Abstract
Purpose
Cyclooxygenase-2 is believed to be an important enzyme in the pathogenesis of colorectal cancer (CRC). Cytosolic phospholipase A2 (cPLA2), also, have been suggested to be related to the carcinogenesis of CRC. The aim of this study was to investigate cPLA2 expression and its relationship with prognostic significance in CRC.
Methods
Eighty-eight patients with colorectal cancer who underwent curative surgery were enrolled in this study. cPLA2 was examined in 88 primary CRCs by immunohistochemistry and we compared their expression with clinicopathologic findings, recurrence and survival in patients with CRC.
Results
The expression of cPLA2 was positive in 54.5% (48/88). The expression of cPLA2 was not correlated with clinicopathologic parameters. However, cPLA2 expression was significantly related with vascular endothelial growth factor expression. Kaplan-Meier analysis didn't show any clinical significance in disease-free survival and overall survival according to cPLA2 expression.
Conclusion
These results suggest that cPLA2 expression was not associated with the prognosis of CRC. However, further large-scale studies are needed to clarify the prognostic effect of cPLA2 in CRC.
Keywords: Cytosolic phospholipase A2, Prognosis, Colorectal cancer
INTRODUCTION
Colorectal cancer (CRC) is one of the most frequent malignancies worldwide, and remains the worldwide leading cause of cancer-related deaths [1,2]. In Korea, it is the second or third most common cancer and has shown a steep increase in recent decades [3].
Recently, much attention has been focused on the involvement of cyclooxygenase (COX) in tumor development and progression [4]. COX is a rate-limiting enzyme in the biosynthesis of prostaglandin (PG) from arachidonic acid (AA), and two isoforms have been characterized, COX-1 and COX-2. COX-2 is an inducible enzyme, but it is known to be constitutively overexpressed and to have an oncogenic effect in a variety of cancers, including colorectal cancer.
In addition to COX-2, PG production is also directly dependant on the availability of free AA. AA is released from membrane glycerophospholipids by fatty acid hydrolysis from its sn-2 position by phospholipase A2 (PLA2) and further metabolized by COX to produce PGs and thromboxanes [5]. PLA2 is a key regulatory enzyme in arachidonic acid metabolism, which leads to the synthesis of PGs via COX pathways. There are several types of PLA2 in human cells. Of these, 85 kDa IVA cytosolic PLA2 (cPLA2) has been suggested to be the major intracellular form and to play an essential role in stimulus induced AA release coupled with COX-2 [6,7]. Because of this key role of cPLA2 in prostaglandin production it has also been suggested to participate in intestinal tumorigenesis. But the clinical impact of cPLA2 expression on oncologic outcomes in CRC is unclear. In our previous data [8], we studied the impact of cPLA2, 15-prostaglandin dehydrogenase, and COX-2 expression on tumor progression in CRC and we demonstrated cPLA2 expression was closely associated with COX-2 expression and might have an important role in tumor progression.
Therefore, as a consecutive study, we investigated the survival of patients with CRC to determine the prognostic significance with respect to cPAL2 expression status.
METHODS
Patients and tissue samples
Eighty-eight patients who had undergone surgical resection for primary sporadic colorectal carcinoma by a single colorectal surgeon at the Department of Surgery, Chosun University Hospital between March 2002 and December 2005 were included in this study. No patient had a history of hereditary CRC syndrome or regularly used aspirin-like drugs. Patients that received pre-operative chemotherapy or radiotherapy were not included. Enrolled patients were followed up until death or December 2008; median post-operative follow-up duration was 44.16 ± 19.50 months. Thirty eight patients had colon cancer and 50 patients had rectal cancers, and mean patient age was 63.97 ± 11.67 years. Samples were graded by pathologists according to the pathological features of the tumors, i.e., histologic grade, lymph node metastasis, distant metastasis, and tumor stage (American Joint Committee on Cancer tumor, nodes, metastasis [TNM] classification). In all cases, archived H&E stained tissue slides were obtained for confirmation of pathological features and for the selection of suitable tissue blocks for immunohistochemical analysis.
