Abstract
Background:
The evidence for the effectiveness of antihypertensive medication use for slowing decline in kidney function in older persons is sparse. We addressed this research question by the application of novel methods in a marginal structural model.
Methods:
Change in kidney function was measured by two or more measures of cystatin C in 1,576 hypertensive participants in the Cardiovascular Health Study over 7 years of follow-up (1989–1997 in four U.S. communities). The exposure of interest was antihypertensive medication use. We used a novel estimator in a marginal structural model to account for bias due to confounding and informative censoring.
Results:
The mean annual decline in eGFR was 2.41 ± 4.91 mL/min/1.73 m2. In unadjusted analysis, antihypertensive medication use was not associated with annual change in kidney function. Traditional multivariable regression did not substantially change these estimates. Based on a marginal structural analysis, persons on antihypertensives had slower declines in kidney function; participants had an estimated 0.88 (0.13, 1.63) ml/min/1.73 m2 per year slower decline in eGFR compared with persons on no treatment. In a model that also accounted for bias due to informative censoring, the estimate for the treatment effect was 2.23 (−0.13, 4.59) ml/min/1.73 m2 per year slower decline in eGFR.
Conclusion:
In summary, estimates from a marginal structural model suggested that antihypertensive therapy was associated with preserved kidney function in hypertensive elderly adults. Confirmatory studies may provide power to determine the strength and validity of the findings.
Keywords: aged, kidney function, hypertension, marginal structural model
1. Introduction
Hypertension is the second leading cause of end-stage renal disease (ESRD) in the U.S. (U.S. Renal Data Systems, 2008). Several studies have demonstrated the beneficial effects of lower blood pressure on kidney function (Klag et al., 1996, Coresh et al., 1998, Young et al., 2002). Although it is widely believed that antihypertensive medications protect kidney function, there is limited evidence from placebo-controlled trials. Several placebo-controlled trials have evaluated the effect of renin-angiotensin system inhibitors, but there have been mixed results across renal outcomes (Casas et al., 2005). In addition, trials of aggressive blood pressure control versus usual control have yielded conflicting results (Klahr et al., 1994, Wright et al., 2002, Ruggenenti et al., 2005, Sarnak et al., 2005).
The evidence for antihypertensive medication and renal outcomes is particularly sparse in older persons with hypertension. Elderly persons are often underrepresented in randomized controlled trials because of concerns about safety, and because their study may be complicated by the presence of multiple comorbidities. In addition, long-term trials are often not feasible, due to both the high cost and burden to participants, and losses to follow-up. To avoid these concerns, investigators often use data collected in an observational study to evaluate medication effects. However, a complication of observational studies is that the intervention is not randomized; therefore it is essential that investigators appropriately deal with the resulting biases.
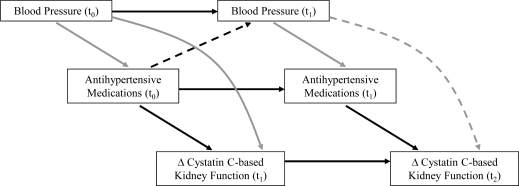
Standard regression techniques may be insufficient in the study of the joint effect of antihypertensive medication use at different times and kidney function, due to the presence of time-dependent confounding. This occurs when a confounder varies over time, and values of the confounder are affected by earlier treatment and associated with the outcome (Hernan et al., 2000, Robins et al., 2000). For example, blood pressure level predicts both the probability of being on antihypertensive medication and change in kidney function (Figure 1: gray arrows); and subsequent blood pressure levels also mediate the relationship between antihypertensive medication use and change in kidney function (Figure 1: dotted arrows). Another common bias in longitudinal studies is informative censoring; this occurs when the outcome is associated with the probability of loss to follow-up. Decline in kidney function is associated with an increased probability of being censored, most often by death. There are systematic differences between those participants who have complete follow-up data and those who do not, and this may introduce bias into the estimate of effect.
