Abstract
The tetraspanin CD37 is widely expressed in B-cell malignancies and represents an attractive target for immunotherapy with mAbs. We have chimerized a high-affinity mouse Ab to CD37 and engineered the CH2 domain for improved binding to human Fcγ receptors. The resulting mAb 37.1 showed high intrinsic proapoptotic activity on malignant B cells accompanied by homotypic aggregation. Furthermore, the Ab-mediated high Ab-dependent cell-mediated cytotoxicity (ADCC) on lymphoma and primary CLL cells. mAb 37.1 strongly depleted normal B cells as well as spiked B-lymphoma cells in blood samples from healthy donors as well as malignant B cells in blood from CLL patients. In all assays, mAb 37.1 was superior to rituximab in terms of potency and maximal cell lysis. A single dose of mAb CD37.1 administered to human CD37-transgenic mice resulted in a reversible, dose-dependent reduction of peripheral B cells. In a Ramos mouse model of human B-cell lymphoma, administration of mAb 37.1 strongly suppressed tumor growth. Finally, a surrogate Fc-engineered Ab to macaque CD37, with in vitro proapoptotic and ADCC activities very similar to those of mAb 37.1, induced dose-dependent, reversible B-cell depletion in cynomolgus monkeys. In conclusion, the remarkable preclinical pharmacodynamic and antitumor effects of mAb 37.1 warrant clinical development for B-cell malignancies.
Introduction
Chemoimmunotherapy incorporating the CD20-specific mAb rituximab has emerged as standard clinical practice for treatment of B-cell non-Hodgkin lymphoma (NHL)1–3 and chronic lymphocytic leukemia (CLL).4,5 However, a significant portion of patients eventually relapse after rituximab treatment, creating a need for alternative approaches. A fully human CD20 Ab, ofatumumab, which was recently approved for the treatment of CLL patients who are refractory to fludarabine and alemtuzumab, is under active investigation as first-line therapy in combination with chemotherapy.6
Although rituximab displays clinically relevant antitumor activity in combination with chemotherapy, direct proapoptotic and Ab-dependent cell-mediated cytotoxicity (ADCC) mediated by rituximab is moderate.7 Work with the first generation of mAbs including rituximab has provided valuable insights into how to engineer novel mAbs with high antitumor efficacy. Advances in our understanding of the interactions of the Abs with their receptors on immune effector cells provide a rationale for generating engineered Abs with high affinity to Fcγ receptors that translates into more potent ADCC. In particular, clinical response to rituximab has been correlated with the presence of certain allelic variants of FcγR exhibiting increased binding to the Ab.8,9 To date, several mAbs optimized for FcγR affinity have been described, including mAbs directed against CD20 (GA101,7 LFB-R603,10 AME-D11); in particular, the CD20 mAb GA101, shows enhanced ADCC relative to rituximab and is under investigation for therapy of B-cell leukemias and lymphomas.7
As an alternative to the prototypic B-cell target CD20, a variety of other leukemia and lymphoma cell-surface Ags have been investigated, including CD19, CD22, CD23, CD37, CD40, and CD80. The tetraspanin CD37 is predominantly expressed on mature B cells, with highest expression levels on peripheral blood B cells, and lower levels on plasma cells. Low-level expression has been reported on T cells, granulocytes, and monocytes, while CD10+ precursor cells are CD37-negative.12–15 Importantly, strong and homogeneous CD37 expression has been detected on the surface of B-cell leukemia and lymphoma cells.16–19 This favorable expression profile led to the exploration of CD37-directed radioimmunotherapy in patients suffering from B-cell lymphoma using the murine CD37 mAb MB-1.20,21 A CD37-specific small modular immunopharmaceutical (SMIP), an IgG1-like single-chain Ab with a truncated constant region, is in early clinical development for CLL.22
We report here on the pharmacologic profile of a novel mAb against human CD37 that we have generated and engineered for improved effector function. mAb 37.1 is an Fc-engineered mouse/human chimeric IgG1 Ab in which 2 amino acids have been replaced in the Fc region to increase affinity to FcγRIIIa.
Methods
Abs
All monoclonal IgG1 Abs (mAbs) against human CD37 used in this study were expressed in DHFR-deficient Chinese hamster ovary (CHO) DG44 suspension cells under serum-free conditions and purified via MabSelect Protein A affinity chromatography (GE Healthcare). mAb 37.1-wt is a mouse-human chimeric IgG1 Ab derived from the murine mAb G28.1. Fc variants of mAb 37.1-wt were constructed by introducing amino acid substitutions in the Fc CH2 domain of the wild-type IgG1 via quick change mutagenesis.23 A surrogate IgG1 mAb 37surr Ab was generated by immunization of mice with cynomolgus monkey CD37-expressing recombinant cells and chimerized and Fc-engineered as for the anti–human CD37 mAbs. Clinical-grade rituximab was obtained from Roche; clinical-grade alemtuzumab was obtained from Berlex.
Fcγ receptors
Ig-like FcγRIIIa (CD16a; allelic variants 158V and 158F) and FcγRI (CD64) were expressed in DHFR-deficient CHO DG44 suspension cells under serum-free conditions and subsequently purified via IgG Sepharose 6 FF affinity chromatography (GE Healthcare).
Cell lines, donors, and patients
Ramos Burkitt lymphoma cells were obtained from ATCC (CRL-1596). Master cell banks and working cell banks were established according to Boehringer Ingelheim Regional Center Vienna standards.
Blood samples from healthy volunteers were obtained from the Austrian Red Cross. Patient samples were provided by the University of Ulm, Germany, and were obtained after informed consent in accordance with the Declaration of Helsinki and with approval of the UK Home Office and the cantonal ethical committee in Austria.
FACS Scatchard analysis
The affinity of the Ab to cellular Ag was calculated by Scatchard analysis using a FACS-based method for quantification of bound Ab, using CD37-positive Ramos Burkitt lymphoma as target cell, as detailed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Surface plasmon resonance
All kinetic interaction experiments between CD37 mAbs and FcγRs CD16 (allelic variants V158 and F158) and CD64, respectively, were performed at 25°C using a Biacore T200 instrument (GE Healthcare), as detailed in supplemental Methods.
