Abstract
We report 2 cases of babesiosis in immunocompetent patients in France. A severe influenza-like disease developed in both patients 2 weeks after they had been bitten by ticks. Diagnosis was obtained from blood smears, and Babesia divergens was identified by PCR in 1 case. Babesiosis in Europe occurs in healthy patients, not only in splenectomized patients.
Keywords: Babesia, Anaplasma, babesiosis, tick-borne infection, parasite, zoonoses, immunocompetent, Europe, dispatch
Babesiosis, a tick-borne infectious disease that occurs worldwide, is caused by species of Babesia, an intraerythrocytic parasite (1). Babesia spp. parasites infect wild and domesticated animals and may cause a malaria-like syndrome. The first human case was described in 1957 in a splenectomized Yugoslavian farmer who died (2). More than 100 Babesia species infect animals, but human infection has been associated with only a few species, mainly B. microti and B. divergens (1–3). B. microti parasites are transmitted by Ixodes scapularis ticks and infect rodents. Since 1957, these parasites have caused hundreds of human babesiosis cases in the United States, the most affected country. Infections are found mainly in healthy persons and manifest as asymptomatic or mild to moderate illness; severe disease, even in immunocompromised or elderly patients, is seldom reported (2,3). B. divergens parasites are endemic to Europe; they are transmitted by I. ricinus ticks and infect bovines (4). In Europe, the disease is rare in humans; ≈40 cases have been reported (2,3,5–7). These cases are almost exclusively severe in immunocompromised patients, especially those whose spleens have been removed (2,3,8). B. divergens parasites are responsible for >70% of these cases (2,8), although the disease is not always confirmed by molecular-based methods.
We report 2 cases of human babesiosis in Colmar, Alsace, a northeastern region of France in which Lyme disease is endemic. The disease was diagnosed in spring 2009 in healthy young persons without history of travel abroad who experienced a marked influenza-like syndrome and recovered. These cases should change the classic description of babesiosis in Europe, in which the disease was thought to affect immunocompromised patients exclusively. Our study indicates that this disease also occurs in Europe among immunocompetent patients.
Case Reports
Patient 1, a 37-year-old woman without known medical history, sought treatment on April 29, two weeks after a tick bite. She had a 38.5°C fever with chills, headaches, and arthromyalgia. Results of a physical examination were normal. Laboratory findings included leukopenia (3,300 leukocytes/µL, 45% polymorphonuclear cells, 37% lymphocytes), aspartate aminotransferase and alanine aminotransferase levels of 136 IU/L and 160 IU/L, respectively; γ-glutamyl transpeptidase 135 IU/L; alkaline phosphatase 131 IU/L; and elevated C-reactive protein level (48 mg/L). Serologic results for Lyme disease, tick-borne encephalitis virus, tularemia, Anaplasma spp., Coxiella burnetii, and Rickettsia spp., as well as blood cultures were negative.
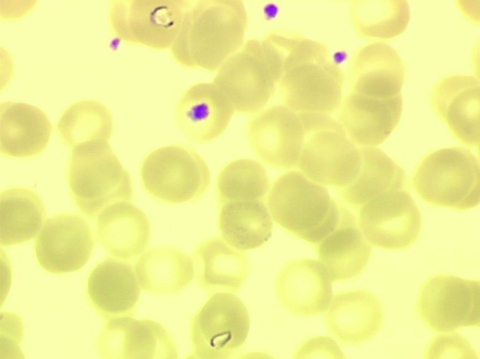
A thin peripheral blood smear stained with May-Grünwald-Giemsa did not show any ehrlichial morulae in granulocytes, as suspected, but a retrospective examination of stored slides on May 22 (the same day that case 2 was characterized) showed pear forms and trophozoites of intraerythrocytic parasites (parasitemia level 0.29%), leading to the diagnosis of babesiosis (Figure 1). The patient had initially received doxycycline (200 mg/d) for a suspected bacterial tick-borne infection, and her symptoms rapidly resolved. The first blood sample was discarded, but on June 16 and June 24, additional serum and whole blood samples were collected in sodium citrate vacutainer tubes (Becton-Dickinson, Franklin Lakes, NJ, USA). The blood smears remained positive until July but were negative in August.
Figure 1.
Two trophozoites, pear-shaped, of Babesia divergens in erythrocytes from case-patient 1 (original magnification ×1,000, May-Grünwald-Giemsa stain).
