Abstract
Objective
In the U.S., seasonal trivalent influenza vaccination (TIV) is currently universally recommended for all pregnant women. However, data on the maternal inflammatory response to vaccination is lacking and would better delineate the safety and clinical utility of immunization. In addition, for research purposes, vaccination has been used as a mild immune trigger to examine in vivo inflammatory responses in nonpregnant adults. The utility of such a model in pregnancy is unknown. Given the clinical and empirical justifications, the current study examined the magnitude, time course, and variance in inflammatory responses following seasonal influenza virus vaccination among pregnant women.
Methods
Women were assessed prior to and at one day (n=15), two days (n=10), or approximately one week (n=21) following TIV. Serum interleukin (IL)-6, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), and macrophage migration inhibitory factor (MIF) were determined by high sensitivity immunoassay.
Results
Significant increases in CRP were seen at one and two days post-vaccination (ps <.05). A similar effect was seen for TNF-α, for which an increase at two days post-vaccination approached statistical significance (p = .06). There was considerable variability in magnitude of response; coefficients of variation for change at two days post-vaccination ranged from 122% to 728%, with the greatest variability in IL-6 responses at this timepoint.
Conclusions
Trivalent influenza virus vaccination elicits a measurable inflammatory response among pregnant women. There is sufficient variability in response for testing associations with clinical outcomes. As adverse perinatal health outcomes including preeclampsia and preterm birth have an inflammatory component, a tendency toward greater inflammatory responding to immune triggers may predict risk of adverse outcomes, providing insight into biological mechanisms underlying risk. The inflammatory response elicited by vaccination is substantially milder and more transient than seen in infectious illness, arguing for the clinical value of vaccination. However, further research is needed to confirm that the mild inflammatory response elicited by vaccination is benign in pregnancy.
Pregnant women are considered at greater risk than the general population for complications, hospitalization, and death due to influenza [1–3]. Based on known risks versus benefits of immunization, routine influenza vaccination is currently recommended by the Centers for Disease Control (CDC) and American College of Obstetricians and Gynecologists (ACOG) for all healthy pregnant women in any trimester [4, 5]. Several studies have shown no harmful effects resulting from influenza vaccination during pregnancy [for review see 2]. However, because vaccination could confer risks that have not been measured or would require larger sample sizes to detect, the wisdom of universal vaccination of pregnant women has been a topic of debate [6, 7].
Studies of TIV in pregnancy have shown no differences among vaccinated versus unvaccinated women and their infants in rates of miscarriage, C-section, gestational age at delivery, birth weight, APGAR scores, length of hospitalization after birth, or fetal malformations and mortality to age four among offspring [8–19]. Maternal vaccination has been associated with 63% reduction in clinical influenza in infants from birth to six months of age [12]. Among infants born during influenza season, maternal vaccination may reduce risk of preterm delivery and small for gestational age birth [20]. Further, maternal influenza infection has been linked to increased risk of schizophrenia in adult offspring [21–23], a risk that vaccination could mitigate. However, given that this link may be mediated by the maternal inflammatory response to infection, it has been postulated that the inflammatory response to vaccination may itself be detrimental to fetal brain development [7]. To our knowledge, there are no available data regarding the magnitude or duration of inflammatory responses elicited by TIV in pregnant women. Thus, better understanding of the maternal inflammatory response elicited by vaccination is highly warranted from a clinical standpoint.
In addition, assuming health benefits, vaccination may provide a useful research model. Inflammatory processes play an important role in the maintenance of successful pregnancy. Pregnancy has been associated with decreased inflammatory responses and maintained/increased antiinflammatory responses to immune challenges in human and animal models [24–29]. It has been postulated that this immune adaptation may prevent rejection of the fetus by the maternal immune system. Thus, a tendency toward inflammatory responding may increase risk of adverse outcomes, including preeclampsia and preterm birth (PTB) [30–33].
Human studies of the inflammatory response system in pregnant women to-date have relied on in vitro models [27, 28]. Because in vitro techniques involve isolation of specific cells, removal of cells from the complex in vivo environment, and exposure to higher levels of antigen than normally occurs in vivo, the clinical relevance of in vitro assessments is often unclear. By providing insight into immune function in the complex, multifaceted, naturally-occurring environment, in vivo models arguably provide data with clearer clinical relevance.