Apoptosis assay
Apoptosis activity was determined in Ramos Burkitt lymphoma cells and primary CLL cells after 24-hour incubation with mAbs in the presence or absence of an IgG cross-linking Ab (goat anti–human IgG, I3382; Sigma-Aldrich) by annexin V staining. Assays were performed in cultured cells in triplicate, and in CLL blood samples from 6 individual donors, as detailed in supplemental Methods. The degree of apoptosis induction is displayed as the total percentage of annexin V–positive cells corrected for spontaneous lysis.
ADCC assay
ADCC assays with cultured Ramos cells or primary CLL cells were performed by 3-hour cocultivation of target (T) cells and IL-2–stimulated effector (E) PBMCs from healthy volunteers (E:T ratio, 25:1). Cytotoxicity was determined by measurement of released lactate dehydrogenase (LDH) and the percentage of specifc cell lysis was calculated according to the formula (E:T cell mix − effector cell control − spontanous release)/(maximal release − spontaneous release). The cocultivation of effector cells with target cells in presence of Ab was performed as detailed in supplemental Methods.
In vivo pharmacokinetic and pharmacodynamic studies in CD37-transgenic mice and cynomolgus monkey
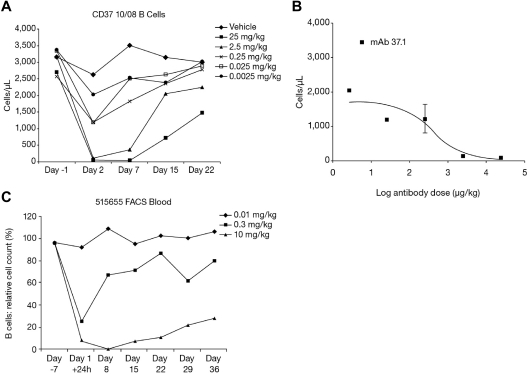
Pharmacodynamic (PD) effects in peripheral blood cells were assessed in HuCD37-transgenic mice, and pharmacokinetic (PK)/PD effects were assessed in peripheral blood of cynomolgus monkeys. Mouse strains and care are fully detailed in supplemental Methods. A single injection of human CD37 mAb (at doses of 0.0025/0.025/0.25/2.5/25 mg/kg) was administered into the lateral tail vein of female HuCD37-transgenic (murine CD37 knockout [ko]; human CD37 knock-in [ki]) mice (3 animals per group). Vehicle-treated animals served as a control group. Peripheral blood cell counts were determined 1 day before dosing and on days 2, 7, 15, and 22 thereafter. B-cell counts were determined by FACS analysis of B220+ lymphocytes. B-cell counts were normalized to control values at the same day and the degree of B-cell depletion relative to control was calculated for each animal.
Cynomolgus monkeys (Macaca fascicularis) received a single dose of mAb 37surr via tail-vein infusion at an infusion rate of ∼ 0.5 mL/min over a 30-minute period. Blood samples were collected from the femoral vein and transferred into tubes containing EDTA as anticoagulant. Blood samples were assayed by flow cytometry using the following marker combinations: CD20/CD21/CD27/CD40 for B cells and CD3/CD4/CD8/CD25 for T cells. B-cell counts were normalized to baseline values on day −7 (7 days before start of treatment) and the degree of B-cell and T-cell depletion relative to baseline was calculated for each animal. For PK analysis of mAb CD37surr, cynomolgus blood samples were obtained from each animal for plasma concentrations at pre-dose, 0.5, 2, 8, and 24 hours after dosing and then on days 4, 8, 15, 22, 29, and 36. A sandwich ELISA was used to quantify total human IgG using a polyclonal anti–human IgG (H&L chain) Ab non–cross-reactive with monkey IgG. Plasma concentration-time data were analyzed by noncompartmental methods with the program ToxKin 3 using individual plasma concentration-time curves.
In vivo antitumor efficacy studies
To establish subcutaneous tumors in mice, Ramos cells were harvested by centrifugation, washed and resuspended in PBS + 5% FCS at 5 × 107 cells/mL. A 100-μL cell suspension containing 5 × 106 cells was then injected subcutaneously into the right flank of the mice (1 site per mouse). Mice were randomly distributed between the treatment and the vehicle control group (15 days after cell injection) when tumors were well established and had reached diameters of 6-8 mm. Abs were administered IV into the tail vein in an administration volume of 100 μL per mouse every third or fourth day. The tumor diameter was measured 3 times a week (Monday, Wednesday, and Friday) with a caliper. The volume of each tumor (in mm3) was calculated according to the formula “tumor volume = length × diameter2 × π/6.” To monitor side effects of treatment, mice were inspected daily for abnormalities and body weight was determined 3 times a week. Animals were killed at the end of the study when the control tumors reached a median size of ∼ 1500 mm3.
Statistical analysis
In PD studies of mAb 37.1 in mice, an ANCOVA with baseline values (day −1) as covariate and the factor dose group (vehicle control, and 0.0025, 0.025, 0.25, 2.5, and 25 mg/kg) was performed for each time point (day 2, 7, 15, and 22). The error term of the ANCOVA was taken as estimate for the variability in the following t tests, comparing the dose groups versus vehicle control. For the adjustment of the respective P values for multiple testing a procedure for ordered alternatives was used. Assuming an increasing effect with increasing dose, the highest dose was tested first and treatment at next lowest dose was only implemented if a statistically significant difference versus the vehicle control group was demonstrated. A P value < .05 was considered to show a difference between the dose groups and vehicle control. In PD studies in the monkey, an ANOVA for repeated measurements with the fixed-factors dose group and time point was performed.
Results
Binding to CD37
Binding to cellular Ag was determined by FACS Scatchard analysis using Ramos Burkitt lymphoma cells as the target. mAb 37.1 bound surface-expressed CD37 with an apparent affinity of 1.3nM (mean, 3 experiments). The Ab bound to recombinant CHO cells expressing human CD37 with similar affinity, but did not bind to the parental CHO cells (data not shown).
