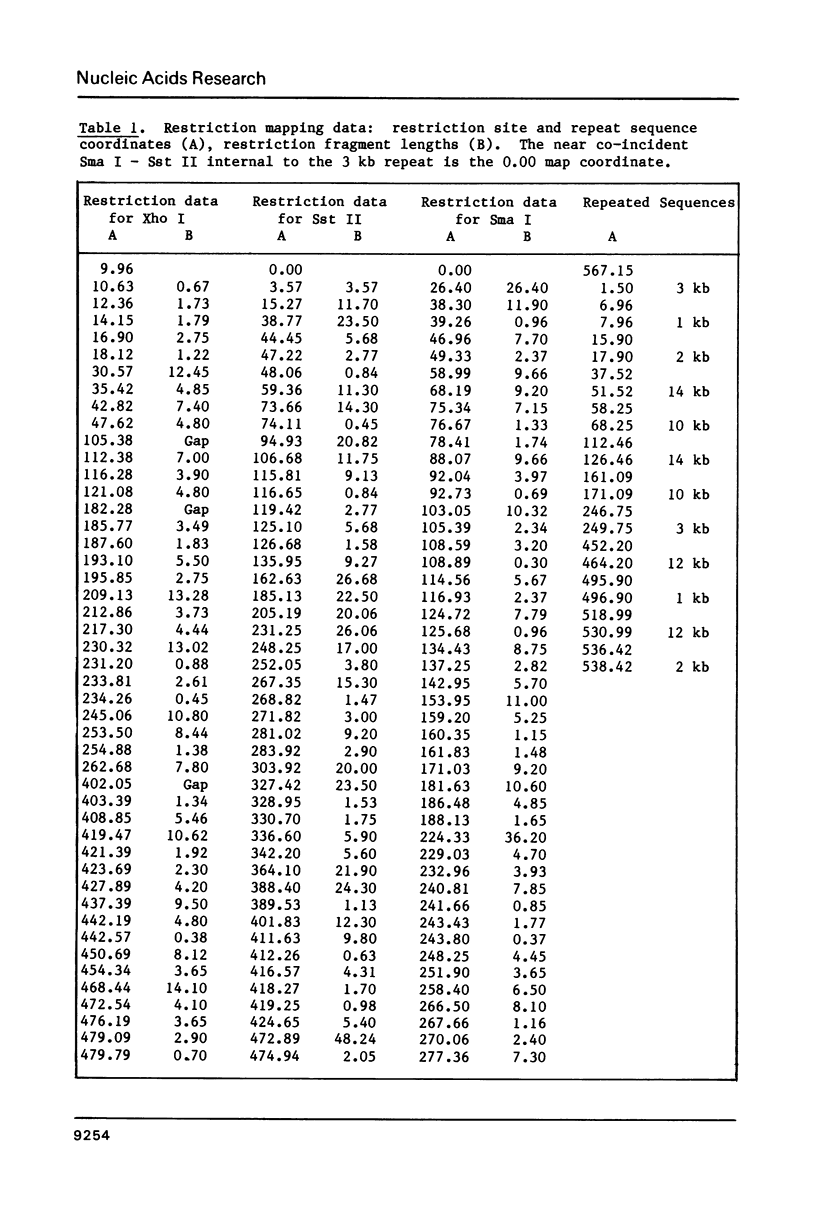
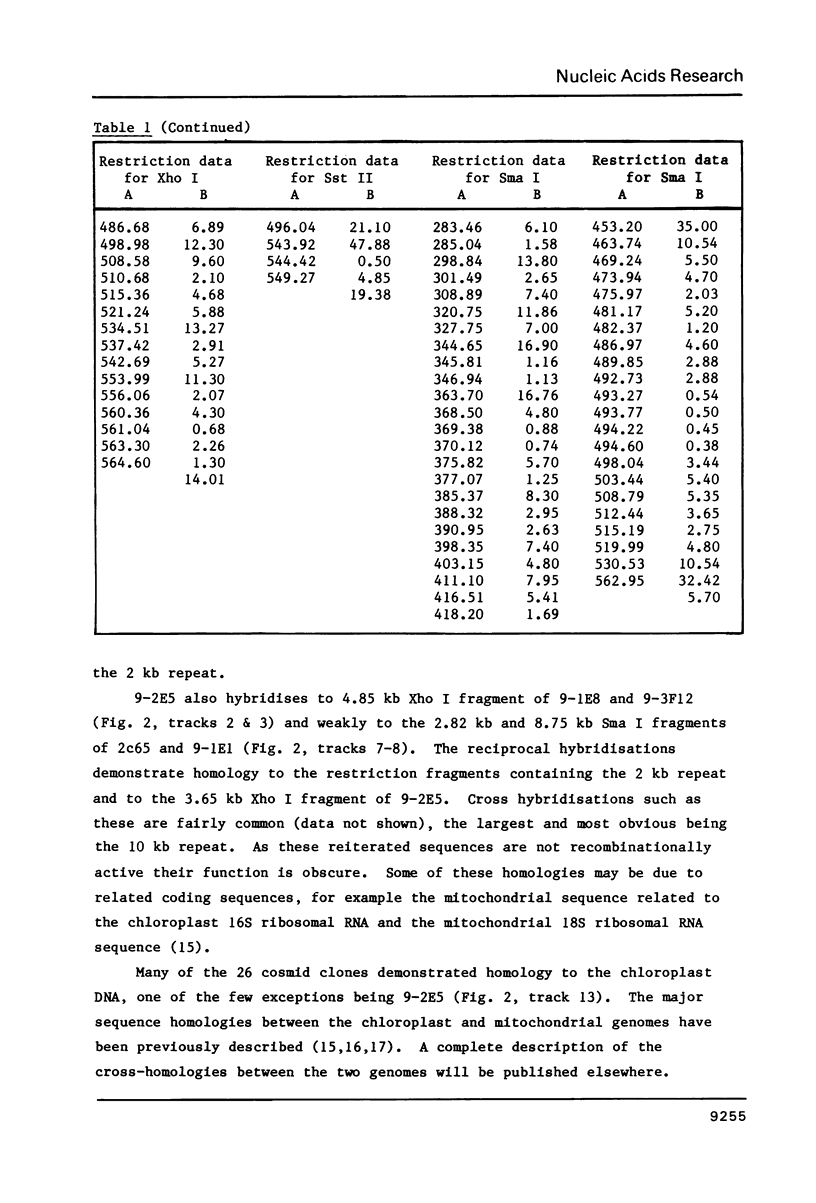
The size of the mitochondrial genome from the fertile cytoplasm of maize has been determined by restriction mapping to be 570 kb. The entire sequence complexity of the genome can be represented on a single circular DNA species (the 'master circle'). The presence of reiterated sequences, active in recombination, results in a complex multipartite organisation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonen L., Gray M. W. Organization and expression of the mitochondrial genome of plants I. The genes for wheat mitochondrial ribosomal and transfer RNA: evidence for an unusual arrangement. Nucleic Acids Res. 1980 Jan 25;8(2):319–335. doi: 10.1093/nar/8.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K. S., Walbot V. Comparison of the Restriction Endonuclease Digestion Patterns of Mitochondrial DNA from Normal and Male Sterile Cytoplasms of ZEA MAYS L. Genetics. 1982 Sep;102(1):109–128. doi: 10.1093/genetics/102.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennicke A. Mitochondrial DNA from Oenothera berteriana: PURIFICATION AND PROPERTIES. Plant Physiol. 1980 Jun;65(6):1207–1210. doi: 10.1104/pp.65.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broach J. R., Guarascio V. R., Jayaram M. Recombination within the yeast plasmid 2mu circle is site-specific. Cell. 1982 May;29(1):227–234. doi: 10.1016/0092-8674(82)90107-6. [DOI] [PubMed] [Google Scholar]
- Chia W., Scott M. R., Rigby P. W. The construction of cosmid libraries of eukaryotic DNA using the Homer series of vectors. Nucleic Acids Res. 1982 Apr 24;10(8):2503–2520. doi: 10.1093/nar/10.8.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconet D., Lejeune B., Quetier F., Gray M. W. Evidence for homologous recombination between repeated sequences containing 18S and 5S ribosomal RNA genes in wheat mitochondrial DNA. EMBO J. 1984 Feb;3(2):297–302. doi: 10.1002/j.1460-2075.1984.tb01799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontarnau A., Hernández-Yago J. Characterization of mitochondrial DNA in citrus. Plant Physiol. 1982 Dec;70(6):1678–1682. doi: 10.1104/pp.70.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble R. J., Gunn R. E., Flavell R. B. Classification of Normal and Male-Sterile Cytoplasms in Maize. II. Electrophoretic Analysis of DNA Species in Mitochondria. Genetics. 1980 Jun;95(2):451–458. doi: 10.1093/genetics/95.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodner R., Tewari K. K. The molecular size and conformation of the chloroplast DNA from higher plants. Biochim Biophys Acta. 1975 Sep 1;402(3):372–390. doi: 10.1016/0005-2787(75)90273-7. [DOI] [PubMed] [Google Scholar]