Immunohistochemistry
A universal immunoenzyme polymer method was used for immunostaining. Four-µm thick sections were cut from formalin-fixed, paraffin-embedded tissue blocks, mounted on poly-lysine-coated slides, dewaxed in xylene, and rehydrated through a graded ethanol series. After deparaffinization, antigen retrieval treatment was performed at 121℃ (autoclave) for 15 minutes in 10 mmol/L sodium citrate buffer (pH 6.0), and sections were then treated with 3% hydrogen peroxide in methanol solution for 20 minutes in order to quench endogenous peroxidase activity. Nonspecific bindings were blocked by treating slides with Ultra V Block (UltraVision Plus Detection System, Thermo Fisher Scientific Inc., Fremont, CA, USA) and incubation for 5 minutes at room temperature. The primary antibodies used were; mouse monoclonal antibody to cPLA2 (1:100 4℃ for overnight; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and rabbit polyclonal antibody to vascular endothelial growth factor (VEGF) (1:100 37℃ for one hour, Santa Cruz Biotechnology Inc.). Each slide was washed four times in phosphate buffered saline (PBS). Biotinylated goat anti-mouse or goat anti-rabbit antibody (UltraVision Plus Detection System) was applied and incubated for 5 minutes at room temperature. Slides were washed again four times in PBS. Streptavidin-Alkaline Phosphate conjugate (UltraVision Plus Detection System) was then applied and incubated for 5 minutes at room temperature. Slides were washed four times in PBS. Fast Red/Naphtol Phosphate substrate was applied to the sections for visualization and incubated 10 to 20 minutes. Sections were counterstained with Mayer's hematoxylin for 20 seconds before air drying and coverslipping. Normal mouse serum IgG (Vector Laboratories Inc., Burlingame, CA, USA) was used in place of the primary antibody as a negative control. Positive control tissue was colon cancers and inflammatory cells stained positive for cPLA2 and VEGF. All experiments were performed in duplicate.
Immunohistochemical staining
Each slide was assessed by a pathologist unaware of patient details. For cPLA2, extent of staining was graded as follows: 0 - staining in less than 1% of tumor cells; 1 - staining in 1 to 20%; 2 - staining in 20 to 50%; and 3 - staining in more than 50%. Overall intensity of staining was also assessed as follows: 0 no staining; 1 weak staining; 2 moderate staining; and 3 strong staining. Final scores (range, from 0 to 9) were obtained by multiplying staining extents and intensities. Final scores were described as follows: 0, no expression; 1 to 3, weak expression; 4 to 6, moderate expression; and 7 to 9, strong expression [7]. For statistical analysis, no expression and weak expression were combined and described as negative for expression, and moderate and strong expression were combined and described as positive for expression.
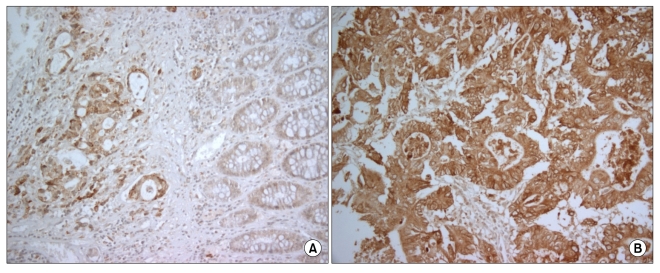
VEGF staining was defined as positive when >10% of tumor cells were stained, and as negative when ≤10% of tumor cells were stained [9]. Representative examples of cPLA2 in normal tissue and cancer tissue are shown in Fig. 1.
Fig. 1.
Immunohistochemical staining. (A) Negative cytosolic phospholipase A2 (cPLA2) in normal tissue. (B) Positive cPLA2 in cancer tissue.