Figure 1:
Illustration of time-dependent confounding of antihypertensive medication use and change in kidney function by blood pressure
In this paper, we apply novel methods in a marginal structural model to account for time-dependent confounding and informative censoring in a longitudinal observational study of antihypertensive therapy and change in kidney function in elderly adults. We used a novel estimator to reduce bias and maximize efficiency. We hypothesized that antihypertensive therapy would be associated with a slower decline in kidney function among hypertensive elderly persons, after accounting for these potential sources of bias.
2. Methods
2.1. Study Population
The Cardiovascular Health Study (CHS) is a community-based study of elderly black and white adults aged 65 years and older at baseline. The primary aim of the CHS is to evaluate risk factors for the development and progression of cardiovascular disease in the elderly (Fried et al., 1991). The study recruited persons from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania in 1989–1990. Black participants were actively recruited during a supplemental enrollment process of CHS during 1992–1993; they comprise 15% of CHS participants. To be considered eligible, persons had to meet the following criteria: 1) age ≥ 65 years; 2) not institutionalized; 3) expected to remain in the current community for 3 years or longer; 4) not under active treatment for cancer; and 5) provided informed consent without requiring a proxy respondent.
Participants completed study visits at enrollment and annually during the follow-up; these visits included an interview, physical examination, health questionnaire, and collection of blood specimens. Hospital discharge summaries and International Classification of Diseases, Ninth Revision (ICD-9) codes were collected for all hospitalizations during the follow-up period. The study was approved by institutional review boards at each site and informed consent was obtained from all participants.
2.2. Kidney Function
Kidney function was measured by cystatin C, an alternative measure of kidney function that is a better estimate of glomerular filtration rate compared with serum creatinine in the elderly (Fliser and Ritz, 2001, Dharnidharka et al., 2002). Cystatin C was measured at baseline, and at 3 and 7 years of follow-up; the primary outcome was the average annual change in cystatin C as measured over the first (baseline to year 3) or second (year 3 to year 7) follow-up period. Cystatin C was measured by a BNII nephelometer (Seimens Healthcare Diagnostics, Deerfield, IL) that utilizes a particle enhanced immunonepholometric assay (N Latex Cystatin-C) (Erlandsen et al., 1999). The assay range is 0.195 to 7.330 mg/L, with the reference range for young, healthy individuals reported as 0.53 – 0.95 mg/L. Intra-assay coefficients of variation (CVs) range from 2.0 – 2.8% and inter-assay CVs range from 2.3 – 3.1%. Samples were measured from frozen and stored at −70°C. Finney et al. (1997), have shown that cystatin-C is stable when frozen, and can withstand several freeze/thaw cycles.
Cystatin C-based estimated glomerular filtration rate (eGFR) was calculated based on the equation (cystatin C−1.19 × 76.7) developed by the CKD-EPI group (Stevens et al., 2008).
2.3. Antihypertensive Medication Use
Antihypertensive medication use was assessed annually; participants were asked to bring their medications to clinical visits (Psaty et al., 1992). Medications included in the present study were angiotensin converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), beta-adrenergic blockers, calcium channel blockers, and diuretics. The exposure was classified as any versus no antihypertensive medication use. Any medication use was defined as the average use of one or more antihypertensive medications between each measure of kidney function (baseline to year 3, year 3 to year 7).
2.4. Other Measures
Age, sex, and race were determined by self-report at baseline; race was categorized as black or white/other because <1% of participants identified as non-black or white race. Blood pressure (systolic and diastolic), height, and weight were annually measured by standard protocol; and body mass index (BMI) was calculated as weight (in kilograms) divided by height (in meters) squared. Smoking status, alcohol consumption, and history of hypertension were determined by self-report. Diabetes was defined as a fasting glucose >125 mg/dL or use of insulin or hypoglycemic medications, and was assessed annually. Disability was defined as a self-reported limitation on one or more activities of daily living and was assessed annually. Frailty was defined as the presence of three of the following abnormalities: unintentional weight loss, self-reported exhaustion, measured weakness, slow walking speed, and low physical activity (Fried et al., 2001). Cognitive function was measured by the Mini-Mental State Examination. Other variables included income (<$15,000, $15,000 to 44,999, $45,000+), post-secondary education, total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, fasting glucose and insulin, creatinine, and C-reactive protein. At the time of the study, cystatin C was not widely available; therefore, creatinine was included as a potential predictor of treatment. Cardiovascular disease (myocardial infarction, stroke, coronary arterial bypass graft, angioplasty), heart failure, hospitalization for cancer, and fracture were assessed annually; methods for adjudication have been described previously (Ives et al., 1995).