- Levings C. S., Sederoff R. R. Nucleotide sequence of the S-2 mitochondrial DNA from the S cytoplasm of maize. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4055–4059. doi: 10.1073/pnas.80.13.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Howe C. J., Stern D. B. Maize mitochondrial DNA contains a sequence homologous to the ribulose-1,5-bisphosphate carboxylase large subunit gene of chloroplast DNA. Cell. 1983 Oct;34(3):1007–1014. doi: 10.1016/0092-8674(83)90558-5. [DOI] [PubMed] [Google Scholar]
- Lonsdale D. M., Hodge T. P., Stoehr P. J. A computer program for the management of small cosmid banks. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):429–436. doi: 10.1093/nar/12.1part2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdale D. M., Thompson R. D., Hodge T. P. The integrated forms of the S1 and S2 DNA elements of maize male sterile mitochondrial DNA are flanked by a large repeated sequence. Nucleic Acids Res. 1981 Aug 11;9(15):3657–3669. doi: 10.1093/nar/9.15.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Thompson W. F. Chloroplast DNA rearrangements are more frequent when a large inverted repeat sequence is lost. Cell. 1982 Jun;29(2):537–550. doi: 10.1016/0092-8674(82)90170-2. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Lonsdale D. M. Mitochondrial and chloroplast genomes of maize have a 12-kilobase DNA sequence in common. Nature. 1982 Oct 21;299(5885):698–702. doi: 10.1038/299698a0. [DOI] [PubMed] [Google Scholar]
- Stern D. B., Palmer J. D. Recombination sequences in plant mitochondrial genomes: diversity and homologies to known mitochondrial genes. Nucleic Acids Res. 1984 Aug 10;12(15):6141–6157. doi: 10.1093/nar/12.15.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. L., Anderson R. S., Bendich A. J. The mitochondrial genome is large and variable in a family of plants (cucurbitaceae). Cell. 1981 Sep;25(3):793–803. doi: 10.1016/0092-8674(81)90187-2. [DOI] [PubMed] [Google Scholar]
- de Zamaroczy M., Faugeron-Fonty G., Bernardi G. Excision sequences in the mitochondrial genome of yeast. Gene. 1983 Mar;21(3):193–202. doi: 10.1016/0378-1119(83)90002-1. [DOI] [PubMed] [Google Scholar]