Abstract
Pulmonary hypertension (PH) is initially a disease of the small, peripheral resistance arteries. Changes in these vessels are best assessed by measurement of pulmonary artery pressure at several levels of flow to generate multi-point pressure-flow curves. This approach is superior to the traditional single-point measurement of pulmonary vascular resistance (PVR) because it allows a flow-independent definition of the resistive properties of that portion of the pulmonary vascular bed and also provides information on its distensibility. In animal models, multi-point pressure-flow curves can be obtained using an isolated, ventilated, perfused lung system. Clinically, cardiopulmonary exercise testing (CPET) with non-invasive echocardiography is feasible and provides realistic values of the resistance and peripheral compliance. Together, these values can be used to better understand and screen for PH and exercise-induced PH.
I. Introduction
Pulmonary hypertension (PH) is defined by a mean pulmonary artery pressure (mPAP) greater than 25 mmHg at rest [1]. In the early stages of the disease, dramatic decreases in cross-sectional area available for flow in the small, peripheral pulmonary arteries occur. These transform the normally low resistance pulmonary circulation into a high resistance circuit. Later, the highly compliant nature of the pulmonary vascular bed is also lost, which has profound consequences for right ventricular function.
Typically, PH is diagnosed on the basis of an invasive right heart catheterization in which mPAP, pulmonary capillary wedge pressure (PCW) and cardiac output (Q) are measured. Pulmonary vascular resistance (PVR) is then calculated at this single functional state as (mPAP-PCW)/Q. However, this measurement fails to take into account either normal or abnormal changes in pressure that occur with changes in flow. For example, PVR decreases with exercise in response to recruitment and pressure-induced dilation of small, peripheral arteries [2]. The failure of PVR to decrease with exercise has recently been described as a clinical entity: exercise-induced pulmonary hypertension [3]. This abnormal functional aspect of the peripheral pulmonary circulation cannot be recognized from single-point calculations of PVR. In addition, when assessing the effects of drugs with the single-point PVR, one can confuse the effect of a drop in pressure (lower mPAP-PCW) with an increase in cardiac output (higher Q) [4].
When PVR is computed from multi-point pressure-flow curves, the result is flow rate-independent and thus a more reliable measure of pulmonary vascular functional state. In addition, the multi-point pressure-flow curves can be used to estimate the distensibility of the small, peripheral arteries [5], which determines their ability to dilate in response to increasing pressure. Pressure-flow curves can be obtained clinically with hemodynamic measurements during cardiopulmonary exercise testing (CPET) [4] or in animal models with isolated, ventilated, perfused lung systems [6]. Like the failure of PVR to decrease with exercise, decreased distensibility may be a clinical entity or risk factor for development of PH.
The development and progression of PH largely depends on the small, peripheral arteries. Thus, accurate techniques for measuring peripheral pulmonary vascular mechanics in patients and animal models are worthy of discussion. Here, we present techniques for measuring peripheral pulmonary artery mechanics from multi-point pressure-flow curves and results from studies in animals and healthy human subjects.
II. Methods
A. Normal and diseased mouse lungs
To demonstrate methods for measuring peripheral pulmonary vascular mechanics in animals, we used male C57BL6/J mice with normal (control: CTL) and diseased (hypoxia-induced PH: HPH) lungs. To create HPH, mice were exposed to 10 days of hypobaric hypoxia such that the partial pressure of oxygen was reduced by half, as previously described [6]. Mice were euthanized with an intraperitoneal injection of 150 mg/kg pentobarbital solution. All protocols and procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Following euthanasia, the trachea, pulmonary artery and left atrium were cannulated for ventilation, perfusate inflow and perfusate outflow, respectively. The lungs were ventilated with room air and perfused with heated RPMI 1640 cell culture medium with 3.5% Ficoll (an oncotic agent). A syringe pump was used to create steady pulmonary vascular flow of perfusate. In parallel, a high frequency oscillatory pump superimposed an oscillatory component on the pulmonary vascular flow. Pressure transducers measured the instantaneous PAP and LAP. Flow rate (Q) was measured with an in-line flow meter. Details on this isolated, ventilated perfused lung setup are available in [6].