2.5. Statistical Methods
Several estimators have been proposed for marginal structural models including g-computation, inverse probability of treatment weighted (IPTW), double robust, and more recently, targeted maximum likelihood estimation (targeted MLE) (Neugebauer and van der Laan, 2005, van der Laan and Rubin, 2006). The IPTW estimator has been widely used because of its relative ease of implementation (Mortimer et al., 2005). The targeted MLE estimator is a novel estimator that is double robust against model misspecification and more efficient compared to IPTW (van der Laan and Rubin, 2006, Bembom et al., 2007, Arnold et al., 2009). The estimator is double robust because it incorporates information from both the outcome model and the treatment mechanism, and will produce consistent estimates if either model is correctly specified. Similar to traditional likelihood methods, targeted MLE is a function of the distribution of the data, although it includes an additional covariate in the regression model that targets the parameter of interest; this covariate is a function of the treatment mechanism. The covariate h(A,W) is the inverse of the conditional probability of the treatment received. For example, in a simple point treatment study, we denote A as the treatment, and W as a set of covariates; h(A,W) is equal to the following in the untreated:
and the following in the treated:
The implementation in R has been described previously by Gruber and van der Laan (2009).
In this study, we combined the targeted MLE estimator to account for the time-independent confounding, IPTW for time-dependent confounding, and IPCW for informative censoring; this combination estimator is referred to as IPTW/IPCW-reduced targeted MLE. We used IPTW for the time-dependent confounding, because the targeted MLE estimator is more difficult to implement longitudinally. This combination estimator takes advantage of the benefits of targeted MLE (increased efficiency and robustness) while using methods that can be implemented easily in standard software and have been published previously (Hernan et al., 2000, Fewell et al., 2004, Gruber and van der Laan, 2009).
2.6. Statistical Analysis
All statistical analyses were restricted to persons with a history of hypertension. We first summarized baseline characteristics of persons taking any or no antihypertensive medications over the first follow-up period. We plotted the distribution of the annual change in eGFR.
We next examined the association between antihypertensive medication use and change in eGFR based on unadjusted and traditional multivariable adjusted regression models. The exposure was the joint antihypertensive medication use over the two follow-up periods, and the outcome was annual change in eGFR. Age, race, gender, diabetes, systolic and diastolic blood pressure, diabetes, creatinine, cardiovascular disease, and heart failure were included as potential confounders. We included confounder measurements that occurred at baseline for the first follow-up and from baseline to year 3 for the second follow-up period. We included other potential confounders from the list of the following candidates: smoking, alcohol consumption, income, post-secondary education, BMI, total, LDL- and HDL-cholesterol, triglycerides, glucose, fasting insulin, C-reactive protein, creatinine-based eGFR, frailty, disability, cognitive function, fracture, or hospitalization for cancer.
Subsequently, we used a marginal structural model approach to estimate the effect of antihypertensive medication use on change in kidney function. Let A(t) indicate antihypertensive use, Y indicate change in eGFR, W(0) indicate the baseline covariates, and L(t) indicate the time-dependent covariates, where t = (0, 1, 2). We fit a model of the form:
where
We separated the potential confounders into those that were modeled as time-dependent (systolic and diastolic blood pressure, diabetes, eGFR-creatinine, cardiovascular disease, and heart failure) and time-independent (age, race, gender, BMI, education, income, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, glucose, insulin, frailty, cognitive function). To calculate the stabilized IPTW weights, we modeled the treatment (antihypertensive medication use) based on a logistic regression model and calculated the weights as:
Aij is antihypertensive medication use, for the ith subject at the jth follow-up, j (j=1, 2), and Lij includes the time-dependent confounders: systolic and diastolic blood pressure, diabetes, creatinine, cardiovascular disease, and heart failure. For j=1, the treatment weights were only a function of the baseline covariates, because history of medication use was unavailable.