Vaccines have been used as a mild immune trigger to examine individual differences in vivo inflammatory responses in non-pregnant adults [34–40]. Differential inflammatory responses to vaccination have been reported in association with depressive symptoms [38] and carotid artery disease [39], conditions with an inflammatory component. Translating this research to pregnancy, differential inflammatory responses to immune triggers may predict risk for preeclampsia and preterm birth. The utility of such a model is dependent on 1) vaccination eliciting a measurable inflammatory response and 2) the presence of sufficient variability in response to allow for differential classification (e.g., high versus low responders). Due to substantial immune adaptation that occurs during pregnancy, with reported down-regulation of inflammatory responding, findings regarding the inflammatory response elicited by vaccination in nonpregnant adults may not extend to pregnant women.
Given both the clinical and empirical justifications for better understanding inflammatory responses elicited by immunization in pregnancy, the current study examined the magnitude, time course, and variance in inflammatory responses following seasonal trivalent influenza virus vaccination (TIV) among pregnant women.
Method
Participants
This study included 46 pregnant women who were assessed prior to and at either one day (n=15), two days (n=10), or approximately one week (6–9 days; n=21) following seasonal influenza virus vaccination. Women were recruited through the Ohio State University Medical Center General Perinatal Clinic. Women were excluded from participation if they reported recent acute illness, chronic health conditions with implications for immune function, or if fetal anomaly or preeclampsia were indicated per medical records.
At a regular prenatal visit, women were informed of the study. Women were asked if they planned to receive the seasonal flu shot. If they were undecided, they were encouraged to speak with their physician about vaccination prior to study enrollment. Women who were eligible and chose to participate completed an informed consent. Participants received compensation for their participation. The study was approved by The OSU Biomedical Institutional Review Board. Data were collected between November 2006 to April 2009.
Demographic and Psychosocial Measures
Demographic and descriptive information regarding height, current weight, pre-pregnancy weight, age, race, education level, marital status, and income was collected. The following health behaviors were assessed at the initial study visit: smoking, participation in regular physical activity (i.e., at least one hour per week of vigorous activity), and frequency of prenatal vitamin use.
Measurement of Serum Inflammatory Markers
At each study visit, whole blood was collected into vacutainer tubes while subjects were in a seated position. All samples were collected between 9:30 am – 1:30 pm. Samples were immediately centrifuged, aliquoted, and placed in −80 C degree freezer storage until analysis. Serum levels of IL-6 and TNF-α were assayed in duplicate with ultra-sensitive multiplex kits from Meso Scale Discovery (MSD) and chemilluminescence methodology using the Immulite 1000 (Siemens Healthcare Diagnostics, Inc., 1717 Deerfield Rd., Deerfield, Il.) Serum levels of MIF were assayed in duplicate using ultra-sensitive multiplex kits from R&D Systems (Minneapolis, MN) per kit instructions.
Physical Measurements
Body mass index (BMI) was calculated (kg/m2) using height as measured by a nurse at the study visit and self-reported weight prior to pregnancy. The general accuracy of self-reported pre-pregnancy weight was confirmed by calculation of expected pre-pregnancy weight based on weight by scale at the study visit.
Influenza Virus Vaccination
Each woman received Fluarix (GlaxoSmithKline) seasonal trivalent influenza virus vaccination. During the 2006–2007 influenza season, each 0.5 mL dose contained 45 μg hemagglutinin (HA), with 15 μg HA of each of the following three virus strains: A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004. During the 2008–2009 influenza season, the vaccination contained A/Brisbane/59/2007 (A/H1N1), A/Uruguay/716/2007 (A/Brisbane/10/2007-like strain) (A/H3N2), and B/Florida/04/2006. The vaccine was administered following baseline blood sampling. Data regarding inflammatory markers at one week post-vaccination were collected during the 2006–2007 influenza season. During the 2008–2009 influenza season, women were assessed at either one day or two days post-vaccination.
Statistical Analyses
Women assessed at one day, two days, and one week post-vaccination were compared in terms of demographic and behavioral characteristics using ANOVA and chi-square analyses to determine the extent to which groups were comparable.