To obtain multi-point pressure-flow curves, lungs were lungs perfused with steady flow at flow rates of 1 to 5 ml/min. Lungs were preconditioned with pulsatile flow from 1 to 5 ml/min at physiological frequencies. Normal ventilation (2 to 10 cm H2O at 90 breaths/min) occurred whenever data were not being collected.
B. Healthy human subjects
After approval by the Ethical Committee of the Erasme University Hospital, healthy volunteers gave a written informed consent to participate in exercise stress tests with echocardiography. Each subject underwent an echocardiographic examination at rest and as workload was increased by 20 W every 2 min until the maximum tolerated. The echocardiography was performed on a semi-recumbent cycle ergometer as previously described [7]. Systolic PAP (sPAP) was estimated from the maximum velocity (V) of continuous Doppler tricuspid regurgitation according to sPAP = 4 × V2 + 5 mmHg where 5 mmHg was the pressure assumed in the right atrium [8]. Mean PAP was calculated as 0.6 × sPpa + 2 [9] and left atrial pressure (LAP) was estimated from the ratio of mitral flow E and tissue Doppler mitral annulus E′ waves [10]. LAP was used as an approximation of PCW. Finally, cardiac output was estimated from left ventricular outflow tract cross sectional area and pulsed Doppler velocity-time integral [11].
C. Data analysis
Multipoint mPAP-Q curves were created for each subject and linear regression was used to determine best fit slope and intercept minimizing the sum of least square error. To compute the distensibility α of the small resistance arteries, each multipoint mPAP-Q plot was fit to the equation
| (1) |
where R0 is the total PVR at rest [5].
All results shown here are presented as mean ± SD. The statistical analysis consisted of a repeated measures analysis of variance. When the F ratio of the analysis of variance reached a P < 0.05 critical value, paired or unpaired modified Student’s t-tests were applied as indicated to compare specific situations [12].
III. Results
A. Normal and diseased mouse lungs
In normal and diseased mouse lungs, multi-point pressure-flow curves are reasonably well fit by either straight lines or (1). By best-fit to a linear equation, we found that in CTL lungs, the average slope was 1.88 ± 0.23 mmHg min/ml, and the intercept 7.2 ± 0.8 mmHg, with a correlation coefficient R2 of 0.99 ± 0.01 (P = 0.00039 ± 0.00064). In HPH lungs, the average slope was similar: 1.71 ± 0.22 mmHg min/ml whereas the intercept was significantly higher: 11.8 ± 0.7 mmHg (P<0.05). In HPH lungs the fit to a linear equation was also excellent (R2 = 0.99 ± 0.002; P = 0.00019 ± 0.00010).
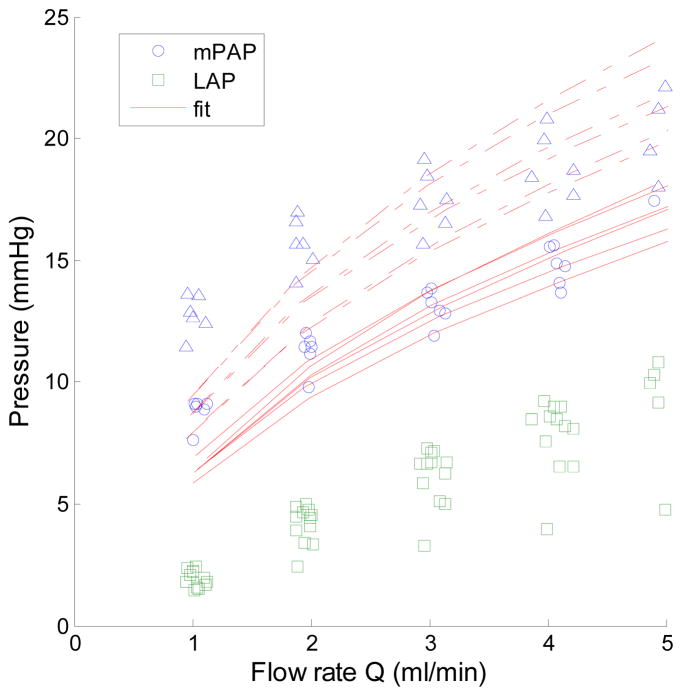
Multi-point pressure-flow curves also were well fit by the distensibility equation, at all but the lowest flow rate (Fig. 1). The average distensibility coefficient α was significantly lower in HPH compared to CTL lungs (4.1 ± 0.5% CTL vs. 3.1 ± 0.5% mmHg−1 HPH; p<0.05). The overall fitting was excellent in both groups (R2 = 0.99 ± 0.009 in CTL and 0.99 ± 0.002 in HPH lungs).
