To the Editor: Spread of multidrug-resistant Neisseria gonorrhoeae is a major public health concern. Effective antimicrobial therapy is a key element in gonorrhea control. However, N. gonorrhoeae has developed resistance to multiple classes of antimicrobial drugs, including β-lactams, tetracyclines, and fluoroquinolones (1–3). Even an extended-spectrum oral cephalosporin-resistant, cefixime-resistant N. gonorrhoeae has emerged, and cefixime has now been withdrawn from use in Japan. Best practice treatment is limited to injectable extended-spectrum cephalosporins, such as ceftriaxone and spectinomycin. The emergence of ceftriaxone-resistant N. gonorrhoeae threatens effective disease control.
We identified a novel ceftriaxone-resistant N. gonorrhoeae isolated from a 31-year-old female commercial sex worker; MIC of ceftriaxone for this isolate was high (2 µg/mL). The woman visited a clinic in Kyoto for a routine examination for sexually transmitted infections in January 2009. Although she had no obvious symptoms or signs, a throat sample collected on her first visit yielded a positive result for N. gonorrhoeae by the strand displacement amplification test (ProbeTec ET, Becton Dickinson, Franklin Lakes, NJ, USA), but a vaginal sample taken at the same time was negative. After 2 weeks, another throat sample was positive for N. gonorrhoeae when cultured on Thayer-Martin medium, and the patient subsequently received 1 g ceftriaxone intravenously. Her pharyngeal sample was also N. gonorrhoeae positive by strand displacement amplification test on the third visit 2 weeks later, and further ceftriaxone treatment was prescribed. However, a culture for test of cure was not conducted because reinfection was considered. A negative result was finally obtained in April 2009.
The culture showed positive reactions in oxidase and catalase tests. Gram staining showed gram-negative diplococci. The ID-test HN-20 Rapid system (Nissui, Tokyo, Japan) classified the bacterium as N. gonorrhoeae. Susceptibility was determined by the agar dilution method (4). For this strain, named H041, MIC of ceftriaxone was high (2 µg/mL), and the strain was highly resistant to penicillin G (4 µg/mL), cefixime (8 µg/mL), and levofloxacin (32 µg/mL). However, it demonstrated susceptibility to spectinomycin (16 µg/mL) and reduced susceptibility to azithromycin (0.5 µg/mL).
To characterize the ceftriaxone-resistant N. gonorrhoeae H041, multilocus sequence typing characterized the strain as ST7363 (5), which is the predominant sequence type (ST) among cefixime-resistant clones (6). N. gonorrhoea multiantigen sequence typing (NG-MAST) was also performed (7). The NG-MAST strategy uses 2 genes, por and tbpB, for porin and a transferrin-binding protein, respectively. NG-MAST indicated that the strain H041 was ST4220 and contained the por2594 allele and the tbpB10 allele. NG-MAST 4220 is a novel ST. However, the tbpB10 allele is the most frequently observed allele (76.5%) among multilocus sequence typing-ST7363 N. gonorrhoeae strains (n = 81) (M. Ohnishi, unpub. data).
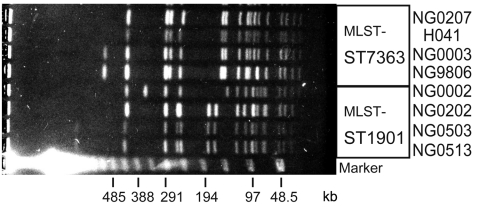
Molecular typing suggested that the novel ceftriaxone-resistant N. gonorrhoeae, H041, is closely related to the ST7363 cefixime-resistant N. gonorrhoeae. Therefore, we compared SpeI-digested genomic DNA banding patterns of strain H041 with those of other N. gonorrhoeae strains by using pulsed-field gel electrophoresis as described (8). Four ST7363 strains, including N. gonorrhoeae H041, and 4 ST1901 strains (another major ST among cefixime-resistant N. gonorrhoeae strains) (6) were analyzed. The banding pattern of SpeI digested H041 genomic DNA was similar to that of other ST7363 strains and indistinguishable from that of cefixime-resistant but ceftriaxone-susceptible NG0207 (Figure).
Figure.
Pulsed-field gel electrophoresis patterns of ceftriaxone-resistant Neisseria gonorrhoeae strain H041 and other multilocus sequence typing (MLST) ST7363 and ST1901 strains. SpeI-digested genomic DNA from ceftriaxone-resistant N. gonorrhoeae H041, 3 of the MLST ST7363 strains and 4 of the MLST ST1901 strains were analyzed by pulsed-field gel electrophoresis. A lambda ladder standard (Bio-Rad, Hercules, CA, USA) was used as a molecular size marker.