Cytokine data were log transformed to normalize the data distribution. Log-transformed data were used for all statistical analyses. Datapoints ≥ 3 standard deviations from the mean change from baseline were considered to be outliers and were excluded from analyses except where both pre- and post-vaccination measures exceeded this limit. Paired t-tests were conducted to compare serum levels of proinflammatory proteins pre- and post-vaccination. To estimate the time course of response, analysis of covariance (ANCOVA) models were fit to responses from each time point with the baseline value included as a covariate. Estimates and standard errors were obtained for each follow-up time and plotted in comparison to the overall baseline mean. Coefficients of variation (CV = 100% × SD/mean) for change from baseline to post-vaccination at each time point and standard deviations of change scores are reported to summarize variability of responses.
Results
Demographic and Behavioral Characteristics
Demographic and behavioral characteristics of the study sample are presented in Table 1. Reflecting the demographic characteristics of the women served at the OSU General Perinatal clinic, women in the study were predominately African-American (61.7%). The average age was = 24.43 (SD = 4.38). Women were predominately in the late first to early second trimester at the time of vaccination [average weeks gestation = 15.1 (SD = 8.1)]. Few women endorsed receiving seasonal influenza vaccination in the previous year (n=5, 10.6%).
Table 1.
Demographic Characteristics
| Follow-Up Timepoint | ||||
|---|---|---|---|---|
| One day (n=15) | Two days (n=10) | One week (n=21) | Total (n=46) | |
| Age | 22.71 (3.92) | 24.31 (4.0) | 25.66 (4.6) | 24.43 (4.38) |
|
| ||||
| Race | ||||
| African-American | 10 (66.7%) | 5 (50%) | 14 (63.6%) | 29 (61.7%) |
| Caucasian | 3 (20%) | 4 (40%) | 7 (31.8%) | 14 (29.8%) |
| Other | 2 (13.3%) | 1 (10%) | 1 (4.5%) | 4 (8.5%) |
|
| ||||
| BMI | 26.94 (6.92) | 28.83 (10.6) | 26.82 (6.01) | 27.28 (7.34) |
|
| ||||
| Weeks Gestation | 13.9 (6.9) | 12.9 (6.8) | 16.9 (9.2) | 15.1 (8.1) |
|
| ||||
| Gravidity | 2.4 (1.5) | 3.6 (2.5) | 3.2 (1.5) | 3.0 (1.8) |
|
| ||||
| Current Smoker | 4 (26.7%) | 3 (30%) | 5 (22.7%) | 12 (25.5% |
|
| ||||
| Hours Sleep Prior to Vaccination | 6.4 (1.9) | 7.0 (1.2) | 7.5 (1.6) | 7.0 (1.7) |
|
| ||||
| Vaccinated Previous Year | 2 (13.3%) | 1 (10%) | 2 (9.1%) | 5 (10.6%) |
Women assessed at one day, two days, and one week post-vaccination were not significantly different in age (F(2,44) = 2.15, p =0.13), body mass index (F(2,44) = 0.50, p =0.61), gravidity (F(2,44) = 1.6) p = 0.21), or race (X2 (4) = 2.01, p =0.74). In terms of health behaviors, women in these three groups did not differ significantly in rates of smoking (X2(2) = 0.21, p =0.90), hours of sleep in the night prior to vaccination (F(2,44) = 1.83), p = 0.17), or rates of vaccination in the previous year (X2(2) = 0.17, p =0.92).