Fig. 1.
Multi-point pressure-flow curves in isolated, ventilated, perfused lungs from healthy control (○: PAP, □ LAP) and chronically hypoxic (△: PAP, □: LAP) mouse lungs with best fits to (1) shown in solid (CTL) and dashed (HPH) lines. Note that HPH lungs have increased pressure at all flow rates due to remodeling of small, peripheral arteries.
B. Healthy human subjects
In human subjects, good quality signals were recovered at all levels of exercise. As shown in Table 1, exercise was accompanied by a four-fold increase in Q, and an increase in sPAP that exceeded 40 mmHg in 19 of 25 subjects. Exercise did not affect LAP or PVR at rest.
Table 1.
Hemodynamic measurements at rest and maximum exercise in 25 normal subjects showing the effect of exercise on HR, sBP, sPAP, and Q.
| Variables | Baseline | Maximum |
|---|---|---|
| HR, bpm | 66 ± 10 | 159 ± 21* |
| sBP, mmHg | 116 ± 9 | 169 ± 17* |
| sPAP, mmHg | 19 ± 5 | 46 ± 11* |
| LAP, mmHg | 8 ± 2 | 9 ± 1 |
| Q, L/min | 4.7 ± 1.0 | 18.0 ± 4.2* |
| 1-pt PVR, WU | 1.2 ± 0.6 | 1.2 ± 0.5 |
Abbreviation: HR: heart rate; sBP: systolic blood pressure; sPAP: systolic pulmonary artery pressure; Q: cardiac output; 1-pt PVR: single-point pulmonary vascular resistance, WU: Wood units, or mmHg/L/min.
: P< 0.05 compared to baseline
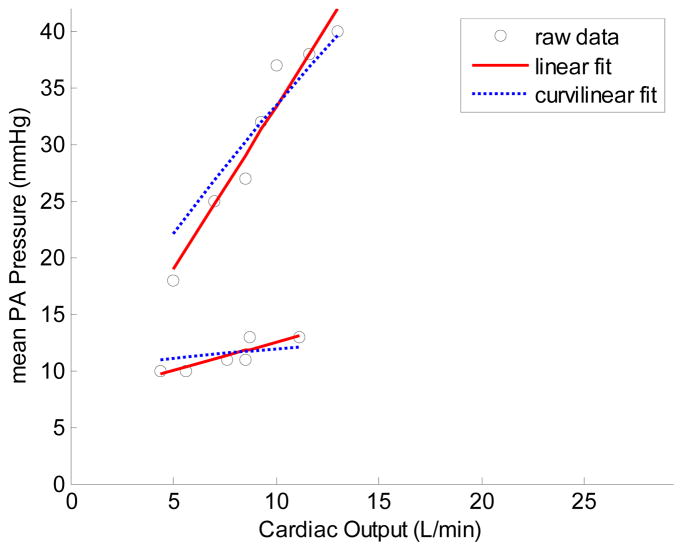
Each mPAP-Q relationship was well described by a linear approximation. The average slope was 1.37 ± 0.65 mmHg min/L, and the intercept 8.2 ± 3.6 mmHg, with a correlation coefficient R2 of 0.92 ± 0.06 (P = 0.0018 ± 0.005). The mPAP-Q relationships also were well fit by the distensibility equation. The average distensibility coefficient α for all subjects was of 0.017 ± 0.018 mmHg−1. The average R2 for the fit to (1) was 0.92 ± 0.06 (P = 0.0020 ± 0.006). Representative multi-point pressure-flow curves with linear and curvilinear fits to (1) are shown in Fig. 2.
