Abstract
Ganglioneuroma is the most differentiated tumor of neural crest origin and rarely arises in the adrenal gland. Ganglioneuroma is typically known to be benign, but very rarely can metastasize to distant sites. We report a case of a 31-year-old man with a huge adrenal mass with hepatic metastases.
Keywords: Ganglioneuroma, Adrenal gland, Adult, Metastasis
INTRODUCTION
Ganglioneuroma is the most differentiated of the neuroblastic tumor. It is a rare benign tumor and is most commonly identified between 10 and 40 years of age, usually in the posterior mediastinum or the retroperitoneum [1]. Ganglioneuroma may evolve as a mature tumor from the very beginning or by spontaneous or treatment-induced differentiating neuroblastoma or ganglioneuroblastoma [2]. It is composed of mature ganglion cells and Schwann cells and though benign, can very rarely metastasize to regional lymph nodes or to distant sites [3]. It is important to know this unusual case to avoid unnecessary wide excision. We report a case of a 31-year-old man who had a huge adrenal mass with hepatic metastases.
CASE REPORT
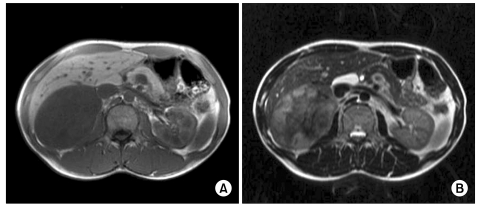
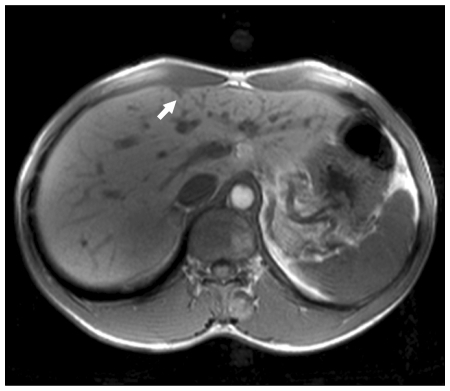
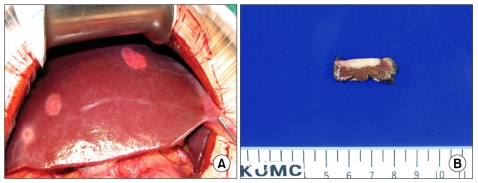
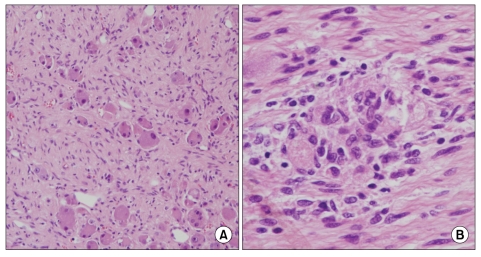
A 31-year-old man was admitted for evaluation of right adrenal mass, which is incidentally found. On admission, he did not have any other specific symptoms or past history. Computed tomography (CT) scan shows a 10.0 cm sized mass located at the suprarenal space and it was closely contacted to the inferior surface of the right hepatic lobe. Calcific and some septated lesions were found in the inferior portion of the mass. On magnetic resonance imaging (MRI), the mass showed low signal intensity on T1-weighted images and heterogeneous signal intensity on T2-weighted images (Fig. 1). Also, several small subcapsular lesions were present on the surface of hepatic segment 4 (Fig. 2). On operation, there were three white subcapsular nodules at the medial segment (Fig. 3A). They measured 3.0 × 2.4 × 1.0 cm, 1.8 × 1.5 × 0.7 cm, and 1.5 × 1.0 × 0.8 cm. Cut surfaces of the hepatic lesion showed pale tan to white and solid scar-like subcapsular masses. The largest lesion measured 2.7 × 2.0 × 0.7 cm (Fig. 3B). Frozen section for the mass was reported to be ganglioneuroma. The main mass and three metastatic lesions were resected out completely. The right adrenal mass was arising from adrenal medulla and measured 13.0 × 10.0 × 6.0 cm. Cut surface of the mass was pale tan to white and solid (Fig. 4). On microscopic examination, the adrenal mass was composed of clusters of mature ganglion cells and surrounding fascicles of Schwann-like cells. Also, there was only a focus of differentiating neuroblasts. So, we diagnosed this lesion as maturing ganglioneuroma (Fig. 5). The hepatic mass showed mature ganglioneuroma (Fig. 6). One year later after operation, there has been no sign of recurrence in the adrenal lesion.