Inflammatory Responses Following Influenza Virus Vaccination
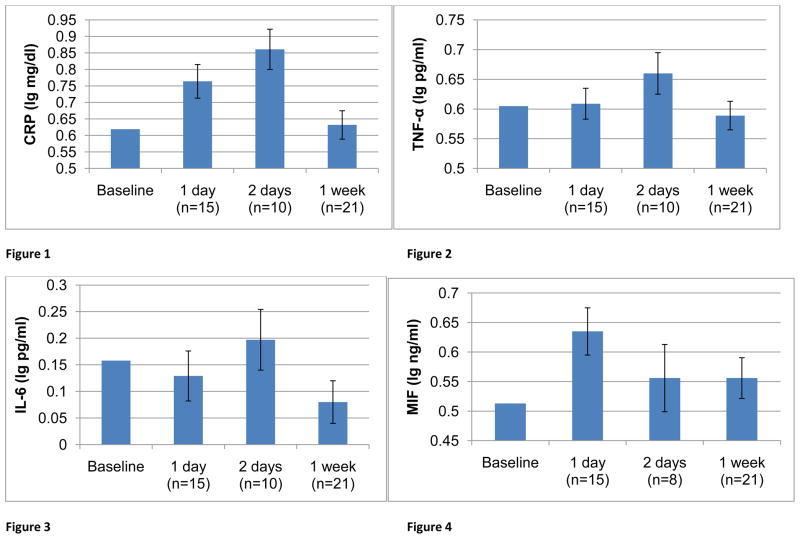
Paired t-tests indicated that compared to baseline, increases in CRP were seen at one day post-vaccination (mean increase 0.19 lg mg/dl (95% CI: 0.08, 0.30), t(14) = 3.76, p = 0.002) and two days post-vaccination; (0.24 lg mg/dl (0.03, 0.45), t(9) = 2.58, p = 0.03; Figure 1). When assessed at one week post-vaccination, CRP levels were not significantly different than baseline; (−0.07 lg mg/dl (−0.15, 0.01), t(21) = −0.41, p = 0.69). For TNF-α, a similar pattern of results was seen (Figure 2). As compared to baseline, no significant increase was seen at one day post-vaccination (−0.0004 lg pg/ml (−0.08, 0.08), t(14) = −0.01, p = 0.991). However, increases in TNF-α approached statistical significance at two days post-vaccination (0.04 lg pg/ml (−0.002, 0.08), t(9) = 2.15, p = 0.06). At one week post-vaccination, TNF-α levels were comparable to baseline (−0.01 lg pg/ml (−0.04, 0.03), t(20) = 0.30, p= 0.77). A decrease in IL-6 at one week post-vaccination also approached statistical significance (mean decrease −0.07 lg pg/ml (−0.15, 0.005), t(19) = −1.97, p = 0.06). Changes in MIF and IL-6 did not reach statistical significance at any timepoint (ps ≥ 0.46 other than IL-6 at one week). MIF measures for two subjects were excluded as outliers (low baseline MIF).
Figure 1–4. Inflammatory responses following seasonal influenza virus vaccination among pregnant women.
Analyses utilized paired t-tests at baseline and follow-up for each set of data. Data are presented using a normalized baseline value for illustrative purposes.
Considerable variability in response to vaccination was evidenced (Table 2). For each marker at each timepoint, the standard deviation in change scores from baseline to post-vaccination was equivalent or greater in magnitude than the mean increase. Coefficients of variation for change at two days post-vaccination ranged from 122% to 728%, with the greatest variability in IL-6 responses at this timepoint.
Table 2.
Mean Change from Baseline and Coefficients of Variation in Change Scores
| Cytokine | Mean (SD) Change from Baseline (log10 scale) | CV in Change from Baseline (log10 scale) | ||||
|---|---|---|---|---|---|---|
| One Day (n=15) | Two Days (n=10) | One Week (n=21) | One Day (n=15) | Two Days (n=10) | One Week (n=21) | |
| CRP | 0.19 (0.20) | 0.24 (0.29) | −.02 (0.19) | 103% | 122% | 1126% |
| IL-6 | −0.02 (0.24) | 0.02 (0.16) | −0.07 (0.17) | 991% | 728% | 227% |
| TNF-α | 0.00 (0.15) | 0.04 (0.06) | −0.01 (0.08) | ---† | 147% | 1521% |
| MIF | 0.04 (0.18) | −0.08 (0.22)* | −0.02 (0.17) | 512% | 283% | 778% |
n=8
absolute mean change < 0.0005; CV not reported
Discussion
These data demonstrate that seasonal influenza virus vaccination elicits a significant inflammatory response among pregnant women. The response was most robust at two days post-vaccination for C-reactive protein, with a similar, though nonsignificant, pattern of response for TNF-α. No statistically significant changes in IL-6 or MIF were evidenced. However, the power to detect effects was limited by sample size. Thus, despite changes in immune regulation previously reported in pregnancy, vaccination resulted in a transient inflammatory response among pregnant women. As there was no nonpregnant comparison group in this study, and participants in previous studies are not demographically equivalent to the current study [39, 40], the magnitude or duration of response during pregnancy versus nonpregnancy is unknown.