Fig. 2.
Multi-point pressure-flow curves for two representative healthy human subjects during CPET with echocardiography showing a wide range of normal pressure-flow relationships and goodness-of-fit to linear and curvilinear (1) shapes.
IV. Discussion
The mechanical properties of the small, peripheral vessels in the pulmonary circulation are important to pulmonary vascular function. In animal models, these can be measured with isolated vessel tests [13], microcomputed tomography imaging at various pressures [14] and estimated from multi-point pressure-flow relationships [5]. Of these, only multi-point pressure-flow curves can be generated in patients and so only this technique is currently clinically useful.
Measurements of resistance and distensibility in normal and diseased mouse lungs indicate that significant functional changes occur in peripheral pulmonary arteries with PH that can be detected via the multi-point pressure-flow curves.
In healthy human subjects, a previous analysis of invasive hemodynamic measurements at exercise showed a mPAP-Q slope of 0.94 ± 9.4 mmHg min L−1 with an extrapolated pressure intercept of 8.2 ± 7.9 mmHg in 63 young adults, and a slope of 2.54 ± 0.77 mmHg min L−1 with a pressure intercept of 2.3 ± 5.4 mmHg in 14 older subjects [2]. The average slope of mPAP-Q of 1.37 mmHg min L−1 in the present study agrees with these previous invasive measurements. This is especially important because non-invasively obtained estimates of mPAP eliminate the need for right heart catheterization, which is associated with morbidity and mortality, especially in PH patients.
The present calculations of α also agree with prior in vitro studies in which a value of 0.02 mmHg−1 was obtained in several species, including humans [3]. We expect that with PH, distensibility will decrease significantly, indicating peripheral vascular stiffening. Calculations of α from single point measurements of Ppa and Q support this conjecture [15] but neither invasive nor non-invasive multi-point Ppa-Q data in patients with PH are currently available.
In spite of previously reported excellent correlations between echocardiographic and invasive measurements of pulmonary artery pressures [16–19], and recent progress in ultrasound technology, poor agreement between the two techniques has also been reported [20], which is a potential limitation to our findings. Nevertheless, we believe that the excellent agreement between our non-invasive estimates of both mPAP-Q slope and α with previous invasive and also in vitro studies is unlikely to have occurred by chance, despite increased noise in the non-invasively obtained data, which causes relatively large standard deviations.
V. Summary
In animal models, multiple techniques are available for measuring peripheral pulmonary vascular mechanics. In humans, non-destructive and preferably non-invasive techniques such as CPET with echocardiography are required. Here we describe robust techniques for the measurement of peripheral pulmonary vascular mechanics in animals and humans and provide representative data in healthy and diseased populations.
The development and progression of PH largely depends on the small, peripheral arteries. More consistent and accurate measurement of peripheral pulmonary vascular mechanics may improve our understanding of PH progression and ultimately lead to better management and treatment of this disease.
Acknowledgments
This work was supported in part by the NIH (R01HL086939, NCC), the Fulbright Foundation (NCC) and FRSM (3.4637.09, RN).
Contributor Information
Naomi C. Chesler, Email: chesler@engr.wisc.edu, Department of Biomedical Engineering at the University of Wisconsin, Madison WI 53706 USA. (608-265-8929; fax: 608-265-9239)
Paola Argiento, Email: paola.argiento@libero.it, Department of Cardiology of Second University of Naples at Monaldi Hospital, 80131 Naples, Italy.
Rebecca Vanderpool, Email: beccav@cae.wisc.edu, Department of Biomedical Engineering at the University of Wisconsin, Madison WI 53706 USA.
Michele D’Alto, Email: mic.dalto@tin.it, Department of Cardiology of Second University of Naples at Monaldi Hospital, 80131 Naples, Italy.