Statistical analysis
The χ2 test and Fisher's exact probability were used to analyze the relationship of cPLA2 according to clinicopathologic characteristics and VEGF expression. The Kaplan-Meier method was used to estimate survival as a function of time and recurrence, survival differences were analyzed with the log-rank test. Significance was defined as P-values of <0.05. The SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
RESULTS
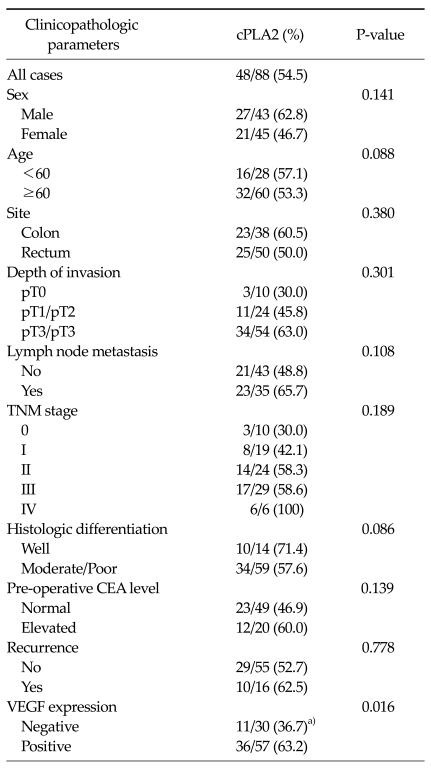
Expression of cPLA2 (Table 1) (revised from our previous study [8])
Table 1.
Correlation of clinicopathologic parameters with cPLA2 expressions
cPLA2, cytosolic phospholipase A2; TNM, tumor, nodes, metastasis; CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor.
a)Significant: negative VEGF expression vs. positive VEGF expression, P = 0.016 (Revised from Lim SC, Cho H, Lee TB, Choi CH, Min YD, Kim SS, et al. Yonsei Med J 2010;51:692-9, with permission of Yonsei University College of Medicine) [8].
cPLA2 staining was observed in the smooth muscle of the muscularis propria and the muscularis mucosa, in some superficial interstitial cells, and in rare endothelial cells. In normal epithelial cells there was no staining or weak cPLA2 staining (Fig. 1A).
cPLA2 expression was positive in 54.5% (48/88) of tumor samples and was positive in 60.5% (23/38) of colon cancers and 50.5% (25/50) of rectal cancers. When classified by the depth of invasion, cPLA2 expression was positive in 30.0% (3/10) of pT0, 45.8% (11/24) of pT1/pT2 and 63.0% (34/54) of pT3/pT4 (P = 0.287). According to the lymph node status, cPLA2 expression was positive in 48.8% of absent lymph node involvement and in 65.7% of present lymph node involvement (P = 0.194). According to TNM stage, cPLA2 expression was positive in 30.3% (3/10) of stage 0, 42.1% (8/19) of stage I, 58.3% (14/24) of stage II, 58.6% (17/29) of stage III and 100% (6/6) of stage IV (P = 0.543).
Also, with regard to pre-operative carcinoembryonic antigen (CEA) levels, the positive expression rate of cPLA2 was 46.9% with normal pre-operative CEA levels and 60.0% with elevated CEA levels. Although cPLA2 expression was not significantly related to the clinicopathologic parameters, cPLA2 expression tended to be high in patients with an advanced stage or unfavorable clinicopathologic features such as pT3/pT4, positive lymph node metastasis and elevated pre-operative CEA levels. Moreover cPLA2 expression was significantly related to VEGF expression (P = 0.016). cPLA2 expression was positive in 63.2% (36/57) of patients who showed positive VEGF expression compared to 36.7% (11/30) of negative VEGF expression.
Correlation with patient survival
For evaluation of patient survival, we precluded stage 0, I and IV patients. So we evaluated the survival analysis in a total of 55 patients with stage II, and III patients of colorectal cancer according to the status of cPLA2 expression.