The utility of vaccination as a model for examining differential risk for adverse outcomes is dependent on sufficient variability in responses, allowing for meaningful classification of individuals as high versus low responders. In the current study, substantial variability was evidenced, as indicated by large standard deviations relative to mean increases and resulting high coefficients of variation. From a research standpoint, the presence of this variability may be of greater importance than the average magnitude of response overall. For example, we have previously reported that pregnant women with greater depressive symptoms exhibited significant inflammatory responses in terms of serum MIF at one week post-vaccination although there was not a statistically significant increase in MIF among women overall [41].
The assessment of in vivo inflammatory responses and factors affecting such responses has important applications in the context of pregnancy. Pregnancy is a period of tight inflammatory control. Attenuation of inflammatory responses during pregnancy has been reported in both human and animal models [24, 26–28] and lack of such adaptation has been associated with adverse pregnancy outcomes. For example, peripheral blood mononuclear cells (PBMCs) stimulated in vitro with antigen or mitogen showed decreased proinflammatory cytokine production and increased antiinflammatory cytokine production during healthy pregnancy ending in full term delivery as compared to nonpregnancy, with the strongest effects seen in the third trimester [28]. In contrast, among pregnancies that subsequently ended in miscarriage or small for gestational age babies, PBMCs exhibited greater proinflammatory cytokine production and reduced antiinflammatory cytokine production as compared to cells from both nonpregnant women and healthy pregnancies [28].
Inflammatory processes may also contribute to gestational hypertension and preeclampsia which affect 6–8% of all pregnancies and are responsible for 40% of medically-indicated preterm deliveries [31]. These disorders are characterized by high levels of circulating inflammatory markers [31, 32, 42–45]. Many features of preeclampsia, including impaired lipid metabolism and endothelial dysfunction, can be induced by proinflammatory cytokines [46] and the clinical severity of preeclampsia correlates with the degree of dysregulation seen in cytokine function [47]. Inflammation associated with infections may disrupt lipid metabolism and cause endothelial damage, predisposing women to the development of hypertensive disorders of pregnancy [48]. Therefore, women prone to exhibit exaggerated inflammatory responses to immune triggers may show increased risk of these disorders and related increased risk of preterm birth.
Studies in pregnant women to-date have relied on in vitro methodology. As compared to in vitro models, examination of similar processes in the in vivo setting provides rich data regarding inflammatory processes in the complex natural environment. Thus, vaccination provides a model that may be useful in understanding differences in risk for pregnancy-related conditions with an inflammatory component, including preterm birth and preeclampsia.
These research directions are suggested in relation to conceptualizing vaccination as a mild inflammatory trigger that may serve as a model for understanding a general propensity toward inflammatory responding. However, there are important gaps in our knowledge of the effects of vaccination on fetal health. Relatedly, the wisdom of universal vaccination for healthy pregnant women is a topic of debate [6, 7]. Due to the known risks of influenza infection in pregnancy and evidence for no effects of vaccination on birth outcomes including preterm labor, rates of C-section, or fetal malformation [8–19], vaccination is considered beneficial and is currently standardly recommended to all pregnant women [1]. There is strong evidence that exposure to infectious agents during the prenatal period influences developmental outcomes including stress reactivity, disease susceptibility, and risk for disorders including schizophrenia and cerebral palsy [49–54]. Thus, by preventing infection, vaccination may mitigate long-term risks for offspring health. The inflammatory response elicited by vaccination is considerably milder and more transient than that elicited by infection [55], arguing for protective benefits of vaccination. However, as demonstrated in the current study, there is substantial variability in the magnitude of response to vaccination. Thus, continued research is needed to further delineate whether the mild inflammatory response elicited by vaccination is benign for fetal development. In particular, birth cohort studies examining maternal cytokine responses to vaccination in conjunction with long-term offspring health outcomes are warranted [7].
Despite current recommendations for routine immunization, historically, vaccination coverage among pregnant women in the U.S. has been low. According to data from the National Health Interview Survey (NHIS), only 11.3% of pregnant women were vaccinated during the 2008–2009 flu season [5]. Reflecting substantial public health efforts and greater public awareness of risk during the 2009–2010 influenza pandemic, coverage of pregnant women was markedly higher; it is estimated that 50.7% of pregnant women received seasonal vaccination and 46.6% received the 2009 H1N1 vaccine [56]. Among pregnant women who did not receive seasonal vaccine during the 2009–2010 season, 47.7% indicated safety concerns for the baby and 45.2% indicated safety concerns for themselves were a factor [56]. Similarly, among women who didn’t receive the novel H1N1 vaccine, 63.6% cited safety concerns for the baby and 61.4% had safety concerns for themselves. Utilizing vaccination as research model promotes current clinical recommendations and may ultimately serve to increase vaccine acceptance by provision of greater safety data.