Robert Naeije, Email: rnaeije@ulb.ac.be, Director of the Department of Pathophysiology of the Erasme University Hospital, Brussels, Belgium.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Circulation. 2009;119 doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Reeves JT, Dempsey JA, Grover RF. Pulmonary circulation during exercise. In: Weir EK, Reeves JT, editors. Pulmonary Vascular Physiology and Physiopathology. Vol. 4. Marcel Dekker; New York: 1989. pp. 107–133. [Google Scholar]
- 3.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation. 2008;118:2183–2189. doi: 10.1161/CIRCULATIONAHA.108.787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelain V, Chemla D, Humbert M, et al. Pulmonary artery pressure flow relations after prostacyclin in primary pulmonary hypertension. Am J Respir Crit Care Med. 2002;165 (3):338–340. doi: 10.1164/ajrccm.165.3.2106033. [DOI] [PubMed] [Google Scholar]
- 5.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol. 2005;288:L419–25. doi: 10.1152/ajplung.00162.2004. [DOI] [PubMed] [Google Scholar]
- 6.Tuchscherer HA, Vanderpool RR, Chesler NC. Pulmonary vascular remodeling in isolated mouse lungs: Effects on pulsatile pressure–flow relationships. Journal of Biomechanics. 2007;40:993–1001. doi: 10.1016/j.jbiomech.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 7.Grunig E, Janssen B, Mereles D, et al. Abnormal pulmonary artery pressure response in asymptomatic carriers of primary pulmonary hypertension gene. Circulation. 2000;102:1145–50. doi: 10.1161/01.cir.102.10.1145. [DOI] [PubMed] [Google Scholar]
- 8.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 9.Chemla D, Castelain V, Humbert M, et al. New formula for predicting mean pulmonary artery pressure using systolic pulmonary artery pressure. Chest. 2004;126:1313–7. doi: 10.1378/chest.126.4.1313. [DOI] [PubMed] [Google Scholar]
- 10.Talreja DR, Nishimura RA, Oh JK. Estimation of left ventricular filling pressure with exercise by Doppler echocardiography in patients with normal systolic function. Am J Soc Echocardiogr. 2007;20:477–479. doi: 10.1016/j.echo.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Christie J, Sheldahl LM, Tristani FE, et al. Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oximetry, and thermodilution. Circulation. 1987;76:539–47. doi: 10.1161/01.cir.76.3.539. [DOI] [PubMed] [Google Scholar]
- 12.Winer BJ, Brown DR, Michels KM. Statistical principles in experimental design. 3. Mc Graw-Hill; New York: 1991. pp. 220–283. [Google Scholar]
- 13.Chesler N, Thompson-Figueroa J, Millburne K. Measurements of mouse pulmonary artery biomechanics. Journal of Biomechanical Engineering. 2004;126(2):309–314. doi: 10.1115/1.1695578. [DOI] [PubMed] [Google Scholar]
- 14.Karau KL, Johnson RH, Molthen RC, et al. Microfocal X-ray CT imaging and pulmonary arterial distensibility in excised rat lungs. Am J Physiol Heart Circ Physiol. 2001;281:H1447–H1457. doi: 10.1152/ajpheart.2001.281.3.H1447. [DOI] [PubMed] [Google Scholar]
- 15.Blyth K, Syyed R, Chalmers J, Foster JE, Saba T, Naeije R, Melot C, Peacock AJ. Pulmonary arterial pulse pressure and mortality in pulmonary arterial hypertension. Respiratory Medicine. 2007;101(12):2495–2501. doi: 10.1016/j.rmed.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg EE. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985;6:359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson JG. Comparison of several noninvasive methods for estimation of pulmonary artery pressure. J Am Soc Echocardiogr. 1989;2:157–171. doi: 10.1016/s0894-7317(89)80053-7. [DOI] [PubMed] [Google Scholar]
- 18.Currie PJ, Seward JB, Chan KL, Fyfe DA, Hagler DJ, Mair DD, Reeder GS, Nishimura RA, Tajik AJ. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985;6:750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 19.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 20.Fisher MR, Chamera E, Housten-Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2009;179:615–621. doi: 10.1164/rccm.200811-1691OC. [DOI] [PMC free article] [PubMed] [Google Scholar]