Binding to Fcγ receptors
Binding parameters of CD37 mAbs interacting with Fcγ receptors CD16-158V, CD16-158F, and CD64 were determined by performing kinetic surface plasmon resonance (SPR) experiments as detailed in supplemental Methods. Data from 1 representative experiment of 3 are summarized in Table 1 and in supplemental Table 1. Non–Fc-engineered mAb 37.1-wt bound CD16-158V and CD16-158F with affinities (KD1) of 215nM and 1205nM, respectively (Table 1; supplemental Figure 2A,C; supplemental Table 1). Our Fc-engineered mAb 37.1 displayed a > 50-fold increase in affinity to CD16-158V (KD1 = 4nM) and a > 40-fold increase in affinity to CD16-158F (KD1 = 28nM), respectively (Table 1; supplemental Figure 2B,D; supplemental Table 1). In addition, we analyzed binding of both mAbs to CD64 and observed an 8-fold increase in affinity of our Fc-engineered mAb 37.1 (KD = 20pM) compared with mAb 37.1-wt (KD = 164pM; Table 1, supplemental Figure 2E-F, supplemental Table 1).
Table 1.
Biacore analysis of mAb 37.1 and mAb 37.1-wt binding to Fcγ receptors
| Fcγ receptor | Dissociation constant, nM | 37.1-wt | 37.1 |
|---|---|---|---|
| CD16 158V | KD1 | 215 | 4 |
| CD16 158 F | KD1 | 1205 | 28 |
| CD64 | KD1 | 0.16 | 0.02 |
Results from 1 representative experiment of 3 are shown.
Proapoptotic activity
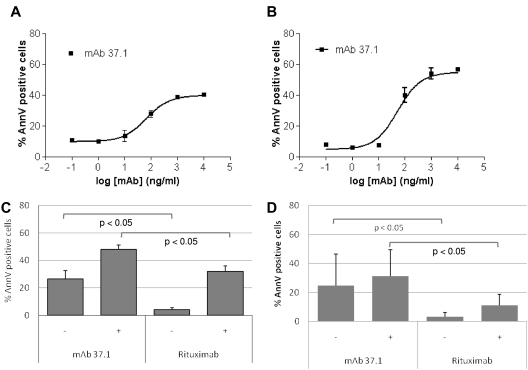
Direct apoptosis induction in the absence of complement factors and effector cells was assessed on Ramos Burkitt lymphoma cells. Results of annexin V staining after 24-hour exposure showed that mAb 37.1 exerted potent proapoptotic activity without IgG cross-linking, which was further increased by cross-linking with anti-IgG (representative examples from 3 independent assays for each condition are shown in Figure 1A-B). The proportions of annexin V–positive cells induced by mAb 37.1 were 37% (range 30%-43%) in the absence and 46% (range 39%-52%) in the presence of a cross-linking Ab. Apoptosis induction was concentration dependent with EC50 values in the range of 40-100 ng/mL.
Figure 1.
Apoptosis assays. Apoptosis induction (% total annexin V positivity) of Ramos lymphoma cells after 24-hour incubation with: (A) increasing concentrations of mAb 37.1 in the absence of an IgG cross-linking Ab; (B) increasing concentrations of mAb 37.1 in the presence of an IgG cross-linking Ab; and (C) 100 μg/mL mAb 37.1 or rituximab in the absence (−) or presence (+) of an IgG cross-linking Ab. Bars represent mean of 4 experiments; SD is indicated. (D) apoptosis induction (% total annexin V positivity above spontaneous apoptosis) of primary CLL cells from 6 donors after 24-hour incubation with 30 μg/mL mAb 37.1, or rituximab in the absence or presence of an IgG cross-linking Ab. Bars represent mean of 6 donors; SD is indicated.
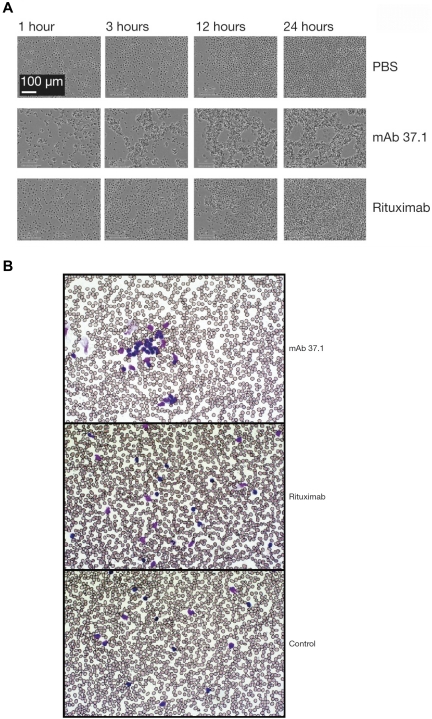
Compared with rituximab, mAb 37.1 displayed significantly (P < .05) higher induction of Ramos cell apoptosis at a saturating mAb concentration of 100 μg/mL, with or without cross-linking (mean of 4 assays is shown in Figure 1C). Importantly, Ramos cells express similar levels of CD20 and CD37 as determined by FACS Scatchard analysis (∼ 110 000 molecules/cell; data not shown). Time-lapse light microscopy of Ramos cells showed that mAb 37.1 induced rapid cell clustering in the absence of IgG cross-linking, with initial signs of clustering already 1 hour after addition of mAb 37.1 and maximal aggregation after 3 hours of incubation (Figure 2A). In contrast, rituximab-treated cells showed similar behavior as buffer controls (Figure 2A).
Figure 2.
Phenotypic analysis. Phenotypic analysis of Ab treatment on Ramos cells (A) and primary CLL cells (B). Ramos cells were incubated with mAb 37.1 or rituximab at a concentration of 30 μg/mL in Image Lock microplates (Essen Instruments) at 37°C in cell culture medium and photomicrographs were recorded as JPEG files at the indicated time points using an automated live-cell imaging system (IncuCyte, Essen Instruments) equipped with a 20× phase-contrast objective. Blood samples from CLL patients were incubated with mAb 37.1 and rituximab for 1 hour and blood smears were analyzed after routine staining (Giemsa). Pictures were taken at room temperature using a Zeiss Axioskop microscope equipped with a 50× Neofluar objective and recorded by a Zeiss AxioCam MR5c without image processing.
