Abstract
Purpose/Objectives
This treatment planning study compares whole breast radiation therapy (WBRT) to accelerated partial breast irradiation (APBI) for different external beam techniques and geometries (free breathing (FB) and deep inspiration breath hold (DIBH)).
Material and Methods
Following approval by our institutional review board, a treatment planning study was performed for 10 patients with left-sided stage 0/I breast cancer enrolled in a Phase I–II study of APBI using intensity modulated radiation therapy (IMRT). Following lumpectomy, patients received planning computerized tomography (CT) scans during FB and using an Active Breathing Control (ABC) device at DIBH. For the FB geometry, standard WBRT and three-dimensional conformal radiation therapy (3DCRT) APBI plans were created. For the DIBH geometry with ABC, WBRT, 3DCRT and IMRT APBI plans were created.
Results
All APBI techniques had excellent planning target volume (PTV) coverage. The maximum PTV dose was reduced from 116% of the prescription dose to 108% with the IMRT (DIBH) APBI plan. The maximum heart doses were >30 Gy for the WBRT techniques, 8.2 Gy for 3DCRT(FB), and <5.0 Gy for 3DCRT(DIBH) and IMRT(DIBH) techniques. The mean left anterior descending artery dose was significantly reduced from 11.4 Gy with WBRT(FB) to 4.2 for WBRT(DIBH) and <2.0 Gy with all APBI techniques.
Conclusions
While PTV coverage was acceptable with all techniques, the plans on the DIBH geometry resulted in a marked reduction in normal tissue dose compared with WBRT planned in the absence of cardiac blocking. Further study is needed to determine if these techniques result in clinical benefits.
Keywords: radiation planning, breast cancer, heart, respiratory motion, active breathing control
INTRODUCTION
National (Radiation Therapy Oncology Group (RTOG)/ National Surgical Adjuvant Breast and Bowel Project (NSABP)), international, and institutional trials are currently ongoing at many centers to investigate the effectiveness of partial breast irradiation (PBI) treated with external beam or brachytherapy for patients with stage 0, I or II disease compared to whole breast radiotherapy for local control and other factors.1–7 Polgar et al have recently reported on the survival and cosmesis data from a randomized trial of PBI (High Dose Rate brachytherapy or electron beam) vs. whole breast radiotherapy. At 5 years, there are no significant differences in survival.8 Because patients with early stage disease have a long life expectancy, it is essential to evaluate not only rates of tumor control but also the dose to healthy tissues received during the delivery of accelerated radiation to the tumor bed.
A number of investigators have evaluated the dosimetric impact of techniques such as active breathing control (ABC) to improve sparing of the heart and/or lung for whole breast treatment for patients.9–11 For example, Sixel et al. showed reductions in the volume of heart receiving greater than 25 Gy from 38 cc (or 4%) to 14 cc (or 1%) for a regular tangential technique.9 Remouchamps et al compared treatment plans for a conventional locoregional technique with patients free-breathing (FB) to the use of a moderate deep inspiration breath hold technique (mDIBH) with an intensity modulation radiation therapy (IMRT) compensation planning technique.10 They found that the use of the mDIBH technique permitted reductions of v30 for 6 of the 9 patients with complete avoidance of the heart possible in 2 of the patients compared to only using IMRT with the patient breathing freely.
Investigators have recently explored the advantages of imaging for the precision of setup for accelerated partial breast irradiation (APBI). For example, Langen et al. evaluated positioning of the seroma cavity with megavoltage CT (MVCT) images obtained with Tomotherapy.12 For the 10 patients studied, they found precision of 2 mm when aligning the MVCT images to the patient’s kilovoltage CT images. Such image guidance techniques, when used daily, make it possible to consider smaller margins.
In this study, we evaluate the resulting normal tissue doses and target coverage for treatment plans along the technology spectrum for guidance and planning for partial breast irradiation. For reference, a whole breast radiotherapy (WBRT) plan is presented for both a patient breathing freely as well as for a deep inspiration breath hold geometry. Three-dimensional conformal radiation therapy (CRT) APBI plans are compared for both free breathing and when improved localization and immobilization are simulated. Finally, the impact of combining improved localization and image guidance with IMRT planning is evaluated. We are specifically interested in the advantages of a breath hold technique to reproducibly spare normal tissues of the heart, LAD, and the ipsilateral lung.
METHODS AND MATERIALS
Beginning November 2004 a phase I–II study, has been conducted at the University of Michigan Comprehensive Cancer Center. This study, approved by our Institutional Review Board, was designed to evaluate APBI using IMRT for early stage breast cancer. All enrolled women were diagnosed with ductal carcinoma in situ (DCIS) or stage I non-lobular breast cancer and underwent lumpectomy with negative margins. Patients with invasive disease had either a negative sentinel lymph node mapping and surgery or formal level 1–2 axillary lymph node dissection with all nodes pathologically negative.
Following lumpectomy, patients underwent a planning computerized tomography (CT) scan at deep inspiration breath hold (DIBH) using an Active Breathing Control (ABC) device (vMax, Sensormedics) and a scan at free breathing (FB) on a 16 slice CT scanner. For each patient, DIBH was at a breath hold of 65–80% of vital capacity (patient dependent) to maximize the heart-chest wall distance. The breath hold state used at simulation was also used for the patient treatment.