Patient 2, a 35-year-old man with an uneventful medical history, was hospitalized on May 21, two weeks after receiving 3 tick bites. He had a 39°C fever, severe headaches, and arthromyalgia. Results of a physical examination were normal. Laboratory findings showed leukopenia (1,860 leukocytes/µL, with 35% polymorphonuclear leukocytes, 49% lymphocytes), marked thrombopenia with 36,000 platelets/mm3, elevated liver enzyme levels (aspartate aminotransferase 70 IU/L, alanine aminotransferase 77 IU/L, γ-glutamyl transferase 161 IU/L, and alkaline phosphatase 86 IU/L), and elevated C-reactive protein (124 mg/L). Tick-transmitted disease serologic results and blood cultures were negative.
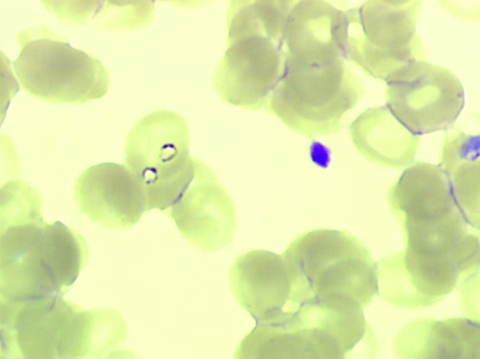
A thin peripheral blood smear stained with May-Grünwald-Giemsa showed intraerythrocytic Babesia spp. (parasitemia level 0.23%) (Figure 2). The patient received azithromycin 500 mg on day 1 then 250 mg/day plus atovaquone, and his illness rapidly resolved. Two samples of serum and whole blood were collected in sodium citrate vacutainer tubes on June 16 and July 21 and sent to the veterinary laboratory of Nantes for B. divergens serologic analysis (indirect immunofluorescent assay by using the gerbil-derived strain B. divergens Rouen F5 antigen), erythrocyte cultures, and DNA extraction (Wizard genomic DNA Purification kit; Promega, Madison, WI, USA) for PCR Babesia spp (9). .
Figure 2.
Two trophozoites of Babesia spp. in 1 erythrocyte from case-patient 2 (original magnification ×1,000, May-Grünwald-Giemsa stain).
Serologic results and cultures remained negative for both patients. However, serologic analysis is neither sensitive nor specific (7,10), and cultures probably were inhibited because blood samples were collected after doxycycline or azithromycin proguanil treatments. The PCR for Babesia spp. is specific for an 18S rDNA 540-base long region of a variable part of the gene with Bab primers GF2 and GR2, was performed (9,11). Results were positive for patient 1. The sequencing of PCR products showed 100% homology with B. divergens human strains GenBank accession nos. FJ944822 and FJ944823 (9). PCR results were negative for patient 2. Samples from patient 2 were collected 1 month after treatment with atovaquone-proguanil, and the blood smear was negative. However, the clinical and biological data and the observation of trophozoites (especially 2 trophozoites in 1 erythrocyte) in the blood smear from patient 2 confirmed by a reference laboratory led us to strongly suspect babesiosis (Figure 2). In this case, the Babesia species remains unknown, and a non–B. divergens species cannot be ruled out, although it is rarer.
Conclusions
Our cases highlight that, in Europe, babesiosis can occur in healthy persons and manifest as moderate illness. The rarity of other reported cases in nonimmunocompromised patients in Europe may be related to the difficulty of diagnosing babesiosis. A stained thin blood smear is rarely performed in Europe after tick bite in healthy patients. The difficulty of detecting intra-erythrocytic forms of babesia coupled with frequent low levels of parasitemia, may result in accurate diagnoses, although acridine orange and fluorescent microscopy may assist in the detection of parasites (1). Other diagnostic tests, such as PCR and serologic analysis, are not routinely performed in France and require a reference laboratory (8).
Babesiosis, although difficult to diagnose, needs to be diagnosed for various reasons: 1) without treatment, babesiosis can lead to severe illness; 2) the disease can persist for a long period without symptoms, which could lead to posttransfusion cases (12); and 3) effective specific treatments are available (atovaquone plus azithromycin, or for severe cases, clindamycin and quinine) (2). These drugs are not usually prescribed in febrile tick-bite cases; doxycycline is the usual drug used to treat tick-borne bacterial diseases. Moreover, patients with moderate infection could benefit from an atovaquone plus azithromycin regimen, which is better tolerated (13).
