Abstract
9cUAB30 is a synthetic analog of 9-cis-retinoic acid with chemopreventive activity in cell lines and in animal models. The purpose of this first-in-human evaluation of 9cUAB30 was to evaluate the single-dose pharmacokinetic profile and toxicity of the compound in healthy volunteers at 3 dose levels. This study enrolled 14 patients to receive a single dose of 5, 10, or 20 mg of 9cUAB30. Plasma and urine samples were collected to assess 9cUAB30 concentrations by a validated LC/MS MS method. 9cUAB30 was well tolerated, with 1 patient experiencing grade 2 toxicity and no grade 3 or 4 toxicities reported. Tmax occurred approximately 3 hours after dose administration with the plasma half-life ranging from 2.79 to 7.21 hours. AUC increased linearly across the examined dose range of 5 to 20 mg; Cmax was proportional to the log of the dose. The plasma clearance ranged from 25 to 39 L/h compared to the renal clearance which ranged from 0.018 to 0.103 L/h. 9cUAB30 has a favorable toxicity and pharmacokinetic profile, with oral availability and primarily hepatic metabolism. Further dose ranging studies with once a day dosing are underway.
Introduction
Retinoids, chemical derivatives of vitamin A, are promising agents for cancer chemoprevention (1, 2). Retinoids (RA) act by binding to retinoic acid receptors (RAR) and/or retinoid X receptors (RXR; ref. 3). These nuclear receptors contain domains for binding to both the ligand molecules and DNA, acting ultimately as ligand-induced transcription factors which regulate cellular processes including differentiation and apoptosis (3, 4). Because of their role in differentiation, apoptosis, and loss of proliferation, RA are able to selectively target highly proliferative cells, making them potential agents for chemoprevention (5).
Current RA, including natural vitamin A derivatives, have unacceptably severe adverse effects, including teratogenicity, mucocutaneous cytotoxicity, and hypertriglyceridemia, the latter two of which are attributed to RAR binding (6, 7). However, as RA suppress tumor growth in multiple tissues, the search for vitamin A derivatives with lower toxicity continues (8).
9cUAB30 is a synthetic analog of 9-cis-RA with little or no RAR-binding activity relative to 9-cis-RA and other RA (9-11). Several analogs of the UAB Class II RA have been synthesized, but 9cUAB30 is the most selective retinoid for activating RXR without elevating serum triglycerides (TG). 9cUAB30 has been assessed in vitro with human cell cultures (5, 12). Human hepatocytes demonstrated no signs of cytotoxicity with treatment of 9cUAB30 up to 50 μmol/L (12), although when human breast cancer cells were treated with 9cUAB30, they showed a significant inhibition of cell proliferation and apoptotic levels 2.5 to 3.5 times the levels of untreated cells (5).
The chemopreventive activity and toxicity of 9cUAB30 has been tested in animal models (3, 9, 10). At a dose level of 200 mg/kg/d diet, 9cUAB30 decreased N-methyl-N-nitrosourea (MNU)-induced mammary cancer incidence by 63% compared to controls (10). When 9cUAB30 was coadministered with tamoxifen, the combination additionally exhibited a significant reduction in the size of established tumors. Animals that received 9cUAB30 (150 mg/kg of diet) had an average of 4.3 tumors per animal; tamoxifen (0.4 mg/kg of diet), 4.6 tumors per animal; 9cUAB30 (150 mg/kg of diet) + tamoxifen (0.4 mg/kg of diet), 2.6 tumors per animals; compared to controls with an average of 6.0 tumors per animal (3). Serum TG levels were measured in rats after 7 days of treatment with the 9-cis-RA 60 mg/kg of diet or 9cUAB30 dosed at 200 or 800 mg/kg of diet. Whereas 9-cis-RA significantly increased (5-fold) serum TGs compared to untreated controls, 9cUAB30 did not affect serum TG levels at either dose (10). The results of these studies suggest that 9cUAB30 is active in preventing carcinogenesis. In addition, toxicity was not reported even when doses that are significantly higher than needed for chemopreventive activity were tested (3, 9-11).
The purpose of this first-in-human evaluation of 9cUAB30 was to evaluate the single-dose pharmacokinetic profile and toxicity of the compound in healthy volunteers at 3 dose levels.