An important limitation of this study is that women were assessed primarily in the first trimester or early second trimester of pregnancy and that no nonpregnant comparison group was assessed. Due to the small sample size, assessment of effects of stage of gestation or statistically controlling for stage of gestation was not possible. Prior data on in vitro inflammatory responses indicate that, as compared to nonpregnant women, pregnant women show attenuation of inflammatory responses which is greatest during the third trimester [28]. The extent to which similar effects are seen in the context of in vivo immune triggers is unknown. In addition, this study did not permit examination of effects of behavioral, demographic, or psychosocial variables on the trajectory of inflammatory responses. Key potential modifiers of this response include race, smoking, sleep, body mass index, prior vaccine exposure, and prior influenza exposure which may be influenced by the number of children in one’s household. A clear future direction using vaccination as an in vivo model is to examine the extent to which these factors predict differential inflammatory responding.
Women in this study were assessed during two different influenza seasons; however, data at one and two days post-vaccination were collected in the same influenza season and this is when an inflammatory response was noted. This time course of response is similar as that noted in nonpregnant samples [34, 40], thus we believe these effects are reliable. However, it is certainly possible that the inflammatory response to vaccination is affected by the specific viral strains in a given flu season, in part due to different rates of prior exposure to specific strains. Future research using larger samples assessed in different vaccine years would help to address this issue. In this study, a decrease in IL-6 from baseline to one-week post-vaccination approached statistical significance (p = 0.06). Additional studies are needed to document if this is a consistent and reliable effect. If so, this may indicate a temporary “overshooting” as the body regains homeostasis following exposure to an inflammatory trigger. Finally, each woman was assessed at only a single follow-up timepoint. Women assessed at each follow-up timepoint were recruited from the same clinic and were demographically similar in terms of age, race, BMI, and parity. However, because the kinetic across different individuals likely differs, even minimal differences between groups may affect responses at a given timepoint. Thus, future research should ideally follow the same women at multiple timepoints to most clearly describe the inflammatory response trajectory following vaccination.
In sum, this study demonstrates that trivalent influenza virus vaccine (TIV) elicits a measurable inflammatory response during pregnancy, and that considerable variability is seen between women in the magnitude of this response. Thus, vaccination may serve as a useful model for examining individual differences in propensity toward inflammatory responses to immune triggers; this model may have implications for understanding risk of adverse pregnancy outcomes. The inflammatory response elicited by TIV is substantially milder and more transient than seen in infectious illness, arguing for the clinical value of vaccination. However, given the current clinical recommendation of routine immunization of healthy pregnant women, further research is warranted to confirm that the mild inflammatory response elicited by vaccination is benign in pregnancy.
Highlights.
Examined inflammatory responses to trivalent influenza virus vaccine (TIV) in pregnant women
Significant increases in serum CRP were seen at one and two days after vaccination
TIV elicits measurable and highly variable inflammatory responses
TIV may be useful as an in vivo model to examine inflammatory processes in pregnancy
Research is needed to confirm that the mild inflammatory response to TIV is benign in pregnancy
Acknowledgments
Manuscript preparation was supported by NICHD (R21HD061644 and R21HD067670). The project described was also supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008 Jan;8(1):44–52. doi: 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 2.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009 Dec;201(6):547–52. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 Influenza A(H1N1) Virus Illness Among Pregnant Women in the United States. Jama-J Am Med Assoc. 2010 Apr 21;303(15):1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The American College of Obstetricians and Gynecologists. Committe Opinion Number 468: Influenza Vaccination During Pregnancy. Obstet Gynecol. 2010;116(4):1006–07. doi: 10.1097/AOG.0b013e3181fae845. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, et al. Prevention and control of influenza with vaccines: Recommendations of the Advisordy Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR. 2010 [PubMed] [Google Scholar]