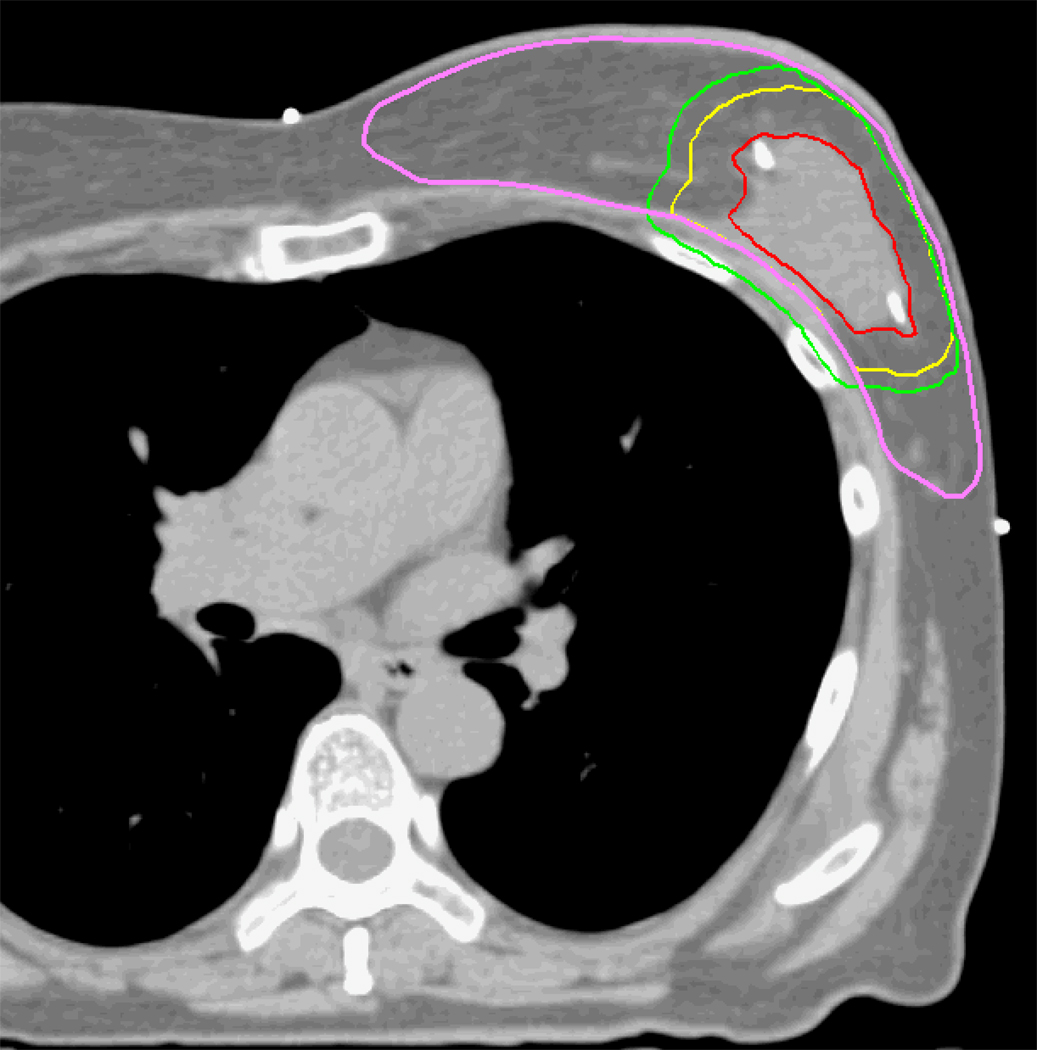
Structures of interest were contoured on the DIBH and FB datasets by physicians and dosimetrists. These structures included the lumpectomy cavity, heart, and left anterior descending (LAD) artery. During a training phase, contours of the LAD were reviewed by a cardiologist to ensure accuracy and consistency of drawing. The ipsilateral and contralateral breasts were contoured by an experienced dosimetrist and reviewed by a physician. Radio-opaque markers were placed to denote the traditional field borders for standard tangent fields during the CT simulation. The breast contour was defined to be 5 mm inside the medial and lateral catheters. The pectoralis major muscle above the ribs was excluded from the breast contour volume. The breast contours were edited back 5 mm from the skin surface to minimize the impact of inaccuracies in the dose calculation for depths less than 5 mm. An example contour is shown in Figure 1. Lungs were auto-contoured with window and level values used in our department protocol. The lumpectomy cavity was contoured by a physician using consistent window and level values for post-surgical tumor beds.
Figure 1.
Example contours and PTV definitions for an example patient with DIBH geometry. The GTV (red), CTV (yellow), PTV(green), and breast (pink) contours are shown.
On the DIBH scans, the clinical target volume (CTV) was defined as a 1 cm expansion of the lumpectomy cavity. The CTV was then expanded 0.5 cm to create the planning target volume (PTV) to account for target motion and uncertainty in target position. In a previous study, we measured the short-term reproducibility of positioning of the breast, demonstrating reproducibility of less than 0.4 cm using an ABC device.13 For the patients in this protocol, a marker was placed on the scar and utilized with the anatomy for daily localization. The 0.5 cm PTV margin was used to simulate the combination of improved positioning of the lumpectomy cavity with the use of ABC and daily image guidance.