Materials and Methods
Subject eligibility and recruitment
Healthy adults between the age of 18 and 65 years with an Eastern Cooperative Oncology Group (ECOG) performance status of 1 or less than 1 (Karnofsky performance status ≥ 80%) were eligible for this study. See Table 1. Subjects were required to have the following: WBC ≥3,000/ μL; platelets ≥100,000/μL, hemoglobin >10 g/dL, bilirubin ≤1.4 mg/dL; aspartate aminotransferase (AST) ≤1.5 × normal; TG ≤1.5 × ULN, and cholesterol ≤1.5 × ULN as well as normal renal and electrolyte levels. Participants were excluded if they were taking St. John’s wort, ketoconazole, vitamin A, tetracycline, lipid-lowering agents, oral corticosteroids or any other RA. Subjects were also excluded if they were taking other investigational agents, had a history of allergic reactions attributed to compounds similar in composition to 9cUAB30, had an uncontrolled intercurrent illness, or were pregnant. The study was approved by the Institutional Review Board, and all subjects provided written informed consent.
Table 1.
Baseline characteristics of enrolled patientsa
| Characteristic | 5 mg (n = 4) | 10 mg (n = 6) | 20 mg (n = 4) |
|---|---|---|---|
| Female, n (%) | 2 (50) | 3 (50) | 2 (50) |
| White, n (%) | 4 (100) | 6 (100) | 4 (100) |
| Age, yb | 55.3 ± 6.1 | 45.0 ± 10.8 | 34.8 ± 8.5 |
| Body mass, kgb | 71.7 ± 15.8 | 81.1 ± 21.9 | 105.3 ±11.3 |
| Body mass index, kg/mb | 26.2 ± 4.5 | 28.6 ± 6.2 | 33.9 ± 3.0 |
| Systolic blood pressure, mmHg | 131.8 ± 9.7 | 126.8 ± 12.0 | 124.3 ± 6.8 |
| Diastolic blood pressure, mmHg | 73.2 ± 5.2 | 77.2 ± 8.5 | 78.2 ± 8.5 |
Presented as % of group or mean ± SD.
P < 0.05 for 5-mg vs. 20-mg groups
Trial design
This pilot clinical trial was designed to characterize the single dose pharmacokinetics of 9cUAB30 in humans at a single dose of 5, 10, or 20 mg of 9cUAB30 given orally in the morning on an empty stomach. The first subject was to receive the 5-mg dose, and after 30 days without a grade 2 or higher toxicity, 3 more subjects were to also receive the 5-mg dose. Without grade 2 or higher toxicities observed among the initial 4 subjects, the 4 subject cohorts of 10 mg and 20 mg were then to be dosed sequentially. Following dosing, subjects were to stay for 24 hours as inpatients, during which time urine samples were collected at 0, 6, 12, 18, and 24 hours after 9cUAB30 administration. Plasma samples were collected at 0, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, 16, 20, and 24 hours postdose. All samples were stored at −70 °C for less than 3 months. Subjects returned on day 8 for pharmacokinetic sample collection and toxicity evaluation and were contacted on day 30 to assess health status and any adverse events related to the study agent. Toxicity was graded using the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE; ref. 13). All adverse events were followed until resolution to grade 1 or less.
Bioanalytical methodology
9cUAB30 was quantitated in heparinized plasma and human urine after storage of up to 3 months using LC-MS/MS detection with a method similar to Kane et al. (14). Liquid-liquid extraction and sample preparation was performed as follows: added 10 μL of 5 μg/mL of 9-cis-RA (internal standard) to 0.5 mL sample, vortexed, added 1 mL of 0.025 mol/L of KOH in ethanol, vortexed, added 5.0 mL of hexane, agitated on an orbital shaker for 10 minutes, centrifuged at 1,200 rpm for 10 minutes, removed and discarded the top organic layer, added 1 mL of acetonitrile and 60 μL of 4 mol/L of HCl, vortexed, added 5 mL of hexane, agitated on an orbital shaker for 10 minutes, centrifuged at 1,200 rpm for 10 minutes, transferred the organic layer to a glass tube, and dried down under N2, reconstituted residue with 50 μL of acetonitrile, vortexed, added 50 μL of 0.5% formic acid, vortexed, placed into autosampler vial fitted with an insert for small volumes.