To assess whether apoptosis could also be induced in primary, patient-derived CLL cells, PBMC preparations from 6 patients were tested at saturating Ab concentration of 30 μg/mL. Similar to effects on Ramos cells, proapoptotic activity seen with mAb 37.1 was significantly higher than with rituximab both in the absence and presence of an IgG cross-linking Ab (P < .05; Figure 1D). Similar to the results seen in Ramos cells, rapid cell clustering induced by mAb 37.1 was observed in blood samples from CLL patients (Figure 2B).
ADCC
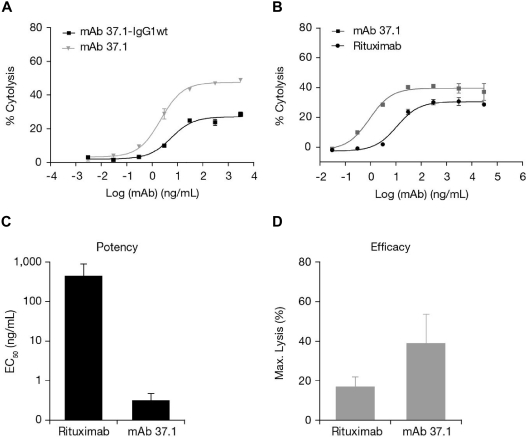
To investigate cell-mediated cytotoxicity, ADCC assays using Ramos target cells and IL-2–stimulated PBMC from healthy donors as effector cells were performed. mAb 37.1 showed strongly enhanced potency and efficacy of cytolysis (Figure 3A) in comparison to the wild-type (non–Fc-engineered) parental Ab. The average EC50 value was 2 ng/mL for mAb 37.1 (mean 3 assays). Maximal cell lysis at saturating Ab concentrations varied from 29% to 71% dependent on donor PBMC. Direct comparison to rituximab revealed clearly improved maximal cell lysis and potency when assessed side by side using the same effector cell preparations (a representative example is shown in Figure 3B).
Figure 3.
ADCC activity. Ab-dependent cell-mediated cytotoxicity (ADCC) activity (% cytolysis) on Ramos cells (E:T ratio 25:1) of mAb 37.1 in comparison with non–Fc-engineered parental mAb (37.1-wt; A) and rituximab (B). ADCC activity on primary CLL cells of 30 μg/mL of mAb 37.1 and rituximab showing (C) potency (EC50 in nanograms per milliliter) and (D) efficacy (maximal cell lysis in %). Bars represent mean of 6 donors; SD is indicated.
In addition, the ADCC activity of mAb 37.1 on primary CLL mononuclear cells from peripheral blood of 6 individual patients was assessed. MAb 37.1 was superior to rituximab with 2- to 3-fold higher maximal cell lysis and ∼ 100-fold improved potency (Figure 3C-D).
Whole-blood assays
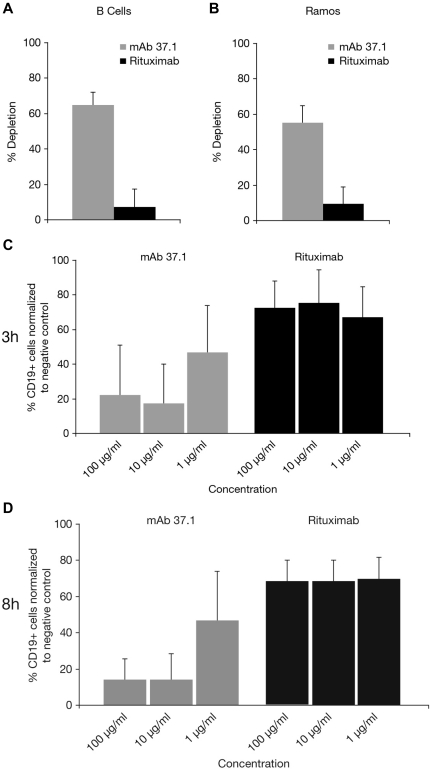
The effect of Abs on normal B cells was assessed in autologous whole-blood depletion assays using blood samples from healthy donors. Whereas rituximab displayed only marginal activity in this assay type, mAb 37.1 demonstrated high efficacy in the range of 50% to 60% depletion in an initial set of 3 different donors (Figure 4A). The effect on Ramos lymphoma cells spiked into the blood samples was assessed in parallel in these 3 samples. The depleting effect of mAb 37.1 was comparable with that on B cells (mean of 57%, Figure 4B) whereas rituximab showed only marginal activity. To further substantiate the effects observed with mAb 37.1, 7 additional blood samples from healthy individuals were investigated. B-cell depletion at Ab concentrations from 3 μg/mL to 300 μg/mL ranged from 39% to 70% (mean: 53%) and thus confirmed efficient B cell–depleting activity of mAb 37.1. Potential effects on other leukocyte subsets were assessed in parallel by enumeration of CD3-positive T cells, CD14-positive monocytes, and granulocytes before and after incubation with mAb 37.1. No depleting activity for mAb 37.1 on these cell types was observed, whereas alemtuzumab, a mAb specific for CD52, displayed T cell–depleting activity (not shown).
Figure 4.
B-cell depletion of whole-blood samples. Depletion (in %) of autologous B cells (A) and spiked Ramos cells (B) assessed by quantitative FACS analysis (TruCount) of whole-blood samples from healthy individuals after 3-hour incubation with 30 μg/mL of mAb 37.1 and rituximab. Bars represent mean of 3 donors; SD is indicated. In whole-blood assays with primary CLL samples, mAb 37.1 shows concentration-dependent CLL cell depletion whereas rituximab displays only marginal activity after 3-hour (C) and 8-hour (D) incubation. Bars represent mean of 8 donors; SD is indicated.