Treatment plans were generated for ten consecutive patients with left-sided breast cancers enrolled on the study. The treatment was planned to a total dose of 38.5 Gy with 3.85 Gy per fraction delivered b.i.d. (at least 8 hours apart) for 10 fractions. The impact of patient geometry (controlled DIBH vs. FB) and treatment technique was evaluated using metrics of target dose homogeneity and the dose to critical structures, especially the heart and LAD. Treatment planning and optimization was performed with the UMPlan treatment planning system and the UM-Opt optimization system, developed at our institution.14 Dose distributions were calculated using a convolution/superposition algorithm with a calculation grid size of 3 mm. For each patient, treatment plans were created using the following techniques and geometries:
WBRT (Standard tangents) – FB
WBRT (Standard tangents) - DIBH
3DCRT APBI – FB
3DCRT APBI – DIBH
IMRT APBI – DIBH
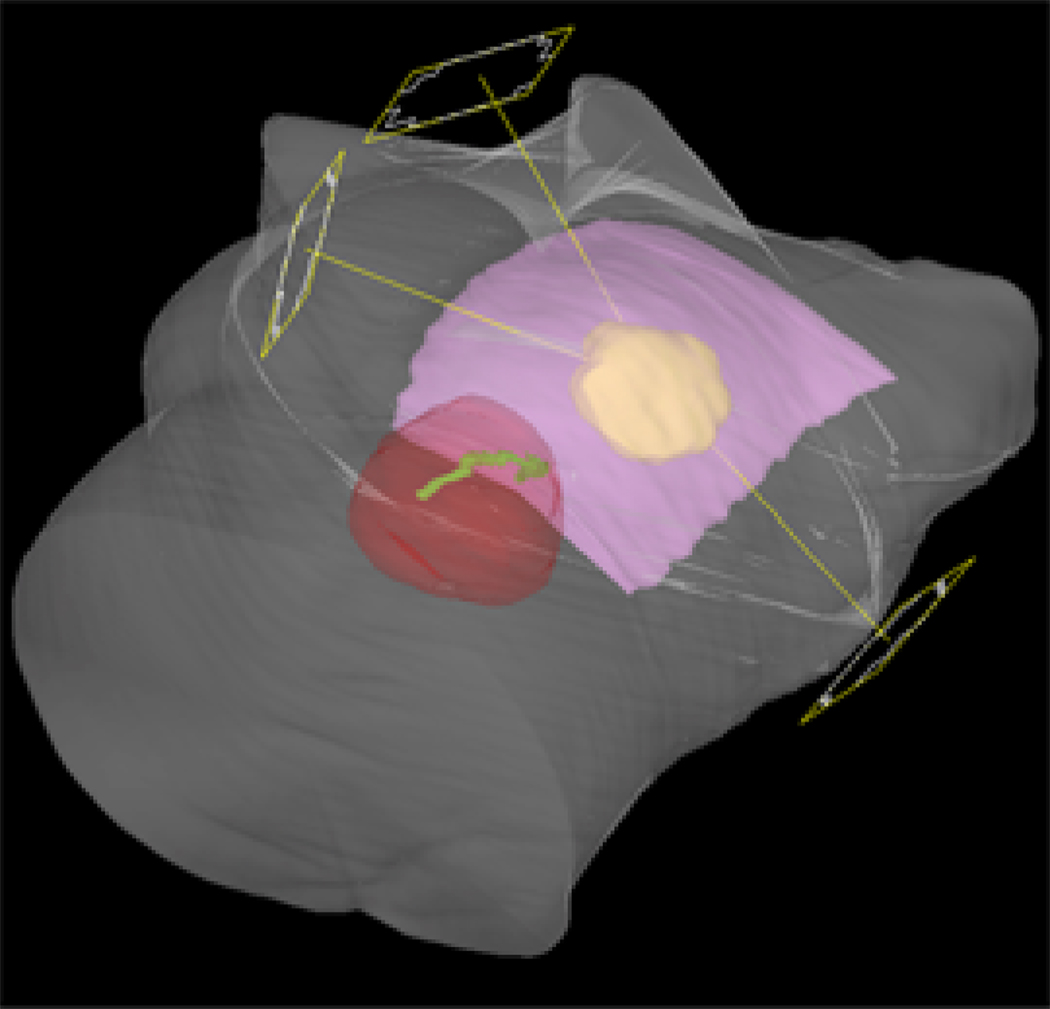
All APBI treatment plans used 6 MV photons with the same 3 or 4 treatment fields (Figure 2) for a given patient. No correction was made to the plans on the free breathing datasets to account for patient motion due to breathing. Instead, the PTV was defined to account for free-breathing as defined below. For the whole breast conventional treatment the target was the whole breast (contoured as defined above) instead of a PTV.
Figure 2.
Example beam arrangement. The contoured breast (pink), CTV (yellow), the heart (red), and the LAD (green) are shown.
The planning goals for the APBI plans were the same for all techniques. The APBI plans were prescribed to a dose of 38.5 Gy. At least 95% of the PTV was to be covered by the prescribed dose. For the forward planned techniques, PTV coverage was a higher priority than the maximum dose. For the forward planned techniques, the dose was renormalized from percent to Gy for comparison. Dose distributions for the IMRT plan were in units of Gy and were not normalized. Techniques 1–4 were created using forward planning (manually) by an experienced dosimetrist.
Technique 1 (WBRT-FB) is a standard conventional plan on the free breathing dataset for each patient calculated to a prescription dose of 50 Gy. No attempts were made to block the heart or the LAD. For comparisons to the APBI plans, dose distributions were biologically corrected from a total dose of 50 Gy with 2 Gy/fraction in our planning system to be equivalent to 38.5 Gy using an alpha-beta ratio of 3 for a total dose of 38.5 Gy with 3.85 Gy/fraction.
Technique 2 (WBRT-DIBH) is a standard conventional plan on the DIBH dataset for each patient calculated to a prescription dose of 50 Gy. Apart from the geometry, it was forward planned in the same way as described for technique 1. No attempts were made to block the heart or the LAD. For comparisons to the APBI plans, dose distributions were biologically corrected from a total dose of 50 Gy with 2 Gy/fraction in our planning system to be equivalent to 38.5 Gy using an alpha-beta ratio of 3 for a total dose of 38.5 Gy with 3.85 Gy/fraction.
Technique 3 is a forward-planned 3DCRT APBI plan on each patient’s free-breathing dataset (the standard geometry for the study) similar to the NSABP-B39/RTOG 0413 study. 4 The PTV is created by a minimum expansion of 1 cm to the CTV. The protocol defines another PTV structure, “PTV_Eval” that is edited 5 mm from the surface so that doses are not calculated in air or in the surface dose region. In this treatment planning study, a 1 cm expansion of the lumpectomy cavity was used to create the CTV and an additional 1 cm expansion of the CTV was used to create the PTV. The PTV expansion of 1 cm represented the patient as freely breathing without daily localization of the target volume. The PTV_Eval structure was also created in this study and used as the reference PTV for comparisons.