- 6.Ayoub DM, Yazbak FE. A closer look at influenza vaccination during pregnancy. Lancet Infect Dis. 2008 Nov;8(11):660–1. doi: 10.1016/S1473-3099(08)70236-6. author reply 1–3. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011 Mar;37(2):284–90. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black SB, Shinefield HR, France EK, Fireman BH, Platt ST, Shay D. Effectiveness of influenza vaccine during pregnancy in preventing hospitalizations and outpatient visits for respiratory illness in pregnant women and their infants. Am J Perinatol. 2004 Aug;21(6):333–9. doi: 10.1055/s-2004-831888. [DOI] [PubMed] [Google Scholar]
- 9.Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005 Apr;192(4):1098–106. doi: 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenberg L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol. 1973 Autumn;2(3):229–35. doi: 10.1093/ije/2.3.229. [DOI] [PubMed] [Google Scholar]
- 11.Heinonen OP, Slone D, Shapiro S. Immunizing agents. In: Kaufman DW, editor. Birth Defects and Drugs in Pregnancy. Boston, MA: Littleton Publishing Sciences Group; 1977. pp. 614–321. [Google Scholar]
- 12.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008 Oct 9;359(15):1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 13.France EK, Smith-Ray R, McClure D, Hambidge S, Xu S, Yamasaki K, et al. Impact of maternal influenza vaccination during pregnancy on the incidence of acute respiratory illness visits among infants. Arch Pediatr Adolesc Med. 2006 Dec;160(12):1277–83. doi: 10.1001/archpedi.160.12.1277. [DOI] [PubMed] [Google Scholar]
- 14.Yeager DP, Toy EC, Baker B. Influenza vaccination in pregnancy. Am J Perinatol. 1999;16(6):283–6. doi: 10.1055/s-2007-993873. [DOI] [PubMed] [Google Scholar]
- 15.Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis. 1993 Sep;168(3):647–56. doi: 10.1093/infdis/168.3.647. [DOI] [PubMed] [Google Scholar]
- 16.Deinard AS, Ogburn P., Jr A/NJ/8/76 influenza vaccination program: effects on maternal health and pregnancy outcome. Am J Obstet Gynecol. 1981 Jun 1;140(3):240–5. doi: 10.1016/0002-9378(81)90267-2. [DOI] [PubMed] [Google Scholar]
- 17.Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis. 1979 Aug;140(2):141–6. doi: 10.1093/infdis/140.2.141. [DOI] [PubMed] [Google Scholar]
- 18.Murray DL, Imagawa DT, Okada DM, St Geme JW., Jr Antibody response to monovalent A/New Jersey/8/76 influenza vaccine in pregnant women. J Clin Microbiol. 1979 Aug;10(2):184–7. doi: 10.1128/jcm.10.2.184-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulka JF. Effectiveness of Polyvalent Influenza Vaccine in Pregnancy - Report of Controlled Study during Outbreak of Asian Influenza. Obstet Gynecol. 1964;23(6):830. [PubMed] [Google Scholar]
- 20.Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011 May;8(5):e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988 Feb;45(2):189–92. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 22.Takei N, Mortensen PB, Klaening U, Murray RM, Sham PC, O’Callaghan E, et al. Relationship between in utero exposure to influenza epidemics and risk of schizophrenia in Denmark. Biol Psychiatry. 1996 Nov 1;40(9):817–24. doi: 10.1016/0006-3223(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991 May 25;337(8752):1248–50. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 24.Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2004;90(1):95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- 25.Aguilar-Valles A, Poole S, Mistry Y, Williams S, Luheshi GN. Attenuated fever in rats during late pregnancy is linked to suppressed interieukin-6 production after localized inflammation with turpentine. Journal of Physiology-London. 2007 Aug 15;583(1):391–403. doi: 10.1113/jphysiol.2007.132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashdown H, Poole S, Boksa P, Luheshi GN. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2007 Apr;292(4):R1667–R74. doi: 10.1152/ajpregu.00274.2006. [DOI] [PubMed] [Google Scholar]
- 27.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, et al. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. J Clin Endocrinol Metab. 2001 Oct;86(10):4933–8. doi: 10.1210/jcem.86.10.7905. [DOI] [PubMed] [Google Scholar]