Superior depletion compared with rituximab was also seen in peripheral blood cells obtained from a cohort of patients with CLL (n = 8). Incubation with mAb 37.1 for 3 hours or 8 hours led to a concentration-dependent B-cell depletion that was superior to rituximab-induced depletion (Figure 4A,D). The mean maximum depletion of ∼ 80% at a mAb concentration of 10 μg/mL was already achieved after 3 hours of incubation (Figure 4C).
In vivo studies
Pharmacodynamic effect on B cells in HuCD37-transgenic mice.
mAb 37.1 lacks cross-reactivity to murine CD37 (data not shown). A transgenic murine CD37 ko/human CD37 ki mouse was therefore generated as a tool to assess the in vivo pharmacodynamic properties of CD37 mAbs. Analysis of isolated transgenic splenocytes demonstrated expression of human CD37 at a density of ∼ 10 000-12 000 sites per cell, and the binding affinity of mAb 37.1 was ∼ 0.3nM and thus comparable with that for human B cells (data not shown).
In transgenic mice, a single injection of mAb 37.1 showed dose-dependent reduction of peripheral B cells, with almost complete depletion at the 2.5 and 25 mg/kg dose levels (Figure 5A). The B-cell nadir occurred between days 2 and 7 after dosing. B-cell reduction in the 25 and 2.5 mg/kg groups was statistically significant on all days during the study (P < .001 for nadir values). At the end of the study (day 22), B-cell counts reached ∼ 50% and 75% of predose values in the 25 and 2.5 mg/kg groups, respectively, indicating that depletion is reversible. Dose-response analysis on day 2 yielded an ED50 of 422 μg/kg (Figure 5B). B-cell depletion was accompanied by a transient reduction of T cells, granulocytes, monocytes, and platelets, but not of RBCs (not shown).
Figure 5.
Dose response in animals. Effect of a single dose of mAb 37.1 (A) on peripheral B-cell count (B220+ cells) in huCD37-transgenic mice. Each curve represents the mean of 3 animals per dose group. (B) Dose response of B cells on day 2 after treatment; absolute B-cell counts for mAb 37.1 are shown. Mean of 3 animals is shown; SD is indicated. (C) Effect of a single dose of mAb 37surr on peripheral blood B-cell count in cynomolgus monkeys. Relative B-cell counts compared with predose (day −7) are depicted. Mean 2 animals per dose group.
Pharmacodynamic effects of a surrogate mAb in cynomolgus monkeys.
As mAb 37.1 lacks cross-reactivity with CD37 orthologs from all monkey species tested, we have developed a cynomolgus-specific chimeric, Fc-engineered surrogate mAb, mAb 37surr, with comparable binding and in vitro functional properties as mAb 37.1. This Ab demonstrated potent ADCC activity against recombinant HEK293 cells expressing cynomolgus CD37, potent proapoptotic activity in the presence and absence of IgG cross-linking, and depletion of naive B cells but not T cells in cynomolgus blood in vitro. Analysis of cynomolgus blood cells revealed similar binding to B and T cells as mAb 37.1 in humans (Table 2). These data validate mAb 37surr as a relevant surrogate Ab for mAb 37.1 to assess CD37-specific Ab effects in the cynomolgus monkey.
Table 2.
Characteristics of mAb 37.1 and mAb 37surr
| Function | Cell type (human/cynomolgus) | mAb 37.1 | mAb 37surr |
|---|---|---|---|
| Apparent affinity, KD | B cells/B cells | 0.4nM | 0.2nM |
| T cells/T cells | 1.7nM | 1.4nM | |
| Ag density, sites/cell | B cells/B cells | 53 000 | 55 000 |
| T cells/T cells | 1900 | 1900 | |
| ADCC (EC50) | Ramos/macCD37-CHO | 0.01nM (2 ng/mL) | 0.02nM (4 ng/mL) |
| Apoptosis, without x-link (EC50) | Ramos/macCD37-CHO | 0.4nM (66 ng/mL) | 9.3nM (1423 ng/mL) |
| Apoptosis, with x-link (EC50) | Ramos/macCD37-CHO | 0.3nM (41 ng/mL) | 10nM (1574 ng/mL) |
| CDC | Ramos/macCD37-CHO | Negative | Negative |
| Whole blood (EC50) | B cells/B cells | 0.2nM (33 ng/mL) | 0.9nM (140 ng/mL) |
ADCC indicates Ab-dependent cell-mediated cytotoxicity; and CDC, complement-dependent cytotoxicity.
The effect of a single infusion of mAb 37surr (doses 10 mg/kg, 0.3 mg/kg, 0.01 mg/kg) on peripheral blood B and T cells was assessed by flow cytometry. At 24 hours after infusion, B-cell numbers decreased sharply in the 2 high-dose animals (from 1213 to 75 cells/μL and from 1596 to 253 cells/μL; Figure 5C). The effect was attenuated in the medium-dose group (295 and 713 cells/μL). No effect was seen in the animals treated with the lowest dose of mAb 37surr. At day 8, no significant effect was seen for the medium- and low-dose animals whereas the B-cell numbers had further declined in the 2 animals treated with the high dose of mAb 37surr (6 and 19 cells/μL). Subsequently, the number of B cells increased in these animals and reached ∼ 30% of predose values on day 36 (end of study). B-cell reduction was statistically significant for the high dose at all time points after infusion (P < .0001).
A decrease of T cells, neutrophils, monocytes, and platelets was observed in animals treated with the high dose of mAb 37surr only. These effects were transient with cell nadirs occurring between days 1 and 8 of the study. The maximum reduction varied between 40% (platelets) and 70% (T cells), effects were not statistically significant. No effect on RBCs was observed.
PK evaluation revealed that the exposure to mAb 37surr was substantial in the intermediate and high-dose group and area under the curve (AUC; 0-840 hours) values increased consideably more than proportionally with dose (see Table 3). In the low-dose group (0.01 mg/kg), all assay results were below the lower limit of quantification (60 ng/mL). In the intermediate-dose group (0.3 mg/kg), PK evaluation was restricted as plasma concentrations were measurable only until 8 hours after administration, and the calculated half-life (6 hours) was not considered as valid (Table 3). The terminal half-life was ∼ 60 hours in the high-dose group (10 mg/kg).