LC-MS/MS data were obtained on an Applied Biosystems/ MDS Sciex API 4000 in positive ion mode for 9cUAB30 and 9-cis-RA (internal standard). The multiple reaction monitoring (mrm) transitions were m/z 295.1 → m/z 165.1 for UAB30, m/z 301.15 → m/z 201.2 for 9-cis-RA. The following settings for the MS/MS were used. Positive ion instrument parameters were: curtain gas (CUR) 12 units, gas1 (GS1) 40 units, gas2 (GS2) 42 units, collision gas (CAD) 7 units, interface heater (IHE) on, nebulizer current 5.00, declustering potential (DP) 40 V, exit potential (EP) 15 V. For 9cUAB30, collision exit potential (CXP) 30 V, collision energy (CE) 71 V, dwell 210 milliseconds. For 9-cis-RA, CXP 10 V, CE 25, dwell 1,000 milliseconds.
A 15 × 4.6-mm SB C18 (Agilent) 1.8-micron HPLC column was used as the analytical column, and 5 μL samples were injected. The mobile phase solvents were A) 0.1% formic acid in water, and B) 0.1% formic acid in acetonitrile. These solvents were mixed and delivered as a gradient at 800 μL/min as follows: 80% A/20% B for 0.5 minutes; ramped to 25% A/75% B over 1.5 minutes and held for 2.0 minutes; ramped to 80% A/20% B over 0.5 minute and held for 2.0 minutes.
Samples were quantitated by linear regression from a 6-point standard curve ranging from 1.56 to 100 ng/mL with a trend line r2 of 0.998 over the range. This quantitative method’s lower limit of quantitation (LLOQ) was 1.56 ng/ mL, and the lower limit of detection (LOD) was 0.78 ng/ mL. Recovery of 9cUAB30 from plasma was greater than 80% compared to water standards. Intraday variability was 0.7% for low-standard triplicates (1.56 ng/mL) and 7.2% for high-standard triplicates (50 ng/mL). The interday variability over 35 days was 14% for a low standard (3.125 ng/mL), and 11% for a high standard (50 ng/ mL). Low, medium, and high concentration quality control (QC) samples were prepared by spiking plasma or urine with a known amount of 9cUAB30, and analytical runs were rejected if QC samples varied by less than 15% from the expected concentration.
Stability
To assess stability, blank human urine and plasma were spiked with a 9cUAB30 stock standard solution (0.2 mL of 1 μg/mL UAB30 + 4.8 mL blank plasma) to obtain con-centrations of 10 ng/mL (plasma and urine), 40 ng/mL (plasma and urine), and 100 ng/mL (urine) and stored at −70 °C. Both the plasma and urine standards were stored for 12 weeks and analyzed in triplicate to assess stability of 9cUAB30 over time.
Pharmacokinetic analyses
Pharmacokinetic variables were determined by noncom-partmental methods with WinNonlin Pro version 5.2 (Pharsight Corporation). Area under the plasma concentration-time curve (AUC) was estimated using the trapezoidal rule from time 0 to peak concentration, and the trapezoidal rule from the peak concentration to the last measurable plasma concentration (AUClast). AUC (0-∞) was then calculated from the time of dosing and extrapolated to infinity. Renal clearance was calculated with the following equation Clr = X/24h/AUC0-24h, where X24h was the total amount excreted in nanograms over 24 hours.
Statistical considerations
The primary objective was to characterize the single-dose pharmacokinetics of 9cUAB30 in normal volunteers. Each of the pharmacokinetic parameters were examined by dose level and in aggregate with basic statistics including means and standard deviations. In general, Jonckheere-Terpstra trend test was performed to determine the significance of the association between increasing dose level and each of the pharmacokinetic measures. The relationships between the parameters AUC and Cmax with dose were especially of interest and were examined using regression models. These measures are by definition 0 for an undosed subject. To fulfill this constraint (that a subject receiving no dose would be predicted to have no measurable Cmax or AUC), models with no intercept were used.
The secondary objectives were to determine the toxicities of 9cUAB30 after administration of a single dose and to correlate the pharmacokinetics of 9cUAB30 with toxicity. All grade II and any grade I toxicities that occur in more than 1 patient have been recorded in Table 2. As only 1 patient had any grade II adverse events, correlation of pharmacokinetics with maximum grade of adverse event was not feasible. A relationship between dose and change in TG levels was tested using the Jonckheere-Terpstra test.