We next calculated the stabilized inverse probability of censoring weights (IPCW) to account for informative censoring. Candidate variables were the same as the list of potential confounders above, as well as cystatin C and antihypertensive medication use. The IPCW were calculated as:
where Cij is an indicator of not being censored for the ith person at the jth follow-up time, and Lij-1 are variables that were associated with censoring, measured prior to censoring at time j.
We applied the targeted MLE to account for the time-independent confounding. In this model, each person was weighted by the IPTW to account for time-dependent confounding, or the product of IPTW and IPCW to also account for the informative censoring. The targeted MLE is implemented in four steps: 1) estimate the conditional expectation of Y, given A and W using a generalized linear model with maximum likelihood, 2) calculate the conditional probability of A, given W, 3) calculate the targeting covariate as described above, 4) update the initial model by adding the targeting covariate and estimate the new coefficients by maximum likelihood. Details of this method have been described previously (van der Laan and Rubin, 2006, Arnold et al., 2009, Gruber and van der Laan, 2009).
To identify the best fit for all models, we used the deletion/selection/addition (D/S/A) algorithm to identify the form of the relationship between the independent and dependent variables, based on polynomial terms (Sinisi and van der Laan, 2004). The D/S/A algorithm uses a combination of forward and backward model fitting and is based on cross-validation. For the non-targeted IPTW variable selection, we forced in the variables that were included in the IPTW-reduced targeted MLE, in order to ensure that the differences were not due to the parameters selected by the D/S/A algorithm. We calculated standard errors based on bootstrap sampling with 1000 replicates.
All analyses were conducted using Stata 10.0 (StataCorp, College Station, TX) and R (The R Foundation, Vienna, Austria).
3. Results
From a total of 5,888 CHS participants, 2,146 reported a history of hypertension. During the first follow-up period, 12% reported being on no antihypertensives, and 88% reported being on an average of 1 or more antihypertensives (Table 1). Persons on antihypertensives were of similar age and had a similar prevalence of smoking as persons on no antihypertensive medications; but were more likely to be female, self-identify as black race, have less education, and have lower income compared with persons on no antihypertensive medications. In addition, persons on antihypertensives were less likely to drink alcohol, had higher BMI, cholesterol, triglycerides, and C-reactive protein levels. Blood pressure levels, LDL-cholesterol, cognitive function and the prevalence of frailty were similar in persons on any or no antihypertensive medications. Persons on antihypertensive therapy were more likely to have diabetes, cardiovascular disease, heart failure, and disability at baseline.
Table 1:
Baseline characteristics of participants in the Cardiovascular Health Study (1989–1990 or 1992–1993, U.S.)
| Antihypertensive Medication Use | ||
|---|---|---|
| None (n = 277) | Any (n = 1,957) | |
| Characteristic | Mean ± SD or N (%) | |
| Age (years) | 72.4 ± 5.6 | 72.8 ± 5.5 |
| Female | 158 (57%) | 1,234 (63%) |
| Black Race | 57 (21%) | 492 (25%) |
| Post-Secondary Education | 111 (40%) | 730 (37%) |
| Income <$15,000 | 112 (45%) | 885 (48%) |
| $15,000 - <$45,000 | 113 (45%) | 784 (43%) |