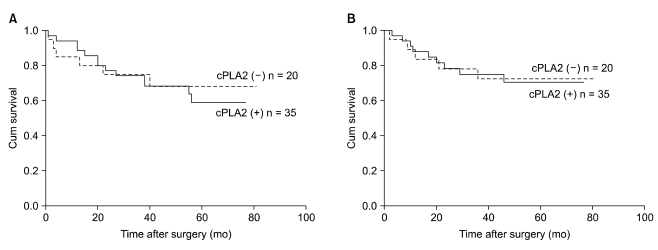
Five-year overall survival was 70.4% in patients showing positive cPLA2 expression and 72.4% in negative cPLA2 expression (P = 0.657). Five-year disease-free survival was 59.2% in patients showing positive cPLA2 expression and 69.3% in negative cPLA2 expression (P = 0.360). Using Kaplan-Meier survival analysis, there was no statistically significant difference in overall survival rates and disease free survival rates according to cPLA2 expression (Fig. 2). When we investigated the survival rate of colon and rectal cancer according to cPLA2 expression respectively, no significant difference was shown, statistically.
Fig. 2.
Overall survival (A) and the disease-free survival (B) curves of patients according to the status of cytosolic phospholipase A2 (cPLA2) expression.
DISCUSSION
We evaluated the expression profiles of cPLA2 in patients with colorectal cancer in a previous study. Consecutively, in this study, we tried to demonstrate that the expression of cPLA2 had prognostic significance in patients with colorectal cancer.
It has been suggested that colonic tumors release free AA and usually produce more PGs compared with normal mucosa tissue [10]. cPLA2 is a key enzyme for PGs and cPLA2 involvement in intestinal tumorigenesis has been suggested. For the first time, Soydan et al. [11] demonstrated that human colon tumors and associated normal mucosa and submucosa contain high molecular weight cPLA2. Other studies have already reported overexpression of cPLA2 found in 35 to 50% of CRC [7,12]. We found that cPLA2 was overexpressed in 54.5% of tissue sample tested but cPLA2 expression was not related to other clinicopathologic parameters, except for VEGF expression. VEGF expression showed a statistically significant correlation with cPLA2 expression (P = 0.016). These results strongly suggest that cPLA2 plays an important role in tumor development and progression in CRC. Similar to our study, it was reported that there was an association between a high number of cPLA2 positive stromal cells and a strong VEGF expression [13].
cPLA2 expression in animal models has been suggested to be pro-tumourigenic. Homozygous deletion of the cPLA2 gene in mice was found to dramatically reduce small intestinal polyp development but not colonic polyp development, suggesting that cPLA2 had an oncologic effect and a role in tumor promotion [14,15]. On the other hand, in azoxymethane-induced mouse models, down regulation of cPLA2 attenuated tumor necrosis factor-alpha mediated apoptosis and facilitated tumor progression [16]. Furthermore, knock-out of cPLA2 was found to enhance tumorigenesis in mice, suggesting a proapoptotic role of cPLA2 expression [17].
As shown in animal models, there are highly variable results on cPLA2 expression in human CRC. Some have suggested that cPLA2 is overexpressed [11-13,18], others, on the other hand, have concluded to the contrary [19]. Furthermore, in another study, researchers reported that the role of cPLA2 seems not to be the rate-limiting step in PGs formation and cPLA2 plays a minor or no role in human colorectal carcinogenesis [20].
One previous study showed that acetylsalicylic acid-induced downregulation of cPLA2 expression supports growth inhibition and apoptosis in colon cancer cells [21]. Panel et al. [7] reported that, in immunohistochemistry of 65 patients with colorectal cancers and Western blot of several colorectal cancer cell lines, cPLA2 was overexpressed in about half of the cases, and that cPLA2 expression was correlated with COX-2 expression, which strongly suggests that cPLA2 plays an important role in tumor development and progression in CRC. But other studies showed that cPLA2 plays an important role in tumor necrosis factor α-induced apoptosis in human colon cancer cells and that colon cancer growth is favored when intracellular AA levels are suppressed by inhibition of cPLA2 [19].