We describe the emergence of ceftriaxone-resistant N. gonorrhoeae, isolated from a pharyngeal specimen from a female commercial sex worker. At 2 µg/mL, the MIC was 4-fold higher than that of the previously reported ceftriaxone nonsusceptible strain (9). Our susceptibility testing suggests that only azithromycin and spectinomycin are effective drugs for treating this strain. In this case, eradication was successful, although N. gonorrhoeae colonization of the pharynx may just be tempory because the pharynx is not an ideal site for N. gonorrhoeae growth. From the routine examinations of commercial sex workers during January–March 2009, 40 N. gonorrhoeae were isolated in the clinic, but no other ceftriaxone-resistant strains were isolated. There is no evidence of dissemination of this strain in Kyoto.
Three independent molecular subtyping methods indicated that the ceftriaxone-resistant H041 strain was N. gonorrhoeae, and it might originate from an ST7363 cefixime-resistant N. gonorrhoeae clone. There are several possible mechanisms for the acquisition of resistance, including formation of a new mosaic type penA allele as penA-X cefixime resistance and acquisition of an extended-spectrum β-lactamase gene. The H041 strain did not produce β-lactamase in a nitrocephin test. Further molecular analysis is needed to elucidate the precise mechanism of the ceftriaxone resistance of the H041 strain.
The emergence of ceftriaxone-resistant N. gonorrhoeae raises concerns for controlling gonorrhea because ceftriaxone is widely recommended and the first-line treatment for gonorrhea around the world. N. gonorrhoeae has a potential to gain an extraordinarily high MIC to ceftriaxone. Surveillance for ceftriaxone-resistant N. gonorrhoeae should be strengthened.
Acknowledgment
We thank Hiroko Matsuoka for her technical assistance.
This study was supported by grants-in-aid from the Ministry of Health, Labour and Welfare of Japan (H21-Shinko-Ippan-001, and −012).
Footnotes
Suggested citation for this article: Ohnishi M, Saika T, Hoshina S, Iwasaku K, Nakayama S, Watanabe H, et al. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan [letter]. Emerg Infect Dis [serial on the Internet]. 2011 Jan [date cited]. http://dx.doi.org/10.3201/eid1701.100397
References
- 1.Tapsall JW, Ndowa F, Lewis DA, Unemo M. Meeting the public health challenge of multidrug- and extensively drug-resistant Neisseria gonorrhoeae. Expert Rev Anti Infect Ther. 2009;7:821–34. 10.1586/eri.09.63 [DOI] [PubMed] [Google Scholar]
- 2.Workowski KA, Berman SM, Douglas JM Jr. Emerging antimicrobial resistance in Neisseria gonorrhoeae: urgent need to strengthen prevention strategies. Ann Intern Med. 2008;148:606–13. [DOI] [PubMed] [Google Scholar]
- 3.Tapsall J. Antibiotic resistance in Neisseria gonorrhoeae is diminishing available treatment options for gonorrhea: some possible remedies. Expert Rev Anti Infect Ther. 2006;4:619–28. 10.1586/14787210.4.4.619 [DOI] [PubMed] [Google Scholar]
- 4.Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing, 19th informational supplement. CLSI document M100–S19. Wayne (PA): The Institute; 2009. [Google Scholar]
- 5.Jolley KA. Multi-locus sequence typing. In: Pollard AJ Maiden MCJ, editors. Meningococcal disease: methods and protocols. Totowa (NJ): Humana Press; 2001. p. 173–86. [Google Scholar]
- 6.Ohnishi M, Watanabe Y, Ono E, Takahashi C, Oya H, Kuroki T, et al. Spreading of a chromosomal cefixime resistant penA gene among different Neisseria gonorrhoeae lineages. Antimicrob Agents Chemother. 2010;54:1060–7. 10.1128/AAC.01010-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin IM, Ison CA, Aanensen DM, Fenton KA, Spratt BG. Rapid sequence-based identification of gonococcal transmission clusters in a large metropolitan area. J Infect Dis. 2004;189:1497–505. 10.1086/383047 [DOI] [PubMed] [Google Scholar]
- 8.Ito M, Yasuda M, Yokoi S, Ito S, Takahashi Y, Ishihara S, et al. Remarkable increase in central Japan in 2001–2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cepharosporins, and fluoroquinolones. Antimicrob Agents Chemother. 2004;48:3185–7. 10.1128/AAC.48.8.3185-3187.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Nakayama H, Huruya K, Konomi I, Irie S, Kanayama A, et al. Analysis of mutations within multiple genes associated with resistance in a clinical isolate of Neisseria gonorrhoeae with reduced ceftriaxone susceptibility that shows a multidrug-resistant phenotype. Int J Antimicrob Agents. 2006;27:20–6. 10.1016/j.ijantimicag.2005.08.021 [DOI] [PubMed] [Google Scholar]