- 28.Marzi M, Vigano A, Trabattoni D, Villa ML, Salvaggio A, Clerici E, et al. Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin Exp Immunol. 1996 Oct;106(1):127–33. doi: 10.1046/j.1365-2249.1996.d01-809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MMK, Hassan NA, Bandar A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol. 1999 Nov;42(5):273–81. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 30.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007 Jan;109(1):121–7. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]
- 31.Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonat M. 2006 Oct;11(5):309–16. doi: 10.1016/j.siny.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–11. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 33.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999 Feb;180(2):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 34.Posthouwer D, Voorbij HAM, Grobbee DE, Numans ME, van der Bom JG. Influenza and pneumococcal vaccination as a model to assess C-reactive protein response to mild inflammation. Vaccine. 2004;23(3):362–5. doi: 10.1016/j.vaccine.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 35.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000 Aug 29;102(9):994–9. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 36.van der Beek MT, Visser LG, de Maat MPM. Yellow fever vaccination as a model to study the response to stimulation of the inflammation system. Vasc Pharmacol. 2002 Aug;39(3):117–21. doi: 10.1016/s1537-1891(02)00297-5. [DOI] [PubMed] [Google Scholar]
- 37.Doherty JF, Golden MHN, Raynes JG, Griffin GE, Mcadam KPWJ. Acute-Phase Protein Response Is Impaired in Severely Malnourished Children. Clin Sci. 1993 Feb;84(2):169–75. doi: 10.1042/cs0840169. [DOI] [PubMed] [Google Scholar]
- 38.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003 Oct;60(10):1009–14. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 39.Carty CL, Heagerty P, Nakayama K, McClung EC, Lewis J, Lum D, et al. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Atertio Thromb Vasc Biol. 2006 Dec;26(12):2738–44. doi: 10.1161/01.ATV.0000248534.30057.b5. [DOI] [PubMed] [Google Scholar]
- 40.Tsai M, Hanson N, Straka R, Hoke T, Ordovas J, Peacock J, et al. Effect of influenza vaccine on markers of inflammation and lipid profile. J Lab Clin Med. 2005;145:323–327. doi: 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory response to in vivo immune challenge during human pregnancy. Brain, Behav, Immun. 2010;24(1):49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;286:R989–R90. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 43.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–9. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 44.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–22. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 45.Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;43:708–14. doi: 10.1161/01.HYP.0000143849.67254.ca. [DOI] [PubMed] [Google Scholar]
- 46.Page NM. The endocrinology of pre-eclampsia. Clin Endocrinol (Oxf) 2002;57:413–23. doi: 10.1046/j.1365-2265.2002.01626.x. [DOI] [PubMed] [Google Scholar]
- 47.Madazli R, Aydin S, Ocak SU, Tolun V, Tolun N. Maternal plasma levels of cytokines in normal and preeclampltic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet Gynecol Scand. 2003;82:797–802. doi: 10.1034/j.1600-0412.2003.00206.x. [DOI] [PubMed] [Google Scholar]
- 48.Herrera JA, Chaudhuri G, Lopez-Jaramillo P. Is infection a major risk factor for preeclampsia? Med Hypotheses. 2001 Sep;57(3):393–7. doi: 10.1054/mehy.2001.1378. [DOI] [PubMed] [Google Scholar]
- 49.Samuelsson A-M, Ohrn I, Dahlgren J, Eriksson E, Angelin B, Folkow B, et al. Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology. 2004;145(11):4897–911. doi: 10.1210/en.2004-0742. [DOI] [PubMed] [Google Scholar]
- 50.Reul JMHM, Stec I, Wiegers GJ, Labeur MS, Linthorst ACE, Arzt E, et al. Prenatal immune challenge alters the hypothalamic-pituitary-adrenalcortical axis in adult rats. J Clin Invest. 1994;93:2600–7. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schendel D, Schuchat A, Thorsen P. Public health issues related to infection and pregnancy and cerebral palsy. Disability Res Rev. 2002;8:39–45. doi: 10.1002/mrdd.10011. [DOI] [PubMed] [Google Scholar]
- 52.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. Br J Obstet Gynaecol. 2003;110(20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 53.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayden FG, Fritz RS, Lobo MC, Alvord WG, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection - Relation to symptom formation and host defense. J Clin Invest. 1998 Feb 1;101(3):643–9. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal Influenza Vaccine Coverage Among Pregnant Women: Pregnancy Risk Assessment Monitoring System. J Womens Health. 2011 May;20(5):649–51. doi: 10.1089/jwh.2011.2794. [DOI] [PubMed] [Google Scholar]