Table 3.
Pharmacokinetic parameters of mAb 37surr in cynomolgus monkey
| Parameter | 0.01 mg/kg | 0.3 mg/kg | 10 mg/kg |
|---|---|---|---|
| C(max) [μg/mL]/[mg/kg] | NC | 24.9 | 24.1 |
| AUC, 0-804 h [μg [times] h/mL]/[mg/kg] | NC | 140 | 1720 |
| t(1/2) [h] | NC | 5.7 | ∼ 60 |
AUC indicates area under the curve; and NC, not calculated.
Antitumor effect in a Ramos xenograft mouse model.
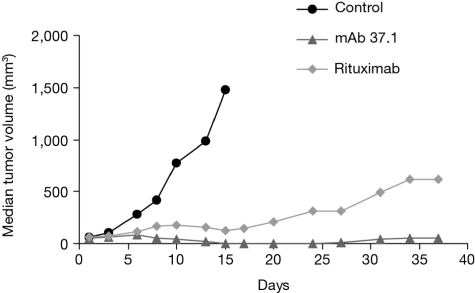
To assess the antitumor efficacy of mAb 37.1 in comparison to rituximab, Ramos cells were injected subcutaneously into the flank of immunodeficient “nude” mice, and mAb treatment at doses of 25 mg/kg given twice weekly IV was initiated when the tumors had reached a median volume of ∼ 60 mm3 (Figure 6). Both Abs caused significant growth retardation compared with vehicle treatment (day 15); the difference between mAb 37.1 and rituximab was not statistically significant.
Figure 6.
Effect on tumor volume. Effect of Ab treatment on growth of established Ramos subcutaneus tumors in NMRI mice. Ten mice per cohort were treated twice weekly with mAb 37.1 and rituximab at 25 mg/kg for a total of 2 weeks. Median tumor volume is shown.
Discussion
The use of mAbs to achieve B-cell depletion has been an important advance in treatment of lymphoid malignancies, and the CD20 mAb, rituximab has been incorporated into standard of care for both NHL and CLL. With increasing exposure of patient populations to rituximab, the frequency of resistance to the agent is on the rise, for example, by emergence of malignant clonal cells with loss of CD20 from the cell surface.24 In addition, rituximab has limited activity in depletion of CLL cells as monotherapy, which is held to be related to the lower density of CD20 on these cells and to the low efficacy of direct rituximab cytotoxicity.25 Recent data from the glyco-engineered CD20 mAb GA101 demonstrated that both an increase in direct cytotoxicity and improved effector cell–mediated cytotoxicity can yield superior preclinical activity against malignant B cells.7 Several clinical trials are under way (http://clinicaltrials.gov) to investigate whether such preclinical superiority will lead to improved benefit for cancer patients.
Several other cell-surface receptors on malignant B cells have been the subject of intense research within the past decade.26 Among those, CD37 is a promising target in B-cell malignancies because of its high expression in many lymphoid cancers and on CLL cells.13,19 A recently developed CD37 IgG-like molecule directed against CD37 exerts proapoptotic and ADCC activity in preclinical settings and showed signs of clinical activity in a phase 1 trial in patients with relapsed, refractory CLL.27,28
Our novel CD37 mAb 37.1 was engineered within the Fc region of the parent IgG1 molecule for increased binding affinity to FcγRIIIa, as has been described for other mAbs.23 Our binding data confirm strongly enhanced binding affinity of the Fc-engineered mAb 37.1 to FcγRIIIa (both CD16-158F and CD16-158V) to a similar extent as reported for CD19 mAbs.29 Interestingly, also the binding affinity to FcγRI (CD64) was enhanced ∼ 8-fold in case of mAb 37.1, whereas Horton and coworkers published only marginally increased binding of their Fc-engineered CD19 mAb to CD64.29
mAb 37.1 shows strongly enhanced cytotoxicity compared with the parent IgG1 molecule on Ramos Burkitt lymphoma cells and CLL cells from patients when tested in a heterologous ADCC assay. Importantly, our CD37 mAb also showed clearly improved ADCC activity when tested side by side with rituximab on Ramos cells, which show similar levels of CD20 and CD37 Ag density. Enhancement of ADCC appeared to be even more pronounced on primary CLL cells than on Ramos cells, which may be explained by a higher number of CD37 molecules than of CD20 on these cells (K.-H.H, unpublished observation, April 2006). In summary, the enhancement of Fcγ receptor binding in this novel CD37 mAb leads to clearly improved ADCC compared with rituximab both on lymphoma cells with similar Ag densities for CD20 and CD37 and on primary CLL cells.
In addition to improved ADCC function, mAb 37.1 was selected for its potential to exert strong and potent direct cell killing. The proapoptotic activity against Ramos cells and primary CLL cells was clearly superior compared with rituximab. mAb 37.1 induces significant apoptosis without IgG cross-linking on Ramos cells and primary CLL cells, which is significantly superior to that of rituximab, which induces only marginal proapoptotic activity in both cell types. After cross-linking, the proapoptotic activity of both mAbs increased, however, less pronounced on primary CLL cells than on Ramos cells. This finding may be explained by a higher heterogeneity of primary CLL cells compared with Ramos cells, differences in levels of Ag expression, and a considerably higher level of spontanous apoptosis in primary cells than in Ramos cells. Apoptotic cell killing by mAb 37.1 does not require IgG cross-linking and thus the mode of direct cell death induction induced by mAb 37.1 resembles that of so-called type II CD20 Abs like tositumumab and GA101. Type II CD20 mAbs are characterized by reduced ability to mediate CDC activity compared with type I Abs, but have been demonstrated to outperform type I Abs with respect to their B cell–depleting activity.7,30 In this context, it is interesting to note that mAb 37.1 likewise lacks CDC activity (data not shown). Furthermore, the observed phenotypic changes on Ramos cells and primary CLL cells after incubation with mAb 37.1 (eg, rapid homotypic aggregation in the absence of a cross-linking mAb) provide evidence that mAb 37.1 can be considered to be a type II CD37 Ab. Homotypic aggregation is a characteristic of type II Abs and is associated with highly effective direct cell killing.31 Additional studies are needed to further elucidate the mode of direct cell killing induced by mAb 37.1, for example, whether a redistribution of target molecules in lipid rafts occurs.