Technique 4 is a forward-planned 3DCRT APBI plan on each patient’s DIBH dataset. As described earlier, the CTV is a 1 cm expansion from the lumpectomy cavity. The PTV is a 0.5 cm expansion to simulate the use of improved immobilization with ABC and the use of daily image guidance.
Technique 5 is an inverse-planned IMRT plan on each patient’s DIBH dataset optimized using the cost function and weights shown in Table 1. In addition to the cost function which is shown, the dose to all structures was minimized. With respect to the PTV distribution, the minimum and maximum dose values to the PTV were specified and therefore affected the overall dose homogeneity. The same CTV and PTV definitions for technique 3 were used to simulate the use of improved immobilization with ABC and the use of daily image guidance. Calculations for comparison were based on the optimized fluence fields.
Table 1.
Cost function for optimization for IMRT.
| Structure | Cost Function | Weighting Factor |
|---|---|---|
| CTV | 100% volume, dose ≥ 38.5 Gy, 99% volume, dose ≤ 40.4 Gy |
100 100 |
| PTV | 95% volume - dose ≥ 38.5 Gy 99% volume, dose ≤ 40.4 Gy |
50 50 |
| LAD | Dose ≤ 3 Gy | 1000 |
| Heart | Dose ≤ 3 Gy | 1000 |
| Uninvolved ipsilateral breast |
Dose ≤ 40.4 Gy Minimize dose |
0.001 |
| Lung | 90 % volume - dose ≤ 5 Gy | 0.001 |
Dose metrics were used to evaluate the quality of the treatment plans. The maximum, and mean target doses were evaluated for all PTVs. Normal tissue metrics of mean and maximum dose were evaluated for the heart, LAD, right breast, left breast volume excluding the PTV, and lungs. Additional evaluation was done of the volume of PTV receiving 95% of the prescription dose. Statistical comparisons were made using the paired t-test and the non-parametric sign-test. Comparisons were made between APBI plans and the WBRT technique, as well as between the APBI with FB and the APBI plans using DIBH. For all statistical tests, p-values less than 5% were considered statistically meaningful, as no multiple comparison correction was applied. Interpretation of meaningful results was consistent between the parametric and non-parametric tests, hence only the p-value from the paired t-tests are reported herein.
RESULTS
This study evaluated the dosimetric impact of different types of advanced technology by investigating patient geometry (FB and DIBH) with and without daily imaging and delivery technique (3DCRT vs. IMRT). The mean volume and standard deviation of the contoured breast was 722±389 and 731±382 cc for the free breathing and DIBH scans, respectively. The mean volumes of the PTVs were 202 and 185 cc for the free breathing and DIBH scans, respectively. The expansion to create the PTV volume on the free breathing data set was 0.5 cm greater than on the DIBH geometry because the DIBH geometry simulation also included the use of daily image guidance.
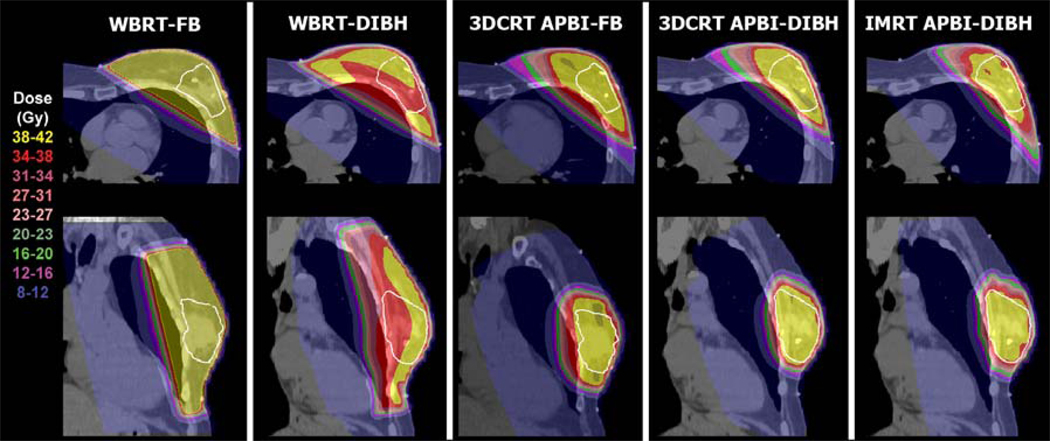
Example dose distributions are shown in Figure 3 for one patient. The greater distance between the heart and breast can be seen by comparing the free-breathing dataset and the DIBH dataset. The differences in the dose distributions between WBRT and APBI techniques can also be seen.
Figure 3.
Example dose distributions for one patient for WBRT and PBI techniques. The PTV is shown in white. Notice the differences in the dose delivered to the heart for the different techniques.
Tables 2–5 summarize the metrics averaged over the 10 patients studied. Acceptable PTV coverage was achieved with all APBI techniques. When compared to WBRT-FB, all APBI plans had improved PTV coverage when assessed by the percent volume covered by 95% of 38.5 Gy (the prescription dose). The results were WBRT-FB 91% and WBRT-DIBH (97%). For the APBI techniques, the results were statistically significant compared to the WBRT-FB technique and the coverage of the PTV was >99% for all APBI techniques. The average maximum target dose was 41.5 Gy for IMRT with DIBH which was significantly less than the average maximum dose of 45.4 Gy with WBRT. All APBI plans resulted in significantly less mean and maximum doses to the heart, LAD and left lung than with WBRT-FB (Figure 4) as determined by p-values <0.05 (Table 2). While there were differences between the mean and maximum doses for the WBRT-FB and WBRT-DIBH techniques, the change in the maximum LAD dose from 29.8 to 15.8 Gy was the only difference that was statistically significant. The average maximum heart dose with WBRT-FB was 36.5 Gy compared to 31.5 Gy for WBRT-DIBH and 8.2, 4.8, and 3.8 Gy for 3DCRT-FB, 3DCRT-DIBH, and IMRT-DIBH, respectively. Average maximum and mean LAD doses were similarly reduced for the APBI techniques compared to WBRT. For the left lung, the v20 dose was significantly reduced from 10.1 Gy with WBRT to 4.1 Gy with the 3DCRT-FB technique. Maximum, mean, v20, v10, and v5 lung doses were all significantly reduced with both DIBH techniques.