| $45,000 | 26 (10%) | 171 (9%) |
| Current Smoker | 29 (10%) | 215 (11%) |
| Current Drinker | 81 (29%) | 442 (23%) |
| BMI (kg/m2) | 26.9 ± 5.2 | 28.0 ± 5.1 |
| Systolic Blood Pressure (mmHg) | 145 ± 22 | 143 ± 22 |
| Diastolic Blood Pressure (mmHg) | 74 ± 13 | 72 ± 12 |
| HDL-Cholesterol (mg/dL) | 54 ± 16 | 53 ± 15 |
| LDL- Cholesterol (mg/dL) | 129 ± 36 | 130 ± 36 |
| Triglycerides (mg/dL) | 138 ± 94 | 151 ± 83 |
| C-reactive Protein (mg/dL) | 4.0 ± 4.5 | 5.8 ± 9.0 |
| Cognitive Function | 90 ± 7 | 90 ± 6 |
| Diabetes | 46 (17%) | 448 (23%) |
| Cardiovascular Disease | 39 (14%) | 347 (18%) |
| Heart Failure | 9 (3%) | 127 (6%) |
| Disability | 13 (5%) | 216 (11%) |
| Frailty | 17 (7%) | 139 (8%) |
Diabetes was defined as fasting glucose >125 mg/dL, or use of insulin or oral hypoglycemics
Participants were classified as current drinkers if they consumed >1 alcoholic beverage per week
Cognitive function was measured on the Mini-Mental State Exam
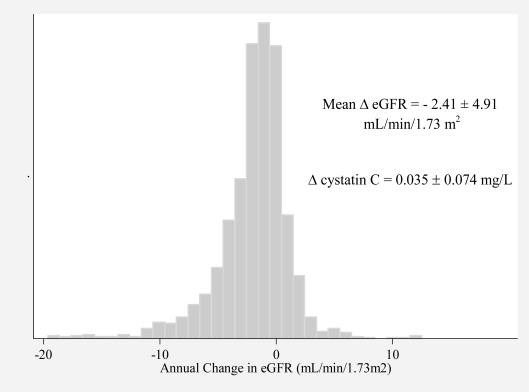
A total of 1,576 participants had cystatin C measured at two or more visits. The mean annual increase in cystatin C in these participants was 0.035 ± 0.074 mg/L (mean ± standard deviation), which corresponds to a decrease in eGFR of 2.41 ± 4.91 mL/min/1.73 m2 (Figure 2). The distribution of annual change in eGFR appeared slightly left skewed.
Figure 2:
Distribution of Annual Change in eGFR in Hypertensive Participants in the Cardiovascular Health Study (1989 – 1997, U.S.)
In unadjusted analysis, antihypertensive medication use was not associated with a change in kidney function (Table 2). Traditional multivariable adjustment for demographics, risk factors, blood pressure, cardiovascular disease, and heart failure did not substantially alter the point estimates for the association of antihypertensive medication use with decline in kidney function.
Table 2:
Antihypertensive medication use and change in kidney function in the Cardiovascular Health Study (1989 – 1997, U.S.)
| Annual Change in eGFR (mL/min/1.73m2) (mean decrease = 2.41 mL/min/1.73m2) | ||||
|---|---|---|---|---|
| None | Antihypertensive Medication Use* | |||
| Estimate | 95% CI | p-value | ||
| Unadjusted | Referent | 0.31 | −0.26, 0.88 | p = 0.30 |
| Traditional Multivariable Regression† | Referent | 0.14 | −0.34, 0.63 | p = 0.57 |
| IPTW Alone † | Referent | 0.41 | −0.20, 1.02 | p = 0.19 |
| IPTW-reduced Targeted MLE† | Referent | 0.88 | 0.13, 1.63 | p = 0.02 |
| IPTW/IPCW †,‡ | Referent | 1.38 | −0.48, 3.24 | p = 0.15 |
| IPTW/IPCW-reduced Targeted MLE†,‡ | Referent | 2.23 | −0.13, 4.59 | p = 0.06 |
Note all coefficients represent the mean annual change in kidney function associated with antihypertensive medication use compared with the mean annual change for someone on no medications
Potential confounders included age, gender, race, systolic and diastolic blood pressure, diabetes, eGFR-creatinine, cardiovascular disease, heart failure, education, income, smoking, alcohol consumption, BMI, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, glucose, insulin, C-reactive protein, frailty, cognitive function, disability, fracture, and cancer.
Potential predictors of censoring included cystatin C, antihypertensives, age, gender, race, systolic and diastolic blood pressure, diabetes, cardiovascular disease, heart failure, education, income, smoking, alcohol consumption, BMI, total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, glucose, insulin, C-reactive protein, frailty, cognitive function, disability, fracture, and cancer.