Table 2.
All grade II adverse events and any grade I adverse events occurring in more than 1 patient (n = 14)
| Toxicity | Grade I (%) | Grade II (%) |
|---|---|---|
| Reduced hemoglobin | 3 (21) | 0 |
| Rash | 2 (14) | 0 |
| Fatigue | 0 | 1 (7) |
| Diarrhea | 2 (14) | 0 |
| Nausea and vomiting | 0 | 1 (7) |
| Dizziness | 0 | 1 (7) |
| Headache | 7 (50) | 0 |
| Elevation in triglycerides | 2 (14) | 0 |
| Elevation in cholesterol | 3 (21) | 0 |
| Nasal congestion | 2 (14) | 0 |
| Decreased anion gap | 2 (14) | 0 |
| Elevated chloride | 2 (14) | 0 |
Results
Subjects
Between November 2008 and May 2009, 14 consented volunteers were treated with single doses of 9cUAB30, 4 subjects at 5 mg, 6 subjects at 10 mg, and 4 subjects at 20 mg. Subjects were 50% male and 50% female and all were Caucasian. 9cUAB30 concentrations were detected in the plasma and urine samples collected from each participant, allowing for the pharmacokinetic analysis of the compound.
The baseline patient characteristics, such as age, gender, race, weight, height, and blood pressure, are summarized in Table 1.
Toxicity
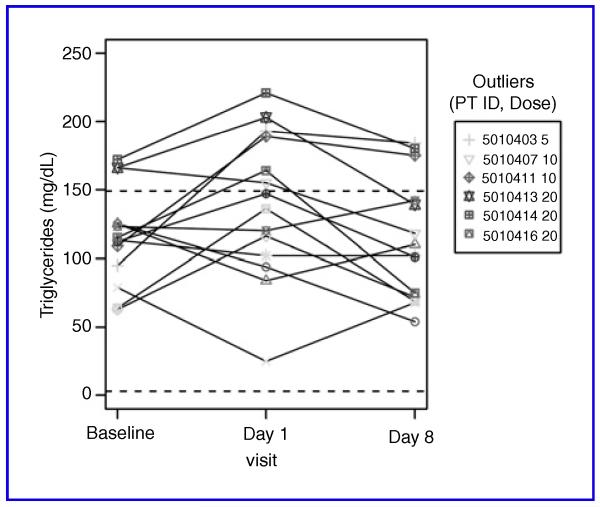
Table 2 lists the frequency of observed adverse events. Grade 1 headache was the most common adverse event, occurring later in day 1. One subject in the 10-mg cohort had grade 1 nasal congestion and the sudden onset of grade 1 and 2 dizziness (positional without any neurologic deficits) followed quickly by grade 1 and 2 nausea, vomiting, and fatigue on day 1. These symptoms were significantly abated by day 2 with some grade 1 dizziness remaining. Due to the occurrence of grade 2 toxicity, this cohort was expanded to a total of 6 subjects (3 men, 3 women) with no further observations of ≥ grade 2 toxicity. No subjects at dose level 5 mg or 20 mg experienced ≥ grade 2 toxicity. The observed laboratory abnormalities included 3 patients with grade 1 cholesterol elevations and 2 patients with grade 1 TG elevations, although several subjects also had decreases in TG levels. Overall, the differences between the treatment groups in change in TG levels from baseline were not statistically significant. Mean TG levels at screening were 116 ± 34.8, 139 ± 53.7 on day 1 (24 hours after 9cUAB30 administration) and 114 ± 44.5 on day 8. Individual TG levels are shown in Figure 1.
Fig. 1.
TG levels for individual subjects at baseline, day 1, and day 8. Normal range lies between the dotted lines.