Table 2.
Comparison of plans to whole breast tangential irradiation with free-breathing (WBRT-FB).
| WBRT - FB | WBRT - DIBH | APBI 3DCRT - FB | APBI 3DCRT - DIBH | APBI IMRT - DIBH | |||||
|---|---|---|---|---|---|---|---|---|---|
| N = 10 | Dose (Gy) |
Dose (Gy) |
P-value | Dose (Gy) |
P-value | Dose (Gy) |
P-value | Dose (Gy) |
P-value |
| PTV | |||||||||
| Maximum | 45.5 | 47.9 | 0.0559 | 44.6 | 0.1429 | 44.9 | 0.2887 | 41.5* | <0.0001 |
| Mean | 39.1 | 41.2* | 0.0131 | 40.6* | 0.0128 | 40.9* | 0.0041 | 39.1 | 0.9558 |
| %Vol>95%d | 90.7 | 97.3 | 0.0592 | 99.6* | 0.0126 | 99.1* | 0.0244 | 99.1* | 0.0221 |
| Heart | |||||||||
| Maximum | 36.5 | 31.5 | 0.1278 | 8.2* | <0.0001 | 4.8* | <0.0001 | 3.8* | <0.0001 |
| Mean | 1.4 | 1.0 | 0.3125 | 0.5* | 0.0208 | 0.4* | 0.0083 | 0.5* | 0.0277 |
| LAD | |||||||||
| Maximum | 29.8 | 15.8* | 0.0252 | 6.1* | 0.0004 | 2.2* | <0.0001 | 1.8* | <0.0001 |
| Mean | 11.5 | 4.2* | 0.0198 | 1.9* | 0.0032 | 0.7* | 0.0020 | 0.8* | 0.0023 |
| Left lung | |||||||||
| Maximum | 39.5 | 41.9 | 0.0583 | 38.1 | 0.0783 | 35.5* | 0.0054 | 35.4* | 0.0017 |
| Mean | 4.2 | 4.9 | 0.2055 | 2.9 | 0.0972 | 1.7* | 0.0053 | 1.8* | 0.0053 |
| V20 | 10.2 | 11.2 | 0.4911 | 4.1* | 0.0060 | 1.7* | 0.0007 | 1.4* | 0.0003 |
| V10 | 13.0 | 14.7 | 0.3055 | 9.2 | 0.1390 | 4.6* | 0.0038 | 4.0* | 0.0010 |
| V5 | 16.1 | 18.7 | 0.1293 | 14.9 | 0.6924 | 8.5* | 0.0215 | 8.2* | 0.0124 |
| Left Breast minus PTV | |||||||||
| Maximum | 45.6 | 47.9 | 0.0702 | 44.5* | 0.0264 | 44.8* | 0.0327 | 41.4* | <0.0001 |
| Mean | 39.6 | 40.8 | 0.1163 | 18.3* | <0.0001 | 17.0* | <0.0001 | 14.8* | <0.0001 |
| %Vol@50%d | 99.6 | 87.7 | 0.3095 | 42.7* | <0.0001 | 39.4* | <0.0001 | 37.2* | <0.0001 |
| Right Breast | |||||||||
| Maximum | 6.3 | 6.8 | 0.1666 | 3.2 | 0.8759 | 2.9 | 0.1480 | 1.8 | 0.1804 |
| Mean | 0.1 | 0.1 | 0.8117 | 0.1 | 0.5409 | 0.1 | 0.4173 | 0.1 | 0.8576 |
Denotes p-value <0.05.
Table 5.
Comparison of APBI-3DCRT with DIBH versus APBI-IMRT with DIBH.
| APBI3DCRT - DIBH | APBI IMRT - DIBH | ||
|---|---|---|---|
| N = 10 | Dose (Gy) |
Dose (Gy) |
P-value |
| PTV | |||
| Maximum | 44.9 | 41.5* | 0.0001 |
| Mean | 40.9 | 39.1* | <0.0001 |
| %Vol>95%d | 99.1 | 99.2 | 0.9293 |
| Heart | |||
| Maximum | 4.8 | 3.8 | 0.3977 |
| Mean | 0.4 | 0.5 | 0.0730 |
| LAD | |||
| Maximum | 2.2 | 1.8 | 0.2948 |
| Mean | 0.7 | 0.8 | 0.4596 |
| Left lung | |||
| Maximum | 35.5 | 35.4 | 0.8994 |
| Mean | 1.7 | 1.8 | 0.4394 |
| V20 | 1.7 | 1.4 | 0.1491 |
| V10 | 4.6 | 4.0 | 0.1120 |
| V5 | 8.5 | 8.2 | 0.5238 |
| Left Breast minus PTV | |||
| Maximum | 44.8 | 41.4* | <0.0001 |
| Mean | 17.0 | 14.8* | 0.0005 |
| %Vol@50%d | 39.4 | 37.2 | 0.2569 |
| Right Breast | |||
| Maximum | 2.9 | 1.8 | 0.3230 |
| Mean | 0.1 | 0.1 | 0.0767 |
Denotes p-value <0.05.
