Abstract
To determine the prevalence of helminthic infections in Pursat Province, Cambodia, we tested fecal specimens from 471 children, 10–14 years of age, in June 2007. The prevalence of infection with echinostome flukes ranged from 7.5% to 22.4% in 4 schools surveyed. Adult worms were identified as Echinostoma revolutum.
Keywords: Parasites, Echinostoma revolutum, echinostomiasis, trematode, prevalence, children, Cambodia, dispatch
Echinostomes (family Echinostomatidae) are intestinal trematodes of birds and mammals, including humans. Echinostomiasis can result in severe epigastric or abdominal pain accompanied by diarrhea, easy fatigue, and malnutrition (1). Heavy worm loads may lead to death due to intestinal perforation or marked malnutrition and anemia, as has been reported for infection caused by an echinostome species, Artyfechinostomum malayanum (under the name Artyfechinostomum mehrai), in India (1).
A total of 20 species of echinostomes that belong to 8 genera (Echinostoma, Echinochasmus, Acanthoparyphium, Artyfechinostomum, Episthmium, Himasthla, Hypoderaeum, and Isthmiophora) infect humans worldwide (1). Echinostoma revolutum, the most widely distributed species, is found from Asia and Oceania to Europe and the Americas (1). The first reported human infection was in Taiwan in 1929 (2). The prevalence of E. revolutum flukes in Taiwan during 1929–1979 varied from 0.11% to 0.65% (3). Small E. revolutum–endemic foci or a few cases of human infection were discovered in the People’s Republic of China, Indonesia, and Thailand until 1994 (4,5). However, no information is available about human E. revolutum infection after 1994, even in areas where the parasite was previously endemic.
In Cambodia, humans are commonly infected with intestinal nematodes and protozoa, including hookworms, Strongyloides stercoralis, Ascaris lumbricoides, Trichuris trichiura, and Giardia lamblia (6,7). However, with the exception of the blood fluke Schistosoma mekongi, infection with trematodes or cestodes has seldom been reported (8). Echinostomatid eggs have been detected in schoolchildren in 2 provinces, Battambang and Kampongcham (9,10), but adult worms were not collected for identification. The Korea Association of Health Promotion, South Korea, and The National Institute of Malaria, Entomology, and Parasitology, Ministry of Health, Cambodia, have been conducting an international collaboration to control intestinal helminthiases in schoolchildren in Cambodia (2006–2011). In June 2007, we conducted a fecal survey in 4 primary schools in Pursat Province, Cambodia, and found that an average of 11.9% of schoolchildren had positive test results for echinostome eggs. Adult worms recovered after the children received treatment with praziquantel and underwent purgation with magnesium salts were identified as E. revolutum. We report echinostomiasis as an endemic trematode infection among schoolchildren in Pursat.
The Study
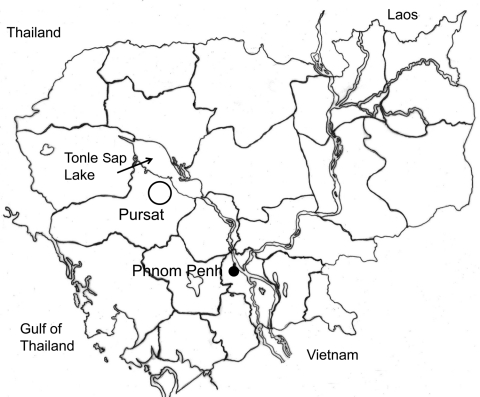
The surveyed areas were lakeside (the Tonle Sap Lake) villages in Pursat Province (Figure 1) where ≈12,000 persons, including 3,500 schoolchildren, live. For this study, 471 children (237 boys), 10–14 years of age, from 4 primary schools were selected. One fecal sample from each child was collected in June 2007. Samples were transported to the Malaria Station in Pursat within 2–3 days of collection and stored at 4°C until examination. The Kato-Katz thick smear technique was used to detect helminth eggs. Examination of feces and anthelmintic treatment were officially approved by the Ministry of Health, Cambodia, under the agreement of the Korea-Cambodia International Collaboration on Intestinal Parasite Control for Schoolchildren in Cambodia.