Fig. 1.
Axial magnetic resonance images show a large mass in the right adrenal gland, which reveals hypointense signal intensity on T1-weighted image (A) and slightly hyperintense and heterogeneous signal intensity on T2-weighted image (B).
Fig. 2.
Axial T1-weighted axial magnetic resonace image shows a small wedge-shaped hypointense nodule in the subcapsular location of medial segment (arrow).
Fig. 3.
The liver shows three white and round subcapsular metastatic lesions (A). Cut surface of one of the wedge resected liver tissue shows pale tan to white scar-like lesion with infiltrative border at subcapsular area (B).
Fig. 4.
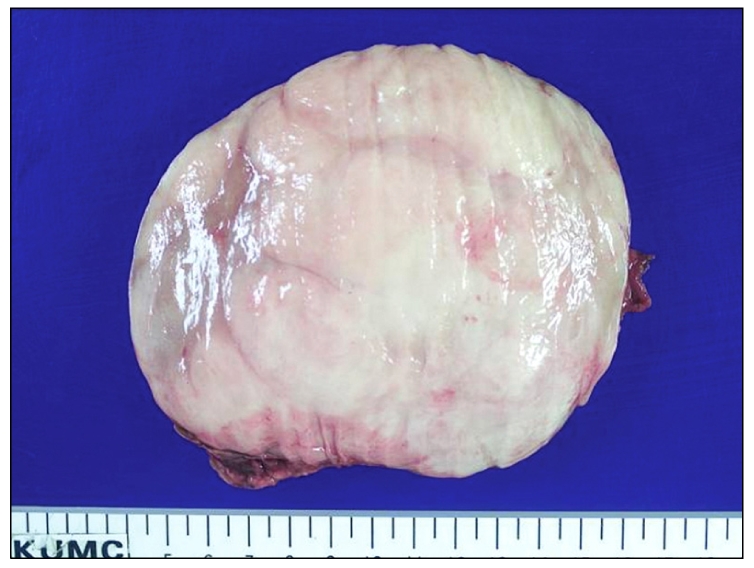
Cut surface of the right adrenal gland showed a round well demarcated pale tan, solid, and firm mass.
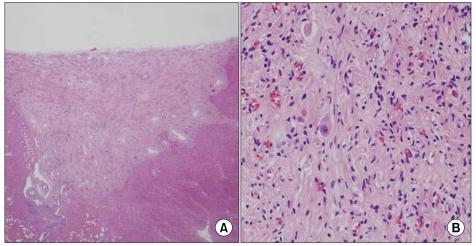
Fig. 5.
Section of the adrenal mass shows scattered ganglion cells surrounded by fascicles of schwann-like spindle cells (A, H&E, ×100). There is a focus of differentiating neuroblasts (B, H&E, ×200).
Fig. 6.
Section of the liver tissue shows scar-like lesion at low magnification (A, H&E, ×40) and scattered ganglion cells surrounded by fascicles of schwann-like spindle cells at high magnification (B, H&E, ×100).