Previous serosurveys from tick-exposed patients or healthy blood donors in France (7), Germany (14), and Switzerland (15) have demonstrated antibodies against Babesia spp. antigens ranging from 1.0% to 11.5%. These data suggest that Babesia spp. infections probably occur more frequently in Europe than previously believed and may affect healthy patients. Although most patients may be asymptomatic, our 2 cases demonstrate that babesiosis can result in a serious influenza-like syndrome in previously healthy patients. In Europe, babesiosis is probably underdiagnosed; thus, we suggest that when patients have influenza-like or malaria-like syndromes after confirmed or suspected tick bites, a blood smear be performed regardless of whether the patient is immunocompromised. Blood smear can identify not only Babesia spp. infection but also Anaplasma spp. infection, another emerging and underdiagnosed tick-borne illness. In cases of new European Babesia spp. infections, a deeper characterization of the strains by erythrocytes cultures and standardized PCR, as well as a systematic study of the patients’ immune systems, should be undertaken to enable a better understanding of this disease.
Biography
Dr Martinot is a physician at the Department of Internal Medicine and Rheumatology, Hospital Pasteur, Colmar, France. His specialty is infectious diseases and primary research interests are tick-borne diseases, procalcitonin, and infections in immunocompromised patients (HIV).
Footnotes
Suggested citation for this article: Martinot M, Zadeh MM, Hansmann Y, Grawey I, Christmann D, Aguillon S, et al. Babesiosis in immunocompetent patients, Europe. Emerg Infect Dis [serial on the Internet]. 2011 Jan [date cited]. http://dx.doi.org/10.3201/eid1701.100737
References
- 1.Homer MJ, Persing DH. Human babesiosis. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne diseases of humans. Washington: ASM Press; 2005. p. 343–60. [Google Scholar]
- 2.Vannier E, Krause PJ. Update on babesiosis. [PMID: 19727410]. Interdiscip Perspect Infect Dis. 2009;9845–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JA, Vannier E. Babesia species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases, 7th ed, Philadelphia: Elsevier; 2010. p. 3539–45. [Google Scholar]
- 4.L’Hostis M, Dumon H, Dorchies B, Boisdron F, Gorenflot A. Large scale survey of bovine babesiosis due to Babesia divergens in France. Vet Rec. 1995;136:36–8. 10.1136/vr.136.2.36 [DOI] [PubMed] [Google Scholar]
- 5.Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38:1219–37. 10.1016/j.ijpara.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Zintl A, Mulcahy G, Skerret HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16:622–36. 10.1128/CMR.16.4.622-636.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters TP. Human babesiosis. Ann Trop Med Parasitol. 1998;92:489–501. PMID: 9683900 [DOI] [PubMed]
- 8.Meliani P, Khatibi S, Randazzo S, Gorenflot A, Marchou B. Human babesiosis. Med Mal Infect. 2006;36:499–504. 10.1016/j.medmal.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 9.Malandrin L, Jouglin M, Sun Y, Brisseau N, Chauvin A. Redescription of Babesia capreoli (Enigk and Friedhoff, 1962) from roe deer (Capreolus capreolus): isolation, cultivation, host specificity, molecular characterization and differentiation from Babesia divergens. Int J Parasitol. 2010;40:277–84. 10.1016/j.ijpara.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 10.Rosenblatt JE. Laboratory diagnosis of infections due to blood and tissue parasites. Clin Infect Dis. 2009;49:1103–8. 10.1086/605574 [DOI] [PubMed] [Google Scholar]
- 11.Bonnet S, Jouglin M, L’hostis M, Chauvin A. Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg Infect Dis. 2007;13:1208–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubernot DM, Lucey CT, Lee KC, Conley GB, Holness LG, Wise RP. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration. Clin Infect Dis. 2009;48:25–30. 10.1086/595010 [DOI] [PubMed] [Google Scholar]
- 13.Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR III, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–8. 10.1056/NEJM200011163432004 [DOI] [PubMed] [Google Scholar]
- 14.Hunfeld KP, Lambert A, Kampen H, Albert S, Epe C, Brade V, et al. Seroprevalence of Babesia infections in humans exposed to ticks in midwest Germany. J Clin Microbiol. 2002;40:2431–6. 10.1128/JCM.40.7.2431-2436.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foppa IM, Krause PJ, Spielmann A, Goethert H, Gern L, Brand B, et al. Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg Infect Dis. 2002;8:722–6. [DOI] [PMC free article] [PubMed] [Google Scholar]