Figure 1.
Surveyed area (circle) near Tonle Sap Lake, Pursat Province, Cambodia.
Four children who had positive test results for echinostomatid eggs and who had occasional, vague abdominal pain and discomfort were selected for anthelmintic treatment and adult worm recovery at the Malaria Station. After we obtained consent from their parents and the school guardian, the children’s infections were treated with a single oral dose of 10 mg/kg praziquantel (Shinpoong Pharmceutical Co., Seoul, South Korea), and purged with 20 g magnesium sulfate. Whole diarrheic feces were collected 3–4 times and pooled individually. The diarrheic feces were processed as previously described (11). Worms were collected by using a wooden applicator and washed several times in water. They were fixed with 10% formalin under coverslip pressure, stained with acetocarmine, and identified by morphologic features.
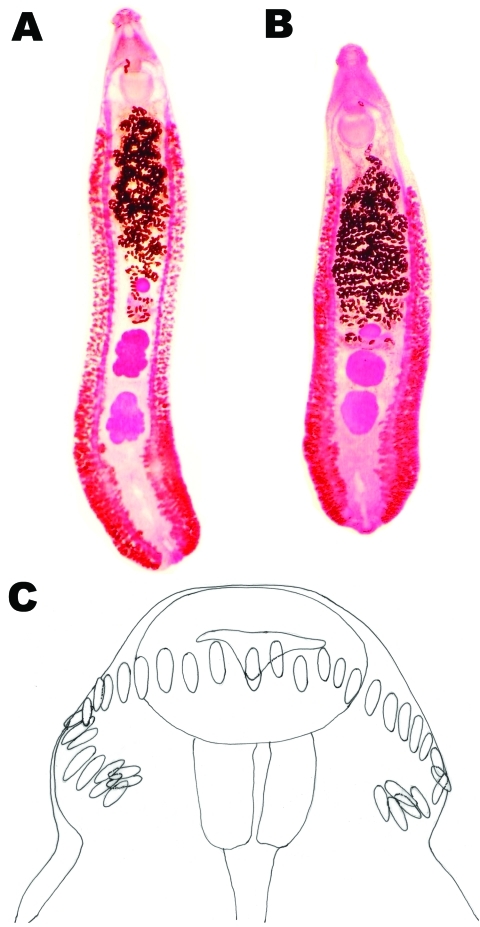
A total of 17.4% of samples were positive for helminth eggs. Echinostomatid eggs were found most frequently, followed by hookworm and Trichuris trichiura eggs (Table 1). The percentages of echinostome eggs were significantly higher in school A than in schools B, C, and D (Table 1). However, prevalence did not differ significantly (p<0.01) between boys and girls (data not shown). A total of 20 echinostome adults (12, 3, 3, and 2 worms) were recovered from 4 children who showed 48–120 eggs per gram of feces (Table 2). The worms were leaflike, elongated (Figure 2), and an average of 8.8 mm long (8.0–9.5 mm) and 1.7 mm wide (1.2–2.1 mm) (n = 10). When first passed in the feces, they were pinkish red and coiled in a “c” or “e” shape. The eggs in uteri were an average of 105 μm long (97–117 μm) and 63 μm wide (61–65 μm) (n = 10). On the basis of these characteristics, the worms were identified as E. revolutum (Froelich, 1802) Looss, 1899.
Table 1. Prevalence of intestinal helminths among schoolchildren, Pursat Province, Cambodia, June 2007*.
