Abstract
Long-standing pulmonary hypertension causes significant peripheral and proximal arterial remodeling and right ventricular dysfunction. The clinical metric most often used to assess the progression of PH is the pulmonary vascular resistance (PVR). However, even when measured from multi-point pressure-flow curves, PVR provides information only on the peripheral arterial function, not the proximal arterial function and gives only an incomplete description of all the forces that oppose right ventricular (RV) flow output. Pulmonary vascular impedance spectra (PVZ) capture the impact of proximal and peripheral arterial structure and function on RV function. Analyses of ventricular-vascular coupling give insight into the efficiency of mechanical and metabolic interactions between the right ventricle and the pulmonary vasculature. Here we review techniques for measuring PVZ in humans and animal models and for determining RV function.
I. Introduction
Pulmonary hypertension is defined by a mean pulmonary artery pressure (PAP) greater than 25 mmHg at rest [1]. In the subset of pulmonary hypertension cases termed pulmonary arterial hypertension (PAH), these high pressures are caused by narrowing of the small, peripheral pulmonary arteries that control pulmonary vascular resistance (PVR). When the larger arteries of the pulmonary circulation are exposed to high pressures for long times, they too transform to become thicker and less compliant. Together, these changes pulmonary vascular resistance and compliance significantly impair right ventricular (RV) function and cause RV failure [1].
In comparison to the systemic circulation, the pulmonary circulation is highly pulsatile. That is, the ratio of pulse pressure over mean pressure in the pulmonary artery is about one, whereas in the aorta, pulse pressure is about 40% of mean pressure; instantaneous flow varies from a maximum at mid-systole to around zero in diastole. As a consequence, the ratio of pulsatile ventricular work to total (steady plus pulsatile) ventricular work is ~25%, ~2.5-fold more than in the systemic circulation [2]. Thus, any useful measures of pulmonary vascular and right ventricular function must take into account the pulsatile nature of flow and pressure. In addition, measures of right ventricular function must take into account the dynamic interactions between the ventricle and vasculature.
The pulmonary vascular impedance spectrum (PVZ) is a complex, frequency-dependent function of pulsatile pulmonary pressure and flow that is sensitive to changes in proximal, intermediate and peripheral arterial resistance and compliance. Thus, it is a comprehensive measure of pulmonary vascular function. In addition the impedance is the dynamic right ventricular afterload; as such, some have suggested that it is a superior predictor of RV failure in PAH compared to PVR [3].
There are many techniques available to assess RV function but only a few are based on hemodynamics and energy requirements. Here we review techniques for measuring RV afterload (i.e., PVZ) in humans and animal models, and for quantifying the RV function in terms of required pressure and flow output and the dynamics of interaction between the ventricle and the vasculature.
II. Pulmonary Vascular Impedance
A. Definition
PVZ is a measure of opposition to pulsatile flow in the pulmonary circulation. PVZ spectra have a magnitude (Z) and a phase (Θ), each of which are functions of frequency (Figure 1). Typically, Fourier series analysis is used to calculate PVZ from pressure and flow waves measured at a single location in the circulation in response to the native heart beat. Fourier analysis is possible because the pulmonary circulation behaves nearly linearly. That is, a purely sinusoidal flow oscillation produces a sinusoidal pressure oscillation of the same frequency but with a phase shift.
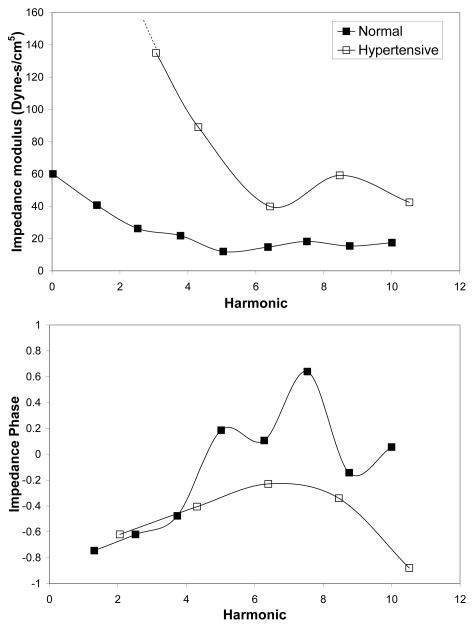
Fig. 1.
Representative healthy and diseased (PAH) pulmonary input impedance spectra in adult humans [5].
To calculate PVZ, pressure and flow waves generated by the right ventricle are decomposed into mean values and a series of harmonic (sine) waves at multiples of the heart rate frequency [2]. For example, if the heart rate is 60 bpm (i.e., 1 Hz), the 1st through 10th harmonics occur at frequencies 1 Hz through 10 Hz. Alternatively, in an isolated lung setup, one can impose pulsatile flow at a range of frequencies with a mechanical pump as pioneered by Caro and McDonald [4]. In either case, the impedance magnitude Z is the modulus of the pressure divided by the modulus of the flow at a given frequency. The impedance phase Θ is the phase delay between pressure and flow waves at a given frequency.