9cUAB30 pharmacokinetic parameters and stability
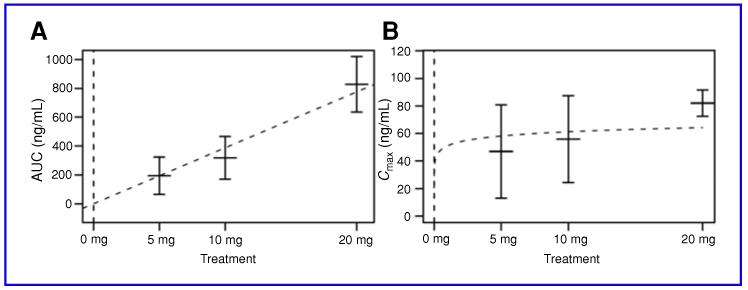
When taken orally, 9cUAB30 appeared at quantifiable levels in subject plasma, with Tmax occurring approximately 2 to 3 hours after dose administration. After Tmax, 9cUAB30 levels declined steadily. Plasma half-life increased in a dose-dependent manner (P = 0.0015), averaging 2.79 hours in the 5-mg group, 5.76 hours in the 10-mg group, and 7.21 hours for the 20-mg group. In regression analysis, AUC increased linearly across the examined dose range of 5 to 20 mg (see Fig. 2A); a linear relationship between AUC and dose was found to be significant (β = 38.7, P < 0.001). Cmax, although increasing with increased dose, was not linear with dose (see Fig. 2B); it was found to be proportional to the log of dose (β = 4.43, P < 0.001). The average plasma clearance ranged from 25 to 39 L/h compared to the average renal clearance which ranged from 0.018 to 0.103 L/h. The examined pharmacokinetic parameters are summarized in Tables 3 and 4. In addition, 9cUAB30 is stable for 12 weeks at −70 °C in both plasma and urine.
Fig. 2.
A and B, means and standard error bars of AUC and Cmax by treatment group shown, along with fitted curves representing chosen models.
Table 3.
9cUAB30 pharmacokinetic parameters (n = 13)
| Tmax,h | Half-life, h | Cmax, ng/mL | AUC 0-inf, ng/mL hr |
Cl, L/h | V, L | Clr, L/h | |
|---|---|---|---|---|---|---|---|
| 5 mga (n = 3) | 3.12 ± 3.25 | 2.79 ± 0.80 | 46.9 ± 34.5 | 193 ±114 | 39.3 ± 34.3 | 138 ± 86.0 | 0.085 ± 0.050 |
| 10 mg (n = 6) | 2.08 ± 1.02 | 5.76 ± 0.76 | 55.9 ± 39.4 | 318 ± 184 | 40.4 ± 19.7 | 337 ± 174 | 0.035 ± 0.049 |
| 20 mg (n = 4) | 2.88 ± 1.31 | 7.21 ± 1.04 | 82.0 ± 9.77 | 827 ±196 | 25.1 ± 5.00 | 258 ± 49.0 | 0.232 ± 0.060 |
Most 9cUAB30 plasma concentrations were detectable, but below the limit of quantitation in 1 subject in the 5-mg dose cohort and this patient was excluded from analysis.
Table 4.
9cUAB30 urine concentrations (n = 14)
| Baseline | 0–6 h, ng/mL | 6–12 h, ng/mL | 12–18 h, ng/mL | 18–24 h, ng/mL | |
|---|---|---|---|---|---|
| 5 mg (n = 4) | 0.00a | 7.88 ± 3.87 | 1.49 ± 2.18 | 0.00a | 0.00a |
| 10 mg (n = 6) | 0.00a | 4.68 ± 4.32 | 2.72 ± 4.25 | 0.73 ± 1.78 | 0.48 ±1.17 |
| 20 mg (n = 4) | 0.00a | 48.0 ± 6.68 | 34.3 ± 17.4 | 34.9 ± 7.64 | 23.5 ± 13.0 |
These represent values lower than the limit of detection.
Discussion
This first-in-human pharmacokinetic study demonstrates that 9cUAB30 is orally available and has both a favorable pharmacokinetic and toxicity profile. Observed toxicities were not likely secondary to 9cUAB30. Grade 1 headache was common, but all subjects with headache stated the exclusion of any caffeine-containing products throughout day 1 was likely a major contributor. All grade 2 toxicity occurred in 1 patient treated at the 10-mg dose level. Further discussion with the 1 subject with grade 2 events (nausea, vomiting, dizziness) revealed that work colleagues had the occurrence of similar symptoms around the same time (over the course of a weekend) which they attributed to a gastrointestinal virus. There was no apparent pattern in the serum TGs from baseline to day 2 or day 8.