| School |
No. children examined |
No. (%) positive results for helminth eggs; 95% CI egg positive rate |
||||
| Echinostomes† |
Hookworms |
Trichuris trichuira eggs |
Others‡ |
Total§ |
||
| A | 116 | 26 (22.4); 14.8–30.0 | 4 (3.4); 0.1–6.7 | 1 (0.9); 0.0–2.6 | 1 (0.9); 0.0–2.6 | 31 (26.7); 18.6–34.8 |
| B | 117 | 12 (10.3); 4.8–15.8 | 12 (10.3); 4.8–15.8 | 0 | 3 (2.6); 0.0–5.5 | 26 (22.2); 14.7–29.7 |
| C | 118 | 9 (7.6); 2.8–12.4 | 0 | 0 | 1 (0.8); 0.0–2.4 | 10 (8.5); 3.5–13.5 |
| D | 120 | 9 (7.5); 2.8–12.2 | 7 (5.8) | 0 | 0 | 15 (12.5); 6.6–17.2 |
| Total | 471 | 56 (11.9); 9.0–14.8 | 23 (4.9); 3.0–6.8 | 1 (0.2); 0.0–0.6 | 5 (1.1); 0.2–2.0 | 82 (17.4); 14.0–20.8 |
*Determined by examination of feces using the Kato-Katz technique. Boldface indicates significant differences between schools A and B (p = 0.01); A and C (p = 0.004), and A and D (p = 0.004), as analyzed by z test.CI, confidence interval. †Most of these are presumed to be eggs of Echinostoma revolutum worms. ‡Includes eggs of Enterobius vermicularis (schools A and B) and Hymenolepis nana (school C) worms. ¶Total no. of schoolchildren positive for >1 helminth species.
Table 2. Recovery of Echinostoma revolutum worms from schoolchildren, Pursat Province, Cambodia, June 2007*.
| Child no. | Age, y | No. echinostome eggs/g† | No. E. revolutum specimens recovered‡ |
|---|---|---|---|
| 1 | 13 | 48 | 12 |
| 2 | 13 | 120 | 3 |
| 3 | 10 | 120 | 3 |
| 4 | 13 | 96 | 2 |
*All children were female. Fecal samples were collected individually 2–3 hours after administration of MgSO4. †Eggs/g of feces; amount in a typical sample was assumed to be 41.7 mg. ‡All recovered worms were adult worms that contained eggs.
Figure 2.
Echinostoma revolutum specimens recovered from schoolchildren in Pursat Province, Cambodia, which had 2 testes in the postequatorial region. A) An adult worm (8 mm long) showing lobulated testes. B) Another adult worm showing globular testes. C) Head collar of an adult specimen armed with 37 collar spines arranged in a single row, including 5 end-group spines on each side.
The major sources of E. revolutum infection in humans are freshwater clams (Corbicula producta) in Taiwan and snails (Physa occidentalis or Lymnaea sp.) in Thailand (1,5). According to school personnel, the children were fond of eating undercooked snails or clams of unidentified species sold on the road to their homes after school. They stated that the mollusks are caught near Tonle Sap Lake. Reasons for the higher prevalence in school A than schools B, C and D are unclear.
Conclusions
Of the schoolchildren living near Tonle Sap Lake, Pursat Province, Cambodia, who participated in this study, 7.5%–22.4%, depending on school, were infected with E. revolutum. E. revolutum trematodes are endemic parasites in this area of Cambodia and a likely source of infection is freshwater snails or clams from the lake. The public health significance of echinostomiasis and educational and prevention efforts should be highlighted.
Echinostomiasis is not only an endemic infectious disease in Asian countries, including Cambodia, but also can be imported by overseas travelers from the United States or Europe. An outbreak of echinostomiasis was reported among US travelers returning from Kenya and Tanzania, although the source of infection was uncertain (12). This diagnosis should also be considered in patients with abdominal pain and diarrhea who have traveled to Southeast Asia and eaten snails or clams.
Despite the dangerous nature of echinostomes, the study of echinostomiasis has been neglected for many decades (13,14), possibly because physicians and laboratory personnel lack knowledge about this trematode parasite. In addition, no easy diagnostic technique is available to detect echinostome eggs, except for routine fecal examination. However, some microscopists seem to overlook or misinterpret the presence of echinostome eggs, particularly in Kato-Katz fecal smears. Even if echinostome eggs are detected, the specific diagnosis is not possible unless the adult worm is collected and identified. Thus, both the training of microscopists and emphasis on the clinical significance of echinostomiasis are urgently needed.
Acknowledgments
We thank the staffs of the National Institute of Malaria, Entomology, and Parasitology, Ministry of Health, Cambodia, for preparing the Kato-Katz smears for the schoolchildren. We also thank Hyun-Mi Kim, Yu-Jung Kim, Jae-Young Park, Geun-Hoon Lee, Hoo-Gn Jeong, and Chong-Kyun Shin, Korea Association of Health Promotion, for their help in reading Kato-Katz fecal smears.