To evaluate the advantage of improved geometry with DIBH for a standard WBRT technique, all APBI techniques were compared to the WBRT-DIBH results (Table 3). Statistically significant dose reductions were possible for all APBI techniques (including APBI 3DCRT-FB) for all lung metrics. The PTV maximum doses were reduced with the APBI-IMRT-DIBH treatment plan. The doses to the heart and LAD were also reduced for the APBI techniques. The differences were statistically significant for the maximum doses to the heart and for the DIBH techniques for the maximum dose to the LAD. For example, the doses ranged from a maximum of 31.5 Gy with WBRT-DIBH to 8.2 Gy with PBI-3DCRT-FB, 4.8 Gy with APBI-3DCRT-DIBH, and 3.8 with APBI-IMRT-DIBH.
Table 3.
Comparison of plans to WBRT at DIBH to evaluate the impact of the geometry. Dose values are in Gray.
| WBRT - DIBH | APBI 3DCRT - FB | APBI 3DCRT - DIBH | APBI IMRT - DIBH | ||||
|---|---|---|---|---|---|---|---|
| N = 10 | Dose (Gy) |
Dose (Gy) |
P-value | Dose (Gy) |
P-value | Dose (Gy) |
P-value |
| PTV | |||||||
| Maximum | 47.9 | 44.6* | 0.0185 | 44.9* | 0.0237 | 41.5* | 0.0004 |
| Mean | 41.2 | 40.6 | 0.2388 | 40.9 | 0.6497 | 39.1* | 0.0018 |
| %Vol>95%d | 97.3 | 99.6* | 0.0027 | 99.1 | 0.0866 | 99.2 | 0.0689 |
| Heart | |||||||
| Maximum | 31.5 | 8.2* | 0.0016 | 4.8* | 0.0001 | 3.8* | <0.0001 |
| Mean | 1.0 | 0.5 | 0.1869 | 0.4 | 0.0722 | 0.5 | 0.1655 |
| LAD | |||||||
| Maximum | 15.8 | 6.1 | 0.0810 | 2.2* | 0.0103 | 1.8* | 0.0083 |
| Mean | 4.2 | 1.9 | 0.2247 | 0.7* | 0.0496 | 0.8 | 0.0527 |
| Left lung | |||||||
| Maximum | 41.9 | 38.1* | 0.0033 | 35.5* | 0.0007 | 35.4* | 0.0009 |
| Mean | 4.9 | 2.9* | 0.0070 | 1.7* | 0.0003 | 1.8* | 0.0003 |
| V20 | 11.2 | 4.1* | 0.0010 | 1.7* | <0.0001 | 1.4* | <0.0001 |
| V10 | 14.7 | 9.2* | 0.0284 | 4.6* | 0.0004 | 4.0* | <0.0001 |
| V5 | 18.7 | 14.9 | 0.1847 | 8.5* | 0.0020 | 8.2* | 0.0008 |
| Right Breast | |||||||
| Maximum | 6.8 | 3.2 | 0.0580 | 2.9* | 0.0415 | 1.8* | 0.0305 |
| Mean | 0.1 | 0.1 | 0.1718 | 0.1* | 0.0402 | 0.1 | 0.9487 |
Denotes p-value <0.05.
To further evaluate the advantage of DIBH for APBI techniques alone, the 3DCRT-DIBH and IMRT-DIBH techniques were compared to the APBI 3DCRT-FB technique (Table 4). The maximum PTV dose was reduced for all DIBH techniques to be closer to the prescription dose of 38.5 Gy. The maximum heart dose was significantly reduced from 8.2 to 4.8 and 3.8 Gy for the 3DCRT and IMRT techniques, respectively. All left lung dose metrics were also significantly reduced. For the left breast tissue minus the PTV, the mean dose was reduced from 18.3 Gy for the 3DCRT-FB technique to 17.0 and 14.8 Gy with the 3DCRT-DIBH, and IMRT-DIBH techniques, respectively. Similar reductions were seen for the percent volume receiving 50% of the prescription dose.
Table 4.
Comparison of plans to APBI-3DCRT-FB to evaluate the impact of patient geometry for all PBI techniques.
| APBI3DCRT with FB | APBI3DCRT with DIBH | APBI IMRT with DIBH | |||
|---|---|---|---|---|---|
| N = 10 | Dose (Gy) |
Dose (Gy) |
P-value | Dose (Gy) |
P-value |
| PTV | |||||
| Maximum | 44.6 | 44.9 | 0.2437 | 41.5* | 0.0008 |
| Mean | 40.6 | 40.9 | 0.0414 | 39.1* | <0.0001 |
| %Vol>95%d | 99.6 | 99.1 | 0.4734 | 99.2 | 0.4072 |
| Heart | |||||
| Maximum | 8.2 | 4.8 | 0.0183 | 3.8 | 0.0523 |
| Mean | 0.5 | 0.4* | 0.0024 | 0.5 | 0.6827 |
| LAD | |||||
| Maximum | 6.1 | 2.2 | 0.1392 | 1.8 | 0.1505 |
| Mean | 1.9 | 0.7 | 0.1243 | 0.8 | 0.1610 |
| Left lung | |||||
| Maximum | 38.1 | 35.5* | 0.0135 | 35.4* | 0.0023 |
| Mean | 2.9 | 1.7* | 0.0001 | 1.8* | 0.0010 |
| V20 | 4.1 | 1.7* | <0.0001 | 1.4* | 0.0002 |
| V10 | 9.2 | 4.6* | 0.0004 | 4.0* | 0.0003 |
| V5 | 14.9 | 8.5* | 0.0007 | 8.2* | 0.0008 |
| Left Breast minus PTV | |||||
| Maximum | 44.5 | 44.8 | 0.3675 | 41.4* | 0.0008 |
| Mean | 18.3 | 17.0* | 0.0112 | 14.8* | 0.0002 |
| %Vol@50%d | 42.7 | 39.4* | 0.0446 | 37.2* | 0.0147 |
| Right Breast | |||||
| Maximum | 3.2 | 2.9 | 0.1211 | 1.8 | 0.2444 |
| Mean | 0.1 | 0.1 | 0.2114 | 0.1 | 0.0784 |
Denotes p-value <0.05.