The half-life ranged from 2.8 hours at the 5-mg dose to 7.2 hours at the 20-mg dose. Plasma concentrations for the 5-mg dose were near the lower end of assay sensitivity by approximately 8 hours and undetectable by 12 hours. From a practical standpoint, the half-life is the time required for the plasma concentration to decrease by 50%, and the calculated half-life will be affected by assay sensitivity and dose, particularly at the lower ranges of both. So, while plasma concentrations at later time points were undetectable due to limitations in assay sensitivity, values were unlikely to be zero and the shorter half-life observed at the 5-mg dose is most likely explained by undetectable plasma concentrations at later time points rather than a dose-dependent half-life.
9cUAB30 is orally available with a Tmax occurring approximately 2 to 3 hours after administration of the dose. Subjects were fasted so the effect of food on 9cUAB30 absorption is unknown. Also notable is the large volume of distribution and small proportion of 9cUAB renally excreted, suggesting extensive tissue binding and hepatic elimination, consistent with other RA.
A single 20-mg dose of 9cUAB30 achieves maximum plasma concentrations of approximately 70 ng/mL in healthy human volunteers. Preclinical studies of 9cUAB30 demonstrated transcriptional activation of an RXR-responsive promoter reporter construct at an EC50 = 118 nmol/L (34 ng/mL), and RXR receptor-binding assays in competition with radiolabelled 9cRA at an IC50 = 284 nmol/L (82 ng/mL; ref. 15). Hansen and colleagues demonstrated that 0.1 μmol/L (29 ng/mL) of 9cUAB30 inhibits colony formation to 62% of untreated cells (16). Although 1 μmol/L concentration of 9cUAB30 (corresponding to 294 ng/mL) was required to prevent KLF4-mediated tumor initiation (17), this is only 3 times the plasma concentration achieved in this single dose study, and plasma concentrations associated with RXR activation and inhibition of colony formation were achieved. A single 20-mg dose of 9cUAB30 achieves plasma concentrations of approximately 80 ng/mL. Assuming 1 compartment, first-order elimination, a dose of 60 mg would be required to achieve maximal concentrations of 0.294 μg/mL. Given the lack of toxicity at the 20-mg dose, escalation to 60 mg may be feasible. This study suggests linearity over the 5- to 20-mg dose range, however, if nonlinearity of dose is observed at subsequent dose levels, as typically occurs with drugs eliminated via the liver, a lower dose would likely achieve these concentrations.
Conclusion
This is a first-in-human study of the novel retinoid 9cUAB30. Preliminary data suggest both a favorable toxicity profile as seen in the preclinical testing in animals, 9cUAB30 did not significantly increase change in serum TGs and pharmacokinetic profile, with oral availability and primarily hepatic metabolism. The Cmax for the 20-mg dose level was 80 ng/mL, exceeding concentrations needed for activation of the RXR receptor in preclinical models. Further doseranging studies with once a day dosing are underway.
Acknowledgment
The authors thank the study participants for making this research possible. The authors also thank the University of Wisconsin Carbone Comprehensive Cancer Center (UWCCC) 3P laboratory for use of its facilities to complete this research.
Grant Support
This research was supported by a contract from the National Cancer Institute’s Division of Cancer Prevention, a component of the National Institutes of Health in the U.S. Department of Health and Human Services. Supported by the NCI, N01-CN-35153-6 (Phase I and II Clinical Trials of Chemoprevention Agent), Grant M01 RR03186 from the General Clinical Research Centers Program of the National Cancer for Research Resources, National Institutes of Health. This work is supported in part by NIH/NCI P30 CA014520-UW Comprehensive Cancer Center Support.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Zanardi S, Serrano D, Argusti A, Barile M, Puntoni M, Decensi A. Clinical trials with retinoids for breast cancer chemoprevention. Endocr Relat Cancer. 2006;13:51–68. doi: 10.1677/erc.1.00938. [DOI] [PubMed] [Google Scholar]