Biography
Dr Sohn is a professor in the Department of Parasitology and Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, South Korea. His major research area is foodborne zoonotic parasites, including echinostomes, heterophyids, and gnathostomes.
Footnotes
Suggested citation for this article: Sohn W-M, Chai J-Y, Yong T-S, Eom KS, Yoon C-H, Sinuon M, et al. Echinostoma revolutum infection in children, Pursat Province, Cambodia. Emerg Infect Dis [serial on the Internet]. 2011 Jan [date cited]. httphttp://dx.doi.org/10.3201/eid1701.100920
References
- 1.Chai JY. Echinostomes in humans. In: Fried B, Toledo R, editors. The biology of echinostomes. New York: Springer; 2009. p. 147–83. [Google Scholar]
- 2.Anazawa K. On a human case of Echinostoma revolutum and its infection route [in Japanese]. Taiwan Igakkai Zasshi. 1929;288:221–41. [Google Scholar]
- 3.Lu SC. Echinostomiasis in Taiwan. Int J Zoonoses. 1982;9:33–8. [PubMed] [Google Scholar]
- 4.Radomyos P, Radomyos B, Tungtrongchitr A. Multi-infection with helminthes in adults from northeast Thailand as determined by post-treatment fecal examination of adult worms. Trop Med Parasitol. 1994;45:133–5. [PubMed] [Google Scholar]
- 5.Yu SH, Mott KE. Epidemiology and morbidity of food-borne intestinal trematode infections. Trop Dis Bull. 1994;91:R125–52. [Google Scholar]
- 6.Sinuon M, Anantaphruti MT, Socheat D. Intestinal helminthic infections in schoolchildren in Cambodia. Southeast Asian J Trop Med Public Health. 2003;34:254–8. [PubMed] [Google Scholar]
- 7.Copelovitch L, Ol OS, Taraquinio S, Chanpheaktra N. Childhood nephrotic syndrome in Cambodia: an association with gastrointestinal parasites. J Pediatr. 2010;156:76–81. 10.1016/j.jpeds.2009.06.049 [DOI] [PubMed] [Google Scholar]
- 8.Ohmae H, Sinuon M, Kirinoki M, Matsumoto J, Chigusa Y, Socheat D, et al. Schistosomiasis mekongi: from discovery to control. Parasitol Int. 2004;53:135–42. 10.1016/j.parint.2004.01.004 [DOI] [PubMed] [Google Scholar]
- 9.Lee KJ, Bae YT, Kim DH, Deung YK, Ryang YS, Kim HJ, et al. Status of intestinal parasites infection among primary school children in Kampongcham, Cambodia. Korean J Parasitol. 2002;40:153–5. 10.3347/kjp.2002.40.3.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park SK, Kim DH, Deung YK, Kim HJ, Yang EJ, Lim SJ, et al. Status of intestinal parasite infections among children in Bat Dambang, Cambodia. Korean J Parasitol. 2004;42:201–3. 10.3347/kjp.2004.42.4.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai JY, Park JH, Han ET, Guk SM, Shin EH, Lin A, et al. Mixed infections with Opisthorchis viverrini and intestinal flukes in residents of Vientiane Municipality and Saravane Province in Laos. J Helminthol. 2005;79:283–9. 10.1079/JOH2005302 [DOI] [PubMed] [Google Scholar]
- 12.Poland GA, Navin TR, Sarosi GA. Outbreak of parasitic gastroenteritis among travelers returning from Africa. Arch Intern Med. 1985;145:2220–1. 10.1001/archinte.145.12.2220 [DOI] [PubMed] [Google Scholar]
- 13.Graczyk TK, Fried B. Echinostomiasis: a common but forgotten food-borne disease. Am J Trop Med Hyg. 1998;58:501–4. [DOI] [PubMed] [Google Scholar]
- 14.Carney WP, Sudomo M. Purnomo. Echinostomiasis: a disease that disappeared. Trop Geogr Med. 1980;32:101–5. [PubMed] [Google Scholar]