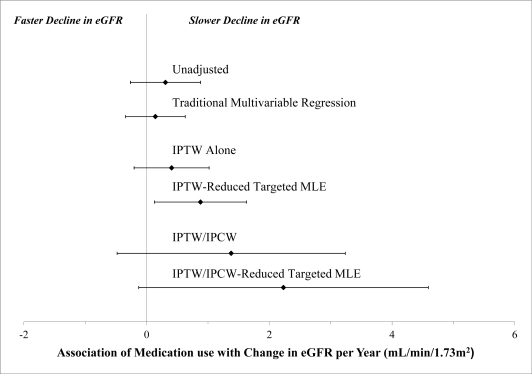
Based on a marginal structural model with IPTW to account for the time-independent and time-dependent confounding, the estimate was modestly further in the protective direction, although did not reach statistical significance (Table 2). Based on a marginal structural model with the IPTW-reduced targeted MLE, the point estimates of effect of antihypertensive medication use was a 0.88 mL/min/1.73m2 slower decline in kidney function (p=0.02), compared to persons on no medications (Table 2). This estimate was larger in magnitude and had a smaller coefficient of variation compared with those obtained using traditional multivariable regression or IPTW-alone (Figure 3)
Figure 3:
Antihypertensive Medication Use and Change in Kidney Function in the Cardiovascular Health Study (1989 – 1997, U.S.), Based on Traditional and Marginal Structural Model Analysis
Accounting for the informative censoring had a substantial effect on the estimates; the mean estimate of effect for antihypertensive medications use was 1.38 mL/min/1.73m2 (p=0.15) slower decline in eGFR based on IPTW/IPCW alone (Table 2); and 2.23 mL/min/1.73m2 (p=0.06), based on the IPTW/IPCW-reduced targeted MLE. Variables associated with censoring were indicators of poor health status: age, lower income, smoking, worse cognitive function, worse kidney function, higher LDL-cholesterol, diabetes, heart failure, frailty, antihypertensive medication use.
There was no evidence for violation of the experimental treatment assignment assumption based on the distribution of weights across the exposure groups. The maximum for the treatment weights was 2.09 at follow-up period one and 1.91 follow-up period two (Table 3). The maximum for the censoring weights was 5.40 at follow-up period one and 6.38 at follow-up period two. All weights were had a mean value of 1.00 (Table 3).
Table 3:
Distribution of Inverse Probability Weights
| Range | Mean | Median | Interquartile Range | |
|---|---|---|---|---|
| IPTW (t = 1) | (0.68 – 2.09) | 1.00 | 1.00 | 0.98 – 1.02 |
| IPTW (t = 2) | (0.52 – 1.91) | 1.00 | 1.00 | 0.99 – 1.01 |
| IPCW (t = 1) | (0.84 – 5.40) | 1.00 | 0.96 | 0.90 – 1.00 |
| IPCW (t = 2) | (0.73 – 6.38) | 1.00 | 1.00 | 0.85 – 1.00 |
4. Discussion
The results of our study were consistent with the hypothesis that antihypertensive medication use may slow decline in kidney function in the elderly. A marginal structural analysis suggested that antihypertensive medication use was protective of kidney function. In contrast, based on traditional regression models, there was no apparent association between antihypertensive medication use and change in kidney function in older persons. Due to the imprecision of the estimates, we cannot exclude the possibility that our findings were due to chance. When considered in the context of prior epidemiological research associating lower blood pressures with improved renal outcomes in the elderly, our study provides modest evidence in support of a beneficial effect of antihypertensive medications on kidney function.
There is a pressing need for effective interventions to slow or prevent decline in kidney function in the elderly; reduced kidney function is highly prevalent in this population and is associated with several adverse health outcomes (Go et al., 2004, Shlipak et al., 2005, Shlipak et al., 2006). Aside from diabetes, hypertension is the strongest risk factor for adverse kidney outcomes (U.S. Renal Data Systems, 2008, Coresh et al., 2003). Chronic hypertension can result in endothelial injury and glomerular capillary damage in the kidney (Rosario and Wesson, 2006). The subsequent reduction in arterial compliance and increased vascular resistance can cause a decline in GFR. Control of systolic blood pressure is associated with a lower risk of decline in kidney function in the elderly. In an analysis of 2,181 elderly men and women enrolled in the placebo arm of the Systolic Hypertension in the Elderly Program, investigators reported the relative risk of a decline in kidney function for persons in the highest quartile of systolic blood pressure was 2.44 (95% CI 1.67, 3.56) compared with the lowest quartile (Young et al., 2002).
Despite the strong evidence for improved kidney function in persons with lower blood pressure, the three large randomized trials that compared intensive blood pressure control with usual care on renal outcomes have reported mixed effects (Klahr et al., 1994, Wright et al., 2002, Ruggenenti et al., 2005, Sarnak et al., 2005). These trials included younger persons with worse kidney function compared to our study. The African American Study of Kidney Disease (AASK) study and the Ramipril Efficacy in Nephropathy 2 (REIN-2) Trial found no benefit of intensive blood pressure control in participants with kidney disease (Wright et al., 2002, Ruggenenti et al., 2005). In the Modification in Diet in Renal Disease (MDRD) Study the intensive blood pressure control did not alter significantly the mean decline in GFR after 3 years of follow-up compared with usual blood pressure control; however, after 10 years of follow-up, investigators reported a reduced risk of kidney failure (Klahr et al., 1994, Sarnak et al., 2005). Several studies have also demonstrated that inhibition of the renin-angiotensin system may provide additional protection for the kidney, beyond the effect on blood pressure control (Jafar et al., 2001, Levey et al., 2005). However, a recent meta-analysis found inconsistent effects of ACE/ARBs compared with placebo across renal outcomes (Casas et al., 2005).
The present study suggests that the IPTW-reduced targeted MLE may have advantages over the IPTW-alone estimator. Inverse probability weighting methods are inefficient (produce large standard errors); this property has been noted previously (Cook et al., 2002, Neugebauer and van der Laan, 2005, Brunelli et al., 2008). The estimates obtained with the combination estimator had smaller standard errors relative to the magnitude of the estimate (coefficient of variation) compared to those obtained with the inverse-weighting methods alone. In addition, the estimates from the combined targeted MLE estimator were larger in magnitude compared to those with the inverse-weighting methods. We speculate that either 1) the functional form of the treatment model was misspecified, or 2) there was unmeasured and/or residual confounding in the IPTW-alone model. These biases were more likely to be reduced in the IPTW-reduced targeted MLE model because of the double robust property of the estimator. Accounting for informative censoring also had an important impact on our estimates. Antihypertensive medication use was associated with a higher probability of censoring, as were other indicators of poor health status; thus, failure to account for this systematic missingness led to an apparent attenuation of the beneficial effect. We hypothesize that antihypertensive medication use may have had a larger effect in those participants with the worst health status; healthier individuals may receive less benefit from medications because they have less loss in function.
This study has limitations which should be considered when interpreting the results. While we included an extensive list of potential confounders, our study may have been limited by unmeasured or residual confounding. An important potential source of residual confounding may be the high variability in blood pressure measurements. The annual blood pressure measurements conducted in the CHS may have varied from the blood pressure measurements conducted in the participants’ regular clinical visits, which were used by their health care providers to make decisions regarding medication use. Additionally, observational studies of medication use are subject to bias due to the co-occurrence of health-seeking behaviors, or the ‘healthy user bias’. However, based on the measured baseline characteristics of users and non-users, those persons on antihypertensive medications appear to be less healthy. In addition, there may be misclassification of self-reported history of hypertension. We did not include information the history of antihypertensive medication use because it was unavailable. Finally, we did not include information on the class or dosage of antihypertensive medication use. As mentioned above, ACE/ARB medications may have improved renoprotective effects compared with other antihypertensives. However, analysis of class effects was limited by low precision and confounding by number of medications.
In summary, estimates from a marginal structural model suggested that antihypertensive therapy was associated with preserved kidney function in hypertensive elderly adults. Future observational studies of antihypertensive medications should use marginal structural models to better account for biases due to time-dependent confounding and informative censoring. The identification of effective interventions to slow decline in kidney function is essential to reduce kidney disease and related morbidity in the later years of life.
Contributor Information
Michelle C. Odden, Oregon State University
Ira B. Tager, University of California, Berkeley
Mark J. van der Laan, University of California, Berkeley
Joseph A.C. Delaney, University of Florida
Carmen A Peralta, University of California, San Francisco.
Ronit Katz, University of Washington.
Mark J. Sarnak, Tufts Medical Center
Bruce M. Psaty, University of Washington
Michael G Shlipak, University of California, San Francisco.
5 References
- Arnold B, Arana B, Mausezahl D, Hubbard A, Colford JM., Jr Evaluation of a pre-existing, 3-year household water treatment and handwashing intervention in rural Guatemala. Int J Epidemiol. 2009;38(6):1651–61. doi: 10.1093/ije/dyp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bembom O, Petersen ML, Rhee S, et al. Biomarker Discover Using Targeted Maximum Likelihood Estimation. Application to the Treatment of Antiretroviral Resistant HIV Infection. UC Berkeley Division of Biostatistics Working Paper Series. 2007 doi: 10.1002/sim.3414. Paper 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli SM, Joffe MM, Israni RK, et al. History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(3):777–82. doi: 10.2215/CJN.04281007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366(9502):2026–33. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- Cook NR, Cole SR, Hennekens CH. Use of a marginal structural model to determine the effect of aspirin on cardiovascular mortality in the Physicians’ Health Study. Am J Epidemiol. 2002;155(11):1045–53. doi: 10.1093/aje/155.11.1045. [DOI] [PubMed] [Google Scholar]
- Coresh J, Longenecker JC, Miller ER, 3rd, Young HJ, Klag MJ. Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S24–30. [PubMed] [Google Scholar]
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- Fewell Z, Hernan MA, Wolfe F, Tilling K, Choi H, Sterne JAC. Controlling for time-dependent confounding using marginal structural models. The Stata Journal. 2004;4(4):402–420. [Google Scholar]
- Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II) Clin Chem. 1997;43(6 Pt 1):1016–22. [PubMed] [Google Scholar]
- Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37(1):79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–76. doi: 10.1016/1047-2797(91)90005-W. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- Gruber S, van der Laan M. Targeted Maximum Likelihood Estimation: A Gentle Introduction. UC Berkeley Division of Biostatistics Working Paper Series. 2009 Paper 252. [Google Scholar]
- Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11(5):561–70. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5(4):278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330(13):877–84. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- Levey AS, Mulrow CD. An editorial update: what level of blood pressure control in chronic kidney disease? Ann Intern Med. 2005;143(1):79–81. doi: 10.7326/0003-4819-143-1-200507050-00013. [DOI] [PubMed] [Google Scholar]
- Mortimer KM, Neugebauer R, van der Laan M, Tager IB. An application of model-fitting procedures for marginal structural models. Am J Epidemiol. 2005;162(4):382–8. doi: 10.1093/aje/kwi208. [DOI] [PubMed] [Google Scholar]
- Neugebauer R, van der Laan MJ. Why Prefer Double Robust Estimates? Illustration with Causal Point Treatment Studies. J Stat Plan Infer. 2005;129(1–2):405–426. doi: 10.1016/j.jspi.2004.06.060. [DOI] [Google Scholar]
- Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: methods and initial experience in the Cardiovascular Health Study. The Cardiovascular Health Study Collaborative Research Group. J Clin Epidemiol. 1992;45(6):683–92. doi: 10.1016/0895-4356(92)90143-B. [DOI] [PubMed] [Google Scholar]
- Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- Rosario RF, Wesson DE. Primary hypertension and nephropathy. Curr Opin Nephrol Hypertens. 2006;15(2):130–4. doi: 10.1097/01.mnh.0000214771.88737.ee. [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365(9463):939–46. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342–51. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1069. Article18. [DOI] [PubMed] [Google Scholar]
- Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin c alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Renal Data Systems . USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2008. [Google Scholar]
- van der Laan M, Rubin D. Targeted maxium likelihood learning. Int J Biostatistics. 2006;2 Article 11. [Google Scholar]
- Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- Young JH, Klag MJ, Muntner P, Whyte JL, Pahor M, Coresh J. Blood pressure and decline in kidney function: findings from the Systolic Hypertension in the Elderly Program (SHEP) J Am Soc Nephrol. 2002;13(11):2776–82. doi: 10.1097/01.ASN.0000031805.09178.37. [DOI] [PubMed] [Google Scholar]