- 2.Crowe DL, Chandraratna RAS. A retinoid X receptor (RXR)-selective retinoid reveals that RXR-α is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands. Breast Cancer Res. 2004;6:R546–55. doi: 10.1186/bcr913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubbs CJ, Hill DL, Bland KI, Beenken SW, Lin TH, Eto I, et al. 9cUAB30, an RXR specific retinoid, and/or tamoxifen in the prevention of methylnitrosourea-induced mammary cancers. Cancer Lett. 2003;201:17–24. doi: 10.1016/s0304-3835(03)00461-0. [DOI] [PubMed] [Google Scholar]
- 4.Love KW, DeAngelis TJ, Berletch JB, Phipps SM, Andrews LG, Brouillette WJ, et al. The novel retinoid, 9cUAB30, inhibits telomerase and induces apoptosis in HL60 cells. Transl Oncol. 2008;1(3):148–52. doi: 10.1593/tlo.08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen NJ, Wylie RC, Phipps SMO, Love WK, Andrews LG, Tollefsbol TO. The low-toxicity 9-cis UAB30 novel retinoid down-regulates the DNA methyltransferases and has anti-telomerase activity in human breast cancer cells. Int J Oncol. 2007;30:641–50. [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka T, De Luca LM. Therapeutic potential of “rexinoids” in cancer prevention and treatment. Cancer Res. 2009;69(12):4945–7. doi: 10.1158/0008-5472.CAN-08-4407. [DOI] [PubMed] [Google Scholar]
- 7.Zusi CF, Lorenzi MV, Vivat-Hannah V. Selective retinoids and rexi-noids in cancer therapy and chemoprevention. Drug Discov Today. 2002;7:1165–74. doi: 10.1016/s1359-6446(02)02526-6. [DOI] [PubMed] [Google Scholar]
- 8.Brtko J, Thalhamer J. Renaissance of the biologically active vitamin A derivatives: established and novel directed therapies for cancer and chemoprevention. Curr Pharm Design. 2003;9:2067–77. doi: 10.2174/1381612033454144. [DOI] [PubMed] [Google Scholar]
- 9.Atigadda VR, Vines KK, Grubbs CJ, Hill DL, Beenken SL, Bland KI, et al. Conformationally defined retinoic acid analogues. 5. Large-scale synthesis and mammary cancer chemopreventive activity for (2E,4E,6Z,8E)-8-(3′,4′-Dihydro-1′(2′H)-naphthalen-1′-ylidene)-3,7-dimethyl-2,4,6-octatrienoic Acid (9cUAB30) J Med Chem. 2003;46:3766–9. doi: 10.1021/jm030095q. [DOI] [PubMed] [Google Scholar]
- 10.Grubbs CJ, Lubet RA, Atigadda VR, Christov K, Deshpande AM, Tirmal V, et al. Efficacy of new retinoids in the prevention of mammary cancers and correlations with short-term biomarkers. Carcinogenesis. 2006;27:1232–9. doi: 10.1093/carcin/bgi308. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Deng W, Bailey SK, Nail CD, Frost AR, Brouillette WJ, et al. Prevention of KLF4-mediated tumor initiation and malignant trans-formation by UAB30 rexinoid. Cancer Biol Ther. 2009;8:289–298. doi: 10.4161/cbt.8.3.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson PJ, Kabirov KK, Izet KM, Lyubimov A. In vitro assessment of P450 induction potential of novel chemopreventive agents SR13668, 9-cis-UAB30, and pentamethychromanol in primary cultures of human hepatocytes. Chem-Biol Interact. 2009;179:263–272. doi: 10.1016/j.cbi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute . Common Terminology Criteria for Adverse Events, version 3.0. National Cancer Institute; 2006. revised August 9. Available from: http://ctep.cancer.gov. [Google Scholar]
- 14.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–8. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muccio DD, Brouillette WJ, Breitman TR, Taimi M, Emanuel PD, Zhang X, et al. Conformationally defined retinoic acid analogues 4. Potential new agents for acute promyelocytic and juvenile myelomonocytic leukemias. J Med Chem. 1998;41:1679–87. doi: 10.1021/jm970635h. [DOI] [PubMed] [Google Scholar]
- 16.Hansen NJ, Wylie RC, Phipps SM, Love WK, Andrews LG, Tollefsbol TO. The low-toxicity 9-cis UAB30 novel retinoid down-regulates the DNA methyltransferases and has anti-telomerase activity in human breast cancer cells. Int J Oncol. 2007;30(3):641–50. [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang W, Deng W, Bailey SK, Nail CD, Frost AR, Brouillette WJ, et al. Prevention of KLF4-mediated tumor initiation and malignant transformation by UAB30 rexinoid. Cancer Biol Ther. 2009;8(3):289–98. doi: 10.4161/cbt.8.3.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]