Both ADCC and apoptosis are considered relevant for the clinical activity of B-cell directed Abs in hematologic malignancies.32,33 However, the relevance of in vitro assays for prediction of responses in vivo appears to be limited because of the artificial nature of the test systems (eg, heterologous effector cells, lack of endogenous IgG levels). To compensate for these limitations, we used whole-blood assays, which are considered to mimic the in vivo situation more closely because of the presence of autologous effector cells and IgG levels, and furthermore are able to reflect the interplay of several modes of action with respect to the depleting activity of the Ab. Results from whole-blood assays, using quantitative FACS methodology, showed that the CD37 mAb described here is ∼ 10-fold more effective in depleting autologous B cells, Ramos lymphoma cell-spiked samples, and primary CLL cells than rituximab. Although a direct comparison with the third-generation, glyco-engineered CD20 mAb GA101 has not been possible to date, the results of whole-blood assays appear to compare favorably with recently published data on GA101.7,25 Taken together, our data and literature data suggest that targeting CD37 on the surface of malignant CLL cells may result in a higher degree of cell depletion than targeting CD20, which ultimately may translate into better clinical efficacy.
No effects on cells with low levels of Ag (T cells, monocytes, granulocytes) were observed in the whole-blood assay using blood from healthy individuals, whereas an Ab known to have T cell–depleting activity (alemtuzumab) showed significant effects in these assays (data not shown). Thus, the level of target Ag expression appears to be below a threshold required for Ab induced cytotoxic effects, at least in the applied in vitro test system.
To assess the in vivo pharmacodynamic activity of our CD37 mAb, 2 different approaches were used. Because of the lack of cross-reactivity of mAb 37.1 with CD37 orthologs of any animal species tested, a transgenic mouse model was established, in which the murine CD37 gene was replaced by human CD37. In addition, a surrogate Ab reactive with cynomolgus CD37 was developed to allow in vivo assessments in an animal species with a FcγR system close to humans.34
A pharmacodynamic study in huCD37-transgenic mice with mAb 37.1 showed that complete B-cell depletion in peripheral blood could be achieved at a single dose of 2.5 mg/kg, with nadirs at days 2-7 and recovery to at least 50% of pretreatment levels within 3 weeks. In the cynomolgus monkey, a single, intermediate dose of 0.3 mg/kg surrogate mAb CD37surr lead to a significant reduction in peripheral B cells, which was complete and long-lasting at the 10 mg/kg dose level. The recovery of peripheral blood B cells indicated that at the doses applied no substantial effects on BM precursor cells were imposed. In both species, a transient reduction of T cells was observed which paralleled that of B cells. In the mouse, these effects were similar to that on B cells, whereas in the monkey a T cell–reducing effect was observed at the 10 mg/kg dose level only, and was far less pronounced than that observed on B cells. The effect on T cells may be attributed to the low level of CD37 expression on T cells.12 It is currently unknown why the effects on transgenic murine T cells were more pronounced than those on cynomolgus T cells. Ag expression levels on T cells were similar in both species and hence do not explain the substantial difference observed in vivo.
It has to be noted that a transient reduction of peripheral T cells has also been observed in clinical trials with CD20 Abs where the CD20 Ag is not expressed on T cells.35 Hence, a transient effect on T cells may also be caused by lymphocyte redistribution effects rather than by cell killing. In our in vitro whole-blood assay, we did not detect any T-cell reducing effect of mAb 37.1 nor mAb CD37surr. Together, our data indicate that depletion of CD37-expressing cells is only transient on nonmalignant peripheral leukocytes, suggesting an acceptable safety profile of these Abs, and supports further investigation in clinical trials in cancer patients.
Targeting CD37 using mAb 37.1 substantially limited the growth of established Burkitt lymphoma in a Ramos xenograft model. The effect was comparable with that achieved with rituximab. However, mice are not considered to be a suitable species with respect to the cytotoxicity-enhancing effect of Fc mutations.7,11 Hence, the ADCC-mediated antitumor effects of mAb 37.1 may be underestimated in the mouse model used in this study.
In conclusion, our data indicate that the novel, Fc-engineered mAb directed against human CD37 described in this article has substantially greater B cell–depleting activity in several in vitro systems than rituximab, and displays profound pharmacodynamic and antitumor effects in vivo. This Ab is a promising candidate for treatment of patients with B-cell malignancies and warrants further clinical evaluation.
Supplementary Material
Acknowledgments
The authors thank all coworkers contributing to the identification, generation, and characterization of mAb 37.1, especially Malgorzata Karner and Irene Schweiger for excellent technical assistance, Ulrich Kunz and Jan-Peter Sandel for PK analysis, and Carina Ittrich for statistical analysis. Writing assistance was provided by Janet Stephenson, and editing of the manuscript was supported by PAREXEL (funded by Boehringer Ingelheim International GmbH).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.-H.H., S.S., G.R.A., and E.B. initiated and supervised studies; Z.K. established assays; K.-H.H., K.K., T.Z., M.V., E.O., A.B., H.L., Z.K., A.J., S.M., and U.H. performed experiments and analyzed data; K.-H.H. wrote the paper; and T.Z., S.S., A.B., S.M., U.H., G.R.A., and E.B. edited the manuscript.
Conflict-of-interest disclosure: K.-H.H., K.K., E.O., A.B., H.L., Z.K., A.J., S.M., U.H., G.R.A., and E.B. are employees of Boehringer Ingelheim. T.Z. received honoraria and research funding. S.S. and T.Z received Honoraria and research funding from Boehringer Ingelheim. M.V. declares no competing financial interests.
The current affiliations for T.Z. are Department of Translational Oncology, National Center for Tumor Diseases (NCT) and German Cancer Research Center, Heidelberg, Germany, and Department of Medicine V, University of Heidelberg, Heidelberg, Germany.