None of these studies have demonstrated the prognostic effect of cPLA2 expression in patients with CRC. Regarding patient survival and cPLA2 expression, we found that cPLA2 expression in CRC was not associated with patient survival. This is the first investigation in which survival of patients is evaluated according to cPLA2 expression in CRC. There was only one report in which quantification of group IIA PLA2, not cPLA2, expression seems to provide valuable prognostic information in stage II CRC [22].
Alterations in the levels and activity of cPLA2 have been associated with cancer pathogenesis in other organs. cPLA2 expression is increased in several human cancers including small bowel, lung, bile duct and liver [12,23-25]. Proliferation of prostate cancer is also dependant on the action of cPLA2 [26].
In conclusion, these results suggest that cPLA2 is constitutively overexpressed and might have an important role in tumor progression. However, cPLA2 expression does not seem to play a role in the prognosis of CRC. Further large scaled studies are needed to clarify the prognostic effect of cPLA2 in CRC.
ACKNOWLEDGEMENTS
This work was supported by the 2010 Chosun University research fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Boyle P, Zaridze DG, Smans M. Descriptive epidemiology of colorectal cancer. Int J Cancer. 1985;36:9–18. doi: 10.1002/ijc.2910360103. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25. doi: 10.1093/bmb/64.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. Seoul: Ministry for Health, Welfare and Family Affairs; 2009. [Google Scholar]
- 4.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–7916. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 5.Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 6.Leslie CC. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- 7.Panel V, Boëlle PY, Ayala-Sanmartin J, Jouniaux AM, Hamelin R, Masliah J, et al. Cytoplasmic phospholipase A2 expression in human colon adenocarcinoma is correlated with cyclooxygenase-2 expression and contributes to prostaglandin E2 production. Cancer Lett. 2006;243:255–263. doi: 10.1016/j.canlet.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 8.Lim SC, Cho H, Lee TB, Choi CH, Min YD, Kim SS, et al. Impacts of cytosolic phospholipase A2, 15-prostaglandin dehydrogenase, and cyclooxygenase-2 expressions on tumor progression in colorectal cancer. Yonsei Med J. 2010;51:692–699. doi: 10.3349/ymj.2010.51.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi JY, Jang KT, Shim YM, Kim K, Ahn G, Lee KH, et al. Prognostic significance of vascular endothelial growth factor expression and microvessel density in esophageal squamous cell carcinoma: comparison with positron emission tomography. Ann Surg Oncol. 2006;13:1054–1062. doi: 10.1245/ASO.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Bennett A, Civier A, Hensby CN, Melhuish PB, Stamford IF. Measurement of arachidonate and its metabolites extracted from human normal and malignant gastrointestinal tissues. Gut. 1987;28:315–318. doi: 10.1136/gut.28.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soydan AS, Tavares IA, Weech PK, Temblay NM, Bennett A. High molecular weight phospholipase A2 and fatty acids in human colon tumours and associated normal tissue. Eur J Cancer. 1996;32A:1781–1787. doi: 10.1016/0959-8049(96)00166-9. [DOI] [PubMed] [Google Scholar]
- 12.Wendum D, Svrcek M, Rigau V, Boëlle PY, Sebbagh N, Parc R, et al. COX-2, inflammatory secreted PLA2, and cytoplasmic PLA2 protein expression in small bowel adenocarcinomas compared with colorectal adenocarcinomas. Mod Pathol. 2003;16:130–136. doi: 10.1097/01.MP.0000052101.58988.1F. [DOI] [PubMed] [Google Scholar]
