Abstract
Purpose
To evaluate the effectiveness of laparoscopic assisted total gastrectomy (LATG), we compared its early surgical outcomes with those of conventional open total gastrectomy (OTG) in patients who were diagnosed as having early gastric cancer preoperatively.
Methods
We retrospectively analyzed early surgical outcomes in 190 consecutive patients who underwent total gastrectomy for early gastric cancer between January 2009 to April 2010. The patients were divided into those who underwent LATG and those who underwent OTG. Their early surgical outcomes were analyzed to evaluate the effectiveness of LATG.
Results
There was no significant difference in postoperative complication rates (P = 0.291). But in the analysis of other early surgical outcomes, we found that LATG could improve time to first flatus (P < 0.001), time to commencement of soft diet (P = 0.034), administration of analgesics (P = 0.024), pain score (Numeric Rating Scale), and hospital discharge (P = 0.045).
Conclusion
Although LATG didn't show better results for postoperative complications than those of OTG, LATG contributes to the improvement of early surgical outcomes, including bowel movement, pain score and hospital discharge. Therefore, we suggest that LATG could be a method to improve early surgical outcomes in patients who need total gastrectomy.
Keywords: Early gastric cancer, Laparoscopic assisted total gastrectomy, Open total gastrectomy
INTRODUCTION
Since laparoscopic gastrectomy was introduced by Kitano et al. [1], laparoscopic (assisted) distal gastrectomy (LADG) has become more commonly performed for early gastric cancer in Korea [2-6]. However, there have been several reports on early surgical outcomes of laparoscopic assisted total gastrectomy (LATG) [7-10]. In these studies, although LATG has been shown to be safe and feasible for early gastric cancer, it has not yet been directly compared with the early surgical outcomes of conventional open total gastrectomy (OTG). In fact, LADG had not yet become popularized compared with LADG, because of its technical difiiculties and fear of postoperative complications. Therefore, the purpose of this study was to evaluate the effectiveness of LATG and to introduce techniques from our experiences.
METHODS
Patients
We retrospectively reviewed the prospectively collected data on 190 consecutive patients who underwent OTG and LATG, for gastric cancer between January 2009 and April 2010 at a single institution. All patients in whom the proximal margin too short to perform gastrojejunostomy had total gastrectomy: these patients were included in this study. Also, all patients who were preoperatively diagnosed with early gastric cancer were enrolled in this study. Our patient population consisted of 63 who underwent LATG and 127 who underwent OTG. After explaining the merits of laparoscopic surgery, the level of difficulty of procedures, and the cost for OTG and LATG, the decision of OTG and LATG resided with the patient.
Surgical techniques of LATG
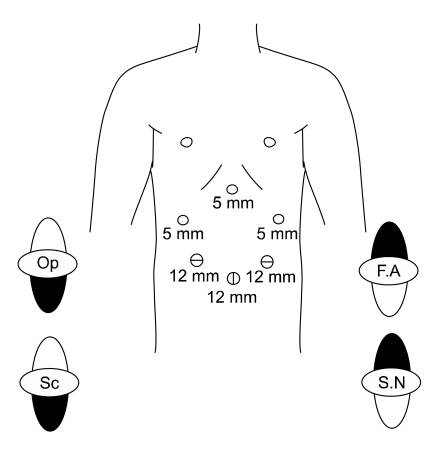
Each patient was placed in thereverse Trendelenburg position. A carbon dioxide pneumoperitoneum was created using the umbilical port, and the pressure was maintained between 12 and 15 mmHg. Five trocars were placed in a U-shape (Fig. 1). The falciform ligament was fixed to the anterior wall of the peritoneum for retraction of the liver using ENDO CLOSE. If the operating field was not sufficient, an additional 5-mm trocar was inserted into the epigastric area to retract the liver.
Fig. 1.
Trocar placement for laparoscopic assisted total gastrectomy. Op, operator; F.A, first assistant; Sc, scopist; S.N, Scrub nurse; 5 mm, 5 mm port; 12 mm, 12 mm port.
Dissection was begun by dividing the greater omentum (4 cm from gastroepiploic arcade) from the mid-portion of the gastroepiploic arcade to the short gastric vessels. Dissection of the lymph nodes around the left gastroepiploic vessels and short gastric vessel was performed. After the dissection of the lymph nodes around the right gastroepiploic area, the infrapyloric area was dissected. In some patients, dissection was advanced to the superior mesenteric vein to include enlarged 14v lymph nodes. Lymph nodes around the suprapyloric area; hepatoduodenal ligament (along the hepatic artery), common hepatic, splenic, celiac, and left gastric arteries; and right and left paracardial areas were dissected in that order.
The duodenal stump is made after clearing on number 5 lymph nodes. The duodenum is transected below the duodenal bulb using an endoscopic linear stapler (Endopath ETS 60, Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). After clearing the lymph nodes, a 4-5 cm midline incision was made from the epigastrictrocar site. A wound protector was applied, and esophagojejunostomy was reconstructed using a circular stapler (Proximate ILS, Ethicon Endo-Surgery Inc.; DST Series EEA, Tyco Healthcare Group LP, North Haven, CT, USA).
Clinical analysis
Clinical data obtained from medical records included patient age, gender, body mass index (BMI), and American Society of Anesthesiologists (ASA) score. Early surgical outcomes included operation time, postoperative complications, intra-operative blood loss, postoperative change in hematocrit, time to first flatus, day of commencement on soft diet, number of administrations of analgesics, Numeric Rating Scale (NRS), and postoperative hospital stay. Pathologic results were analyzed for tumor size, number of retrieved lymph nodes, resection margins, and American Joint Committee on Cancer/International Union Against Cancer staging.
To evaluate the intra-operative blood loss, the attending anesthesiologist recorded the estimated blood loss. This was based on the observation of the number of surgical sponges used, the amount of fluid in the suction device, and a calculation of the amount of irrigant used during the operation. Preoperative hematocrit was checked before undergoing surgery and postoperative hematocrit was checked on postoperative day one at 7:00 AM.
Our postoperative pain control consisted of intravenous patient-controlled analgesia (Fentanyl 2,500 µg, Ketorolactromethamine 180 mg, OndansetronHCl 16 mg). To evaluate the patients' postoperative pain, we calculated the number of additional doses of analgesics until the patient was discharged from the hospital. Also, we applied an NRS for all patients. The NRS was checked on postoperative day (POD) 0 at 11:00 PM, POD 1 at 8:00 AM, POD 1 at 11:00 PM, POD 2 at 8:00 AM, POD 3 at 8:00 AM and POD 5 at 8:00 AM.
Patients were discharged if they had no problems eating a soft diet, showed an absence of inflammatory conditions, including leukocytosis, unstable vital sign and abrupt onset abdominal pain, and were generally comfortable. Also, we left the final decision about discharge up to the patients.
Statistical analysis
Statistical analysis was performed using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). Patient data was analyzed by one-way analysis of variance, followed by a post-hoc Turkey test and the χ2 test. A P-value less than 0.05 was considered statistically significant.
RESULTS
Patient demographics
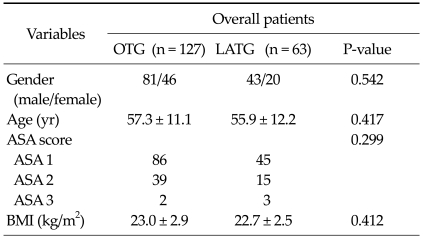
The clinical characteristics of the 190 patients are presented in Table 1. In comparison of patients overall, there was no difference in gender, age, ASA score, and BMI between the LATG and OTG groups.
Table 1.
Clinical characteristics of patients in the OTG and LATG groups
Values are presented as means ± SD or number.
OTG, open total gastrectomy; LATG, laparoscopic assisted total gastrectomy; ASA, American Society of Anesthesiologists; BMI, body mass index.
Early surgical outcomes of total gastrectomy
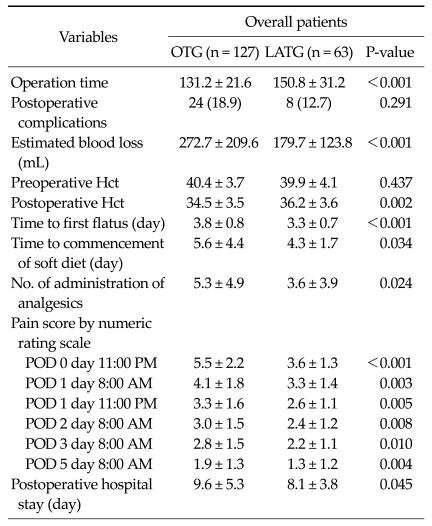
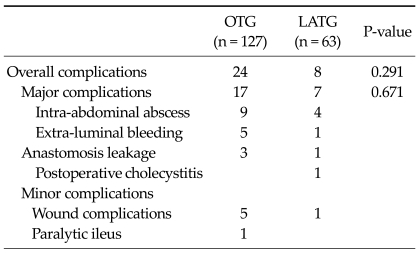
Table 2 presents early surgical outcomes in all patients. Operation time, it took longer to perform for LATG than OTG (LATG vs. OTG; 150.8 minutes vs. 131.2 minutes; P < 0.001). There was no significant difference for postoperative complication rate (LATG 12.7% vs. OTG 18.9%; P = 0.291). There were significant differences for the amount of estimated blood loss (LATG 179.7 mL vs. OTG 272.7 mL; P < 0.001) and postoperative change in hematocrit (Hct) (LATG 36.2 vs. OTG 34.5; P = 0.002). The mean day to first flatus (P < 0.001) and commencement of soft diet (P = 0.034) were checked earlier in the LATG group than in OTG group. The postoperative hospital stay was significantly shorter in the LATG group than in the OTG group (P = 0.045). NRS scores were significantly lower in the LATG group than in the OTG group at POD 0 at 11:00 AM, POD 1 at 8:00 AM, POD 1 at 11:00 PM, POD 2 at 8:00 AM, POD 3 at 8:00 AM, POD 5 at 8:00 AM (P < 0.001, P = 0.003, P = 0.005, P = 0.008, P = 0.010, P = 0.004).
Table 2.
Early surgical outcomes in patients who underwent OTG and LATG
Values are presented as means ± SD or number (%).
OTG, open total gastrectomy; LATG, laparoscopic assisted total gastrectomy; Hct, hematocrit; POD, post operative day.
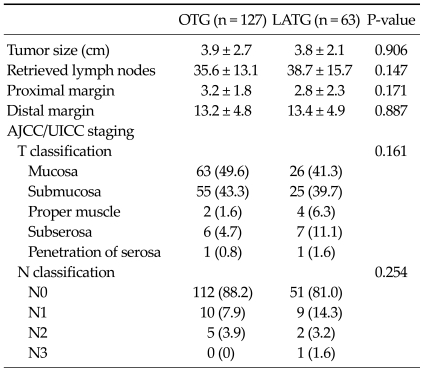
In pathologic results, there were no significant differences for tumor size, the number of retrieved lymph nodes, resection margins, tumor's depth and nodal staging (Table 3).
Table 3.
Pathologic results in patients who underwent OTG and LATG
Values are presented as means ± SD or number (%).
OTG, open total gastrectomy; LATG, laparoscopy assisted total gastrectomy; AJCC/UICC, American Joint Committee on Cancer/International Union Against Cancer.
Details of postoperative complications of LATG
In patients who underwent LATG, postoperative complications occurred in 8 patients. Intra-abdominal abscesses developed in 4 patients. In four of eight patients, extra-luminal bleeding, anastomosis leakage, cholecystitis, and wound complication occurred, respectively. Intra-abdominal abscess were managed by pig-tail insertion and administration of antibiotics. Extra-luminal bleeding was solved by laparoscopic reoperation for bleeding of suprapancreatic branch around the splenic artery. Anastomosis leakage was managed by conservative treatment and upper gastrointestinal series showed closure at postoperative 14 days. Postoperative acalculouscholecystitis was managed by laparoscopic cholecystectomy at postoperative day 9. One wound complication was treated conservatively (Table 4).
Table 4.
Lists of postoperative morbidities in patients who underwent OTG and LATG
Values are presented as number of patients.
OTG, open total gastrectomy; LATG, laparoscopic assisted total gastrectomy.
DISCUSSION
Many studies have reported that LADG with gastroduodenostomy (Billroth I) is as safe as that of open gastrectomy and less invasive than open gastrectomy [2-5]. However, LATG is not a generally accepted approach among many surgeons due to technical difficulties and high complication rate. Up to now, there have been some reports about the technical feasibility and safety compared with conventional open gastrectomy [7-10].
In practical procedures of LATG, we often had difficulty in reconstruction of anastomosis due to limited operation field. One of the difficulties was the process of clamping the distal esophagus through small incision. Another was the process of inserting and extracting of the straight needle into the purse-string clamp. A final difficulty was the process of anvil insertion into the esophagus. Especially in obese patients, we had difficulties in performing these processes.
Although it would be difficult to perform these processes in the LATG, our results showed the feasibility of LATG for postoperative complications as in other previous reports. We speculate that the accumulated experiences of surgeons in laparoscopic gastrectomy will aid us in getting over these difficulties. In our institution, we had experienced 1,100 cases of laparoscopic gastrectomy including LADG with gastroduodenostomy, LADG with Roux-en-Y reconstruction, LATG and total laparoscopic distal gastrectomy with delta-shaped anastomosis by 2008. From the accumulated experiences of experts, we could learn the method to reconstruct anastomosis easily. In reconstruction, we had difficulty in the shortened esophageal stump. To make the esophageal stump as long as we could, we additionally dissected the crus of the diaphragm. To extract the straight needle from the purse-string clamp easily, we rolled up the straight needle in the abdomen (Fig. 2). Also, we were able to obtain a shorter operation field by comparing the end of esophageal stump and the location of midline incision.
Fig. 2.
Rolling up the straight needle in the abdomen.
In the present study, we found that the early surgical outcomes were more favorable for patients who underwent LATG, as assessed by estimated blood loss, change of postoperative Hct, earlier bowel movement, less pain during recovery, and earlier hospital discharge. Although there was a report which longer operation time and CO2 pneumoperitoneum incur the possibility of several hemodynamic consequences in these patients [11]. We speculate that these improved early surgical outcomes resulted from the merits of LATG. We could minimize length of incision and manipulation by performing LATG.
In comparison to the pathologic results, although the current study could not be confirmed for oncologic results of LATG, there were no significant differences of pathologic results between the two groups (Table 3). In the present study, although there was no significance statistically for nodal staging, the proportion of metastatic lymph nodes is higher in LATG group than in OTG group. We assume that is just part of the reason why the LATG group has more advanced gastric cancer in tumor depth. Therefore, it will require a large volume and long-term follow up to evaluate the oncologic results of LATG.
We acknowledge that there is an inherent limitation in our retrospective study. Although the baseline characteristics of patients in OTG and LATG groups were similar, there was the possibility of bias in the study population. Also, although the methods of laparoscopic gastrectomy had been decided on by the selected patients, there was, again, the possibility of bias in the study population. Therefore, it will require a large, randomized, and prospective study to evaluate the effectiveness of LATG.
In conclusion, the present study has suggested that LATG is not only a feasible procedure for postoperative complications but also contributes to the improvement of early surgical outcomes including bowel movement, pain during recovery, and hospital discharge.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 2.Kim W, Song KY, Lee HJ, Han SU, Hyung WJ, Cho GS. The impact of comorbidity on surgical outcomes in laparoscopy-assisted distal gastrectomy: a retrospective analysis of multicenter results. Ann Surg. 2008;248:793–799. doi: 10.1097/SLA.0b013e3181887516. [DOI] [PubMed] [Google Scholar]
- 3.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 4.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 5.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874–880. doi: 10.1016/j.jamcollsurg.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Ryu KW, Kim YW, Lee JH, Nam BH, Kook MC, Choi IJ, et al. Surgical complications and the risk factors of laparoscopy-assisted distal gastrectomy in early gastric cancer. Ann Surg Oncol. 2008;15:1625–1631. doi: 10.1245/s10434-008-9845-x. [DOI] [PubMed] [Google Scholar]
- 7.Asao T, Hosouchi Y, Nakabayashi T, Haga N, Mochiki E, Kuwano H. Laparoscopically assisted total or distal gastrectomy with lymph node dissection for early gastric cancer. Br J Surg. 2001;88:128–132. doi: 10.1046/j.1365-2168.2001.01618.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee SE, Ryu KW, Nam BH, Lee JH, Kim YW, Yu JS, et al. Technical feasibility and safety of laparoscopy-assisted total gastrectomy in gastric cancer: a comparative study with laparoscopy-assisted distal gastrectomy. J Surg Oncol. 2009;100:392–395. doi: 10.1002/jso.21345. [DOI] [PubMed] [Google Scholar]
- 9.Mochiki E, Toyomasu Y, Ogata K, Andoh H, Ohno T, Aihara R, et al. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc. 2008;22:1997–2002. doi: 10.1007/s00464-008-0015-9. [DOI] [PubMed] [Google Scholar]
- 10.Sakuramoto S, Kikuchi S, Futawatari N, Katada N, Moriya H, Hirai K, et al. Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc. 2009;23:2416–2423. doi: 10.1007/s00464-009-0371-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Q, Mao Z, Jin J, Deng Y, Zheng M, Yu B. The safety of CO2 pneumoperitoneum for elderly patients during laparoscopic colorectal surgery. Surg Laparosc Endosc Percutan Tech. 2010;20:54–57. doi: 10.1097/SLE.0b013e3181ce1462. [DOI] [PubMed] [Google Scholar]