Correspondence: Karl-Heinz Heider, Boehringer Ingelheim RCV GmbH & Co KG, Dr Boehringer Gasse 5-11, Vienna, Austria; e-mail: karl-heinz.heider@boehringer-ingelheim.com.
References
- 1.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105(4):1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 4.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23(18):4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 5.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 6.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28(10):1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 9.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Le Garff-Tavernier M, Decocq J, de Romeuf C, et al. Analysis of CD16+CD56dim NK cells from CLL patients: evidence supporting a therapeutic strategy with optimized anti-CD20 monoclonal antibodies. Leukemia. 2011;25(1):101–109. doi: 10.1038/leu.2010.240. [DOI] [PubMed] [Google Scholar]
- 11.Bowles JA, Wang SY, Link BK, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood. 2006;108(8):2648–2654. doi: 10.1182/blood-2006-04-020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Spriel AB, Puls KL, Sofi M, et al. A regulatory role for CD37 in T cell proliferation. J Immunol. 2004;172(5):2953–2961. doi: 10.4049/jimmunol.172.5.2953. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz-Albiez R, Dorken B, Hofmann W, Moldenhauer G. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988;140(3):905–914. [PubMed] [Google Scholar]
- 14.Moore K, Cooper SA, Jones DB. Use of the monoclonal antibody WR17, identifying the CD37 gp40-45 Kd antigen complex, in the diagnosis of B-lymphoid malignancy. J Pathol. 1987;152(1):13–21. doi: 10.1002/path.1711520103. [DOI] [PubMed] [Google Scholar]
- 15.Barrena S, Almeida J, Yunta M, et al. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia. 2005;19(8):1376–1383. doi: 10.1038/sj.leu.2403822. [DOI] [PubMed] [Google Scholar]
- 16.Belov L, de la Vega O, dos Remedios CG, Mulligan SP, Christopherson RI. Immunophenotyping of leukemias using a cluster of differentiation antibody microarray. Cancer Res. 2001;61(11):4483–4489. [PubMed] [Google Scholar]
- 17.Smith JL, Jones DB, Bell AJ, Wright DH. Correlation between histology and immunophenotype in a series of 322 cases of non-Hodgkin's lymphoma. Hematol Oncol. 1989;7(1):37–48. doi: 10.1002/hon.2900070104. [DOI] [PubMed] [Google Scholar]
- 18.Norton AJ, Isaacson PG. Detailed phenotypic analysis of B-cell lymphoma using a panel of antibodies reactive in routinely fixed wax-embedded tissue. Am J Pathol. 1987;128(2):225–240. [PMC free article] [PubMed] [Google Scholar]
- 19.Schuurman HJ, van Baarlen J, Huppes W, Lam BW, Verdonck LF, van Unnik JA. Immunophenotyping of non-Hodgkin's lymphoma. Lack of correlation between immunophenotype and cell morphology. Am J Pathol. 1987;129(1):140–151. [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminski MS, Fig LM, Zasadny KR, et al. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labeled anti-CD37 antibody in B-cell lymphoma. J Clin Oncol. 1992;10(11):1696–1711. doi: 10.1200/JCO.1992.10.11.1696. [DOI] [PubMed] [Google Scholar]
- 21.Press OW, Eary JF, Badger CC, et al. Treatment of refractory non-Hodgkin's lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J Clin Oncol. 1989;7(8):1027–1038. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 22.Andritsos L, Furman R, Flinn I, et al. A phase I trial of TRU-016, an anti-CD37 small modular immunopharmaceutical (SMIP) in relapsed and refractory CLL [abstract]. J Clin Oncol. 2009;27(15s) Abstract 3017. [Google Scholar]
- 23.Lazar GA, Dang W, Karki S, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103(11):4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haidar JH, Shamseddine A, Salem Z, et al. Loss of CD20 expression in relapsed lymphomas after rituximab therapy. Eur J Haematol. 2003;70(5):330–332. doi: 10.1034/j.1600-0609.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 25.Patz M, Isaeva P, Forcob N, et al. Comparison of the in vitro effects of the anti-CD20 antibodies rituximab and GA101 on chronic lymphocytic leukaemia cells. Br J Haematol. 2011;152(3):295–306. doi: 10.1111/j.1365-2141.2010.08428.x. [DOI] [PubMed] [Google Scholar]
- 26.Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp Hematol. 2008;36(7):755–768. doi: 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Lapalombella R, Joshi T, et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110(7):2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furman RR, Andritsos L, Flinn IW, et al. Phase 1 dose escalation study of TRU-016, an anti-CD37 SMIPTM protein in relapsed and refractory CLL [abstract]. Blood (ASH Annual Meeting Abstracts) 2010;116(21) Abstract 56. [Google Scholar]
- 29.Horton HM, Bernett MJ, Pong E, et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 2008;68(19):8049–8057. doi: 10.1158/0008-5472.CAN-08-2268. [DOI] [PubMed] [Google Scholar]
- 30.Beers SA, Chan CH, James S, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood. 2008;112(10):4170–4177. doi: 10.1182/blood-2008-08-172999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alduaij W, Ivanov A, Honeychurch J, et al. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117(17):4519–4529. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang SY, Weiner G. Complement and cellular cytotoxicity in antibody therapy of cancer. Expert Opin Biol Ther. 2008;8(6):759–768. doi: 10.1517/14712598.8.6.759. [DOI] [PubMed] [Google Scholar]
- 33.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44(16):3823–3837. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 34.Zalevsky J, Leung IW, Karki S, et al. The impact of Fc engineering on an anti-CD19 antibody: increased Fcgamma receptor affinity enhances B-cell clearing in nonhuman primates. Blood. 2009;113(16):3735–3743. doi: 10.1182/blood-2008-10-182048. [DOI] [PubMed] [Google Scholar]
- 35.Morschhauser F, Cartron G, Lamy T, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia. Blood (ASH Annual Meeting Abstracts) 2009;114(22) Abstract 884. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.