- 13.Wendum D, Comperat E, Boëlle PY, Parc R, Masliah J, Trugnan G, et al. Cytoplasmic phospholipase A2 alpha overexpression in stromal cells is correlated with angiogenesis in human colorectal cancer. Mod Pathol. 2005;18:212–220. doi: 10.1038/modpathol.3800284. [DOI] [PubMed] [Google Scholar]
- 14.Takaku K, Sonoshita M, Sasaki N, Uozumi N, Doi Y, Shimizu T, et al. Suppression of intestinal polyposis in Apc(delta 716) knockout mice by an additional mutation in the cytosolic phospholipase A(2) gene. J Biol Chem. 2000;275:34013–34016. doi: 10.1074/jbc.C000585200. [DOI] [PubMed] [Google Scholar]
- 15.Hong KH, Bonventre JC, O'Leary E, Bonventre JV, Lander ES. Deletion of cytosolic phospholipase A(2) suppresses Apc(Min)-induced tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:3935–3939. doi: 10.1073/pnas.051635898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong M, Guda K, Nambiar PR, Rezaie A, Belinsky GS, Lambeau G, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–315. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 17.Ilsley JN, Nakanishi M, Flynn C, Belinsky GS, De Guise S, Adib JN, et al. Cytoplasmic phospholipase A2 deletion enhances colon tumorigenesis. Cancer Res. 2005;65:2636–2643. doi: 10.1158/0008-5472.CAN-04-3446. [DOI] [PubMed] [Google Scholar]
- 18.Osterström A, Dimberg J, Fransén K, Söderkvist P. Expression of cytosolic and group X secretory phospholipase A(2) genes in human colorectal adenocarcinomas. Cancer Lett. 2002;182:175–182. doi: 10.1016/s0304-3835(02)00081-2. [DOI] [PubMed] [Google Scholar]
- 19.Dong M, Johnson M, Rezaie A, Ilsley JN, Nakanishi M, Sanders MM, et al. Cytoplasmic phospholipase A2 levels correlate with apoptosis in human colon tumorigenesis. Clin Cancer Res. 2005;11:2265–2271. doi: 10.1158/1078-0432.CCR-04-1079. [DOI] [PubMed] [Google Scholar]
- 20.Dimberg J, Samuelsson A, Hugander A, Söderkvist P. Gene expression of cyclooxygenase-2, group II and cytosolic phospholipase A2 in human colorectal cancer. Anticancer Res. 1998;18(5A):3283–3287. [PubMed] [Google Scholar]
- 21.Yu HG, Huang JA, Yang YN, Luo HS, Yu JP, Meier JJ, et al. Inhibition of cytosolic phospholipase A2 mRNA expression: a novel mechanism for acetylsalicylic acid-mediated growth inhibition and apoptosis in colon cancer cells. Regul Pept. 2003;114:101–107. doi: 10.1016/s0167-0115(03)00084-3. [DOI] [PubMed] [Google Scholar]
- 22.Buhmeida A, Bendardaf R, Hilska M, Laine J, Collan Y, Laato M, et al. PLA2 (group IIA phospholipase A2) as a prognostic determinant in stage II colorectal carcinoma. Ann Oncol. 2009;20:1230–1235. doi: 10.1093/annonc/mdn783. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto S, Shoji M, Setoguchi Y, Kato M, Hashizume S, Ichikawa A, et al. Molecular cloning of the 31 kDa cytosolic phospholipase A2, as an antigen recognized by the lung cancer-specific human monoclonal antibody, AE6F4. Cytotechnology. 1995;17:103–108. doi: 10.1007/BF00749397. [DOI] [PubMed] [Google Scholar]
- 24.Wu T, Han C, Lunz JG, 3rd, Michalopoulos G, Shelhamer JH, Demetris AJ. Involvement of 85-kd cytosolic phospholipase A(2) and cyclooxygenase-2 in the proliferation of human cholangiocarcinoma cells. Hepatology. 2002;36:363–373. doi: 10.1053/jhep.2002.34743. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Yuan YW, Zhang JR, Zhou DY. Up-regulation of c-myc expression in MCF-7/Adr human breast cancer cells and its association with resistance against doxorubicin. Di Yi Jun Yi Da Xue Xue Bao. 2002;22:124–126. [PubMed] [Google Scholar]
- 26.Sved P, Scott KF, McLeod D, King NJ, Singh J, Tsatralis T, et al. Oncogenic action of secreted phospholipase A2 in prostate cancer. Cancer Res. 2004;64:6934–6940. doi: 10.1158/0008-5472.CAN-03-3018. [DOI] [PubMed] [Google Scholar]