Finally, 3DCRT-DIBH and IMRT-DIBH techniques were compared to evaluate the importance of IMRT with the same use of imaging and immobilization (Table 5). IMRT resulted in a significant improvement in decreasing the maximum PTV dose. Another advantage with IMRT was in decreasing the maximum and mean doses to the left breast tissue minus the PTV. The percent volume receiving 50% of the prescription dose was also decreased with IMRT, but the difference was not statistically significant. On the DIBH geometry, there is a greater distance between the heart and the target. In addition, there is a smaller PTV margin.
DISCUSSION
This study evaluated the dosimetric impact of different types of advanced technology by investigating patient geometry (FB and DIBH) with and without daily imaging and delivery technique (3DCRT vs. IMRT). In this work, we evaluated the dosimetric impact of the use of advanced technologies by evaluating patient geometry (free breathing versus DIBH) with and without daily image guidance and the use of IMRT compared to 3DCRT for APBI with improved localization and image guidance. It should be noted that the results from the IMRT optimization can be changed by altering the cost function used for optimization. Higher priorities were set on minimizing dose to the heart and obtaining good target homogeneity. It should also be noted that the IMRT plans were the only ones creating utilizing inverse planning techniques. The results for the non-IMRT plans may differ when using multi-segment optimization methods.
A study by Baglan et al of an external APBI technique was originally designed following experience with brachytherapy.1 Therefore, it is unknown what the exact requirements should be for target homogeneity with external beam techniques. The heterogeneous dose distribution achieved with brachytherapy techniques may not apply for a fractionated treatment delivered to a PTV with an external beam technique. For example, it may be important to require a minimum dose to the target volume to ensure that the target volume is not under-dosed with an external beam technique due to discrepancies from respiratory motion and setup variations. Further clinical studies are needed to determine the acceptable range of dose heterogeneity. With respect to the free-breathing values presented, it is important to note that these treatment plans represent calculations on a single representation of the patient. In reality, the delivered dose will be different due to the impact of breathing. This is addressed in planning by using a larger value for the PTV expansion.
Several investigators have evaluated different external beam delivery methods for APBI and compared the methods to conventional whole breast radiotherapy. Baglan et al described a 3DCRT approach for APBI delivery.1 In their evaluation, a 10 mm margin was included from the CTV to the PTV to account for breathing and setup error based on their evaluation of clip displacement and normal inhale and exhale. For their definition of normal breast tissue (which included muscle), they found that the dosimetric limits for the normal ipsilateral breast tissue required that <25% of the defined breast volume be part of the PTV. This work was part of the foundation for the multi-institutional RTOG 0413 (NSABP B-39) study. Instead of contouring a breast volume on the CT slices, an irradiated volume is drawn based on tangents from catheters placed during simulation.4 They found the technique to be feasible in a multi-institutional trial.
Other investigators have also evaluated the dosimetric impact of accelerated partial breast techniques delivered with 3DCRT and found similar reductions in cardiac doses for patients treated free-breathing. For example, Hiatt et al demonstrated significant improvements in cumulative heart DVHs for a 3DCRT PBI technique compared to an WBRT technique for 8 patients with unfavorable cardiac position.5 The mean heart dose decreased from 4.6 Gy with a WBRT technique to 0.75 Gy with a 3DCRT APBI technique. The APBI technique also had smaller standard deviation compared to the whole breast technique. Patel et al evaluated interstitial brachytherapy, 3DCRT, and helical tomotherapy in prone and supine positions.7 For the 3DCRT part of their study, they found low mean heart doses with all methods of APBI delivery. When evaluating target coverage, they used different expansions for PTV margins: no expansion for their prone tomotherapy technique, 0.5 cm for their supine tomotherapy technique, and a 1.0 cm expansion for their 3DCRT technique. Similar to this study, the dosimetric differences between the 3DCRT APBI techniques in their study can be attributed mainly to the differences in the PTV margins. Kozak et al evaluated a photon only and a mixed photon/electron 3DCRT APBI approach.6 They achieved good results with both methods. Tradeoffs were noted between the techniques with respect to beam complexity and the amount of normal tissues irradiation. With the mixed modality technique, they were often able to use one less beam to achieve the desired results.
Finally, in a prospective clinical study, Formenti et al evaluated a prone 3DCRT APBI technique for 47 patients.3 With their prone technique, none of the heart received doses greater than or equal to 5 Gy. With respect to target coverage, the mean value was 110% of the prescription dose.
Similar to the above investigations, we found acceptable target coverage with all partial breast irradiation techniques. For example, the maximum dose was reduced for patients planned with IMRT (108%) compared to the 3DCRT techniques (116–118%). The volume of PTV receiving 95% of the prescription dose (36.5 Gy) was 99% for all APBI techniques. However, the above studies investigated free-breathing techniques with supine or prone positioning and standard setup margins. In our study we compared APBI along a continuum from conventional to advanced techniques. Dosimetric results from conventional planning and delivery techniques with a standard margin for free breathing and localization were compared to the results with advanced therapy techniques, such as improved localization with daily imaging and immobilization with ABC. Finally, we evaluated the impact of IMRT for planning with improved localization and immobilization. For the five plans created, statistical methods were used to investigate the dosimetric significance of the differences between techniques. We found that the improved localization and immobilization resulted in decreased doses to normal tissues. For the DIBH geometry, the primary dosimetric advantage of IMRT was in reducing the maximum dose to the PTV and in reducing the maximum and mean doses to uninvolved left breast tissue.
For the WBRT techniques, the only statistically significant differences for DIBH compared to FB were with respect to the mean dose and the maximum and mean LAD doses. We found the DIBH geometry permitted further reduction in the maximum heart dose when compared with the free-breathing delivery for the APBI techniques. Given that investigators are still assessing the impact of dose to the heart, it is unclear if such reductions are clinically significant. The use of DIBH can further reduce radiation exposure in those patients whose cardiac positioning would place them at increased risk for radiation-induced toxicity. In our study, we also monitored the dose to the LAD and assigned a cost to dose to that structure during IMRT. Because the volume of the LAD is small, it was a sensitive structure in our optimization. Due to the decreased volume of whole breast irradiated, we found all APBI techniques resulted in significant reductions in LAD dose compared to the WBRT.
CONCLUSIONS
Our data suggest that while PTV coverage was acceptable with all techniques, the 3DCRT and the IMRT plans on the DIBH geometry resulted in a marked reduction in normal tissue dose compared with APBI plans in a free breathing geometry due to the reduced PTV margin when ABC is utilized for immobilization and image guidance techniques are utilized for localization. A trend towards further reduction in maximum doses to the heart, LAD, contralateral breast and ipsi-lateral lung was achieved with IMRT. These results support the need to further investigate the potential benefits as well as limitations of APBI using IMRT in clinical trials as an alternative to WBRT. For patients where dose to the heart is a concern due to position, controlled deep inspiration breath hold techniques (for example with an ABC device) provide the capability to limit the heart dose for treatment delivery. Breath hold techniques can be considered for breast cancer patients for a range of techniques and for accelerated or standard fractionation schedules. The use of daily localization and methods to limit target motion (such as with ABC) can reduce the amount of normal ipsilateral breast irradiated by decreasing the CTV to PTV margin. Clinical studies are required to demonstrate whether or not these techniques provide clinically significant differences for patients in terms of local control and reductions in cardiac toxicity. Data collected in clinical trials should be comprehensively evaluated with respect to the planned and representative delivered doses, including setup and respiratory motion considerations, to improve the understanding of the planning requirements for APBI techniques.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the Annual Meeting of the American Society for Therapeutic Radiology and Oncology, November 2006 (Philadelphia, Pennsylvania).
Conflict of Interest Notification
All authors report no actual or potential conflicts of interest.
Contributor Information
Jean M. Moran, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
Merav A. Ben-David, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
Robin B. Marsh, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
James M. Balter, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
Kent A. Griffith, Department of Radiation Oncology, University of Michigan Cancer Center Biostatistics Core, Ann Arbor, MI
James A. Hayman, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
Lori J. Pierce, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
REFERENCES
- 1.Baglan KL, Sharpe MB, Jaffray D, et al. Accelerated partial breast irradiation using 3D conformal radiation therapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2003;55:302–311. doi: 10.1016/s0360-3016(02)03811-7. [DOI] [PubMed] [Google Scholar]
- 2.Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247–1253. doi: 10.1016/s0360-3016(03)01573-6. [DOI] [PubMed] [Google Scholar]
- 3.Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Vicini F, Winter K, Straube W, et al. A phase I/II trial to evaluate three-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for Stage I/II breast carcinoma: initial report of feasibility and reproducibility of Radiation Therapy Oncology Group (RTOG) Study 0319. Int J Radiat Oncol Biol Phys. 2005;63:1531–1537. doi: 10.1016/j.ijrobp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Hiatt JR, Evans SB, Price LL, et al. Dose-modeling study to compare external beam techniques from protocol NSABP B-39/RTOG 0413 for patients with highly unfavorable cardiac anatomy. Int J Radiat Oncol Biol Phys. 2006;65:1368–1374. doi: 10.1016/j.ijrobp.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Kozak KR, Doppke KP, Katz A, et al. Dosimetric comparison of two different three-dimensional conformal external beam accelerated partial breast irradiation techniques. Int J Radiat Oncol Biol Phys. 2006;65:340–346. doi: 10.1016/j.ijrobp.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 7.Patel RR, Becker SJ, Das RK, et al. A dosimetric comparison of accelerated partial breast irradiation techniques: multicatheter interstitial brachytherapy, three-dimensional conformal radiotherapy, and supine versus prone helical tomotherapy. Int J Radiat Oncol Biol Phys. 2007;68:935–942. doi: 10.1016/j.ijrobp.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Polgar C, Fodor J, Major T, et al. Breast-Conserving Treatment with Partial or Whole Breast Irradiation for Low-Risk Invasive Breast Carcinoma-5-Year Results of a Randomized Trial. Int J Radiat Oncol Biol Phys. 2007;29:694–702. doi: 10.1016/j.ijrobp.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Sixel KE, Aznar MC, Ung YC. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 10.Remouchamps VM, Vicini FA, Sharpe MB, et al. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55:392–406. doi: 10.1016/s0360-3016(02)04143-3. [DOI] [PubMed] [Google Scholar]
- 11.Frazier RC, Vicini FA, Sharpe MB, et al. Impact of breathing motion on whole breast radiotherapy: a dosimetric analysis using active breathing control. Int J Radiat Oncol Biol Phys. 2004;58:1041–1047. doi: 10.1016/j.ijrobp.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Langen KM, Buchholz DJ, Burch DR, et al. Investigation of accelerated partial breast patient alignment and treatment with helical tomotherapy unit. Int J Radiat Oncol Biol Phys. 2008;70:1272–1280. doi: 10.1016/j.ijrobp.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Moran JM, Balter JM, Ben-David MA, et al. Short-term displacement and reproducibility of the breast and nodal targets under active breathing control. Int J Radiat Oncol Biol Phys. 2007;68:541–546. doi: 10.1016/j.ijrobp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessler ML, McShan DL, Epelman MA, et al. Costlets: A Generalized Approach to Cost Functions for Automated Optimization of IMRT Treatment Plans. Optimization and Engineering. 2005;6:421–448. [Google Scholar]