Unlike PVR, PVZ cannot be expressed as a single number; a graphical display is required to show the impedance magnitude and phase as functions of frequency. In healthy humans, impedance falls steeply from a relatively high value at 0 Hz to a first minimum at 2–4 Hz, followed by a second maximum at 6–8 Hz with smaller fluctuations at higher frequencies. At low frequencies, the phase angle is negative, indicating that flow leads pressure; at higher frequencies the phase hovers around zero [2] (Figure 1). In patients with PAH, the impedance magnitude is higher at all frequencies but most dramatically at the fundamental (0th) and lower order harmonics; also, impedance phase typically becomes more negative in patients with PAH (Figure 1) [5].
PVZ magnitude at zero Hz (Z0) is the ratio of mean pressure to mean flow, which corresponds to PVR. At high frequencies when the magnitude of PVZ is relatively constant and the phase is near zero, it can often be safely assumed that wave reflections are negligible. In this case PVZ magnitude is termed characteristic impedance (ZC), which reflects the ratio of inertial effects to compliance effects in the large, conduit arteries.
Because impedance is sensitive to blood flow pulsatility, the normal heart rate (HR) is an important parameter. In particular, the normal heart rate determines the way in which we interpret changes in impedance. In general, the low frequency behavior of the impedance is determined by the resistance vessels, the mid-range frequency behavior is determined by intermediate vessels and the high frequency behavior is determined by the vessels most proximal to the point of measure. For example, in the aorta, the input impedance is determined by the peripheral vasculature for frequencies <0.2 HR, the intermediate arterial network (i.e., the total arterial compliance) for 0.2 HR < frequencies <3 HR, and the aorta for frequencies >3 HR [6]. Similar relationships likely exist for the pulmonary vasculature.
B. PVZ measurement in humans
In order to measure PVZ in humans, right heart catheterization is typically required to obtain the transient pulmonary artery pressure synchronized to pulmonary artery flow. High fidelity pressure measurements can be made by catheters with solid state sensors at the tip [7,8]; lower fidelity data can be obtained by the fluid-filled catheters that are more commonly used clinically [9,10]. Similarly, flow can be measured intravascularly with a specialized sensor for flow at the tip of a catheter [7,8] or extravascularly with ultrasound [9,10].
Using these techniques, PVZ has been calculated healthy humans [11] and in patients with pulmonary hypertension secondary to mitral stenosis, congenital cardiac defects, congestive heart failure and COPD, and idiopathic pulmonary arterial hypertension [5, 12–14]. Generally, PVZ magnitude (including Z0 and ZC) increases and the first minima and maxima of impedance magnitude shifts shifted to higher frequencies (Figure 1). To date, particular changes in PVZ spectra that are diagnostic of particular diseases, their progression or their effective treatment, have not been identified.
C. PVZ measurement in animal models
PVZ has been measured in large and small animals using in vivo and ex vivo techniques. The in vivo techniques are similar to those used in humans; the ex vivo techniques often employ an isolated lung and a pressure or flow pump that replaces the function of the heart. An advantage of ex vivo, isolated lung measurement of PVZ is that the effects of changes in pulmonary arterial, left atrial and airway pressures, as well as flow rate and HR can be independently investigated. Also, changes in PVZ can be correlated with direct measurements of pulmonary arterial mechanics via isolated vessel experiments, whole lung morphometrics or other techniques.
The choice of animal model not only affects the relevant frequency range for PVZ (because native HR depends inversely on animal size) but can also affect the way in which pathology affects metrics of PVZ. For example, changes in ZC after experimental pulmonary embolization have been shown to differ with species: in dog lungs, ZC decreased after embolization with glass beads and autologous blood clots but in pigs and goats, ZC increased [15–19]. In mice, we found no significant changes in ZC with microsphere embolization [20]. PVZ is easier to measure accurately in larger animal models but using rodent models the impact of genetically engineered defects on pulmonary vascular function can be determined.
Finally, computer models can be used to gain insight into the changes in pulmonary vascular mechanics responsible for changes in pulmonary vascular impedance. The simplest models are lumped parameter models such as the three- for four-element Windkessel [21, 22]. These models account for PVR and compliance but cannot capture wave reflections. Transmission line models capture wave reflections but typically do not account for the complexity of pulmonary vascular architecture. Fully three-dimensional computational models of pulsatile blood flow in the lung are possible but the computational costs can be daunting [23]. Models also have the advantage that they can be used to predict changes with growth and development and investigate spatially specific structure-function relationships.
III. Right Ventricular Function
Useful and well established parameters for assessing ventricular function include cardiac output, ejection fraction, end-systolic pressure-volume relation, end-diastolic pressure-volume relation, preload recruitable stroke work, mechanical efficiency, relaxation factor and maximum and minimum dP/dt [24–26]. However, quantifying ventricular function without attention to pulmonary vascular function ignores the important dependency of ventricular output on arterial impedance.