DISCUSSION
Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma are tumors of varying maturity derived from the primordial neural crest cells that form the sympathetic nervous system [4]. The most common site of origin of neuroblastoma is the adrenal medulla (35% of cases) [5]. Neuroblastoma may undergo spontaneous regression and maturation to more mature neuroblastic tumors [6]. Regression of known neuroblastoma most often occurs in stage 4S tumors and takes 6 to 12 months on average [7]. Maturation also occurs; how often these tumors mature is unknown [8]. Ganglioneuroma is the most differentiated benign tumor of neural crest origin. Although the most common site is not the adrenal medulla but the posterior mediastinum (41.5%), the involvement of ganglioneuroma in the adrenal gland is relatively rare (21%) [9]. Ganglioneuromas most often manifest as an asymptomatic mass, which are discovered on routine radiographic studies for other lesions [10]. On MRI, ganglioneuroma shows low signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighited images [6]. Because neuroblastoma and ganglioneuroblastoma may mature to ganglioneuroma, certainly some ganglioneuromas arise from neuroblastoma and ganglioneuroblastoma [6]. Ganglioneuromas are also documented to have arisen in the neuroblastomas after chemotherapy [2]. This case is very unique because the patient doesn's have any past history of neuroblastoma or ganglioneuroblastoma and shows multiple hepatic metastases. Like this case, there was a previous report of adrenal ganglioneuroma with regional lymph node metastasis [3]. Also, there were several reports of metastatic ganglioneuromas which were found after therapy for neuroblastoma or ganglioneuroblastoma. It is believed that these tumors represent metastases of neuroblastoma or ganglioneuroblastoma, which are maturated to ganglioneuroma later. Treatment of choice is complete surgical resection if possible [6]. Usually, these patients have an excellent prognosis. But local recurrence and malignant transformation into malignant peripheral nerve sheath tumors have been reported, so periodic radiologic surveillance should be performed after resection [10].
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Banks E, Yum M, Brodhecker C, Goheen M. A malignant peripheral nerve sheath tumor in association with a paratesticular ganglioneuroma. Cancer. 1989;64:1738–1742. doi: 10.1002/1097-0142(19891015)64:8<1738::aid-cncr2820640830>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Ambros IM, Zellner A, Roald B, Amann G, Ladenstein R, Printz D, et al. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med. 1996;334:1505–1511. doi: 10.1056/NEJM199606063342304. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan R, Koliyadan KS, Krishnand G, Bhat SS. Retroperitoneal ganglioneuroma with lymphnode metastasis: a case report. Indian J Pathol Microbiol. 2007;50:32–35. [PubMed] [Google Scholar]
- 4.Joshi VV. Peripheral neuroblastic tumors: pathologic classification based on recommendations of international neuroblastoma pathology committee (modification of shimada classification) Pediatr Dev Pathol. 2000;3:184–199. doi: 10.1007/s100240050024. [DOI] [PubMed] [Google Scholar]
- 5.Morris JA, Shcochat SJ, Smith EI, Look AT, Brodeur GM, Cantor AB, et al. Biological variables in thoracic neuroblastoma: a Pediatric Oncology Group study. J Pediatr Surg. 1995;30:296–302. doi: 10.1016/0022-3468(95)90577-4. [DOI] [PubMed] [Google Scholar]
- 6.Lonergan GJ, Schwab CM, Suarez ES, Carlson CL. Neuroblastoma, ganglioneuroblastoma, and ganglioneuroma: radiologic-pathologic correlation. Radiographics. 2002;22:911–934. doi: 10.1148/radiographics.22.4.g02jl15911. [DOI] [PubMed] [Google Scholar]
- 7.MacMillan RW, Blanc WB, Santulli TV. Maturation of neuroblastoma to ganglioneuroma in lymph nodes. J Pediatr Surg. 1976;11:461–462. doi: 10.1016/s0022-3468(76)80204-7. [DOI] [PubMed] [Google Scholar]
- 8.Jaffe N. Neuroblastoma: review of the literature and an examination of factors contributing to its enigmatic charcter. Cancer Treat Rev. 1976;3:61–82. doi: 10.1016/s0305-7372(76)80005-9. [DOI] [PubMed] [Google Scholar]
- 9.Jain M, Shubha BS, Sethi S, Banga V, Bagga D. Retroperitoneal ganglioneuroma: report of a case diagnosed by fine-needle aspiration cytology, with review of the literature. Diagn Cytopathol. 1999;21:194–196. doi: 10.1002/(sici)1097-0339(199909)21:3<194::aid-dc9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 10.Geoerger B, Hero B, Harms D, Grebe J, Scheidhauer K, Berthold F. Metabolic activity and clinical features of primary ganglioneuromas. Cancer. 2001;91:1905–1913. doi: 10.1002/1097-0142(20010515)91:10<1905::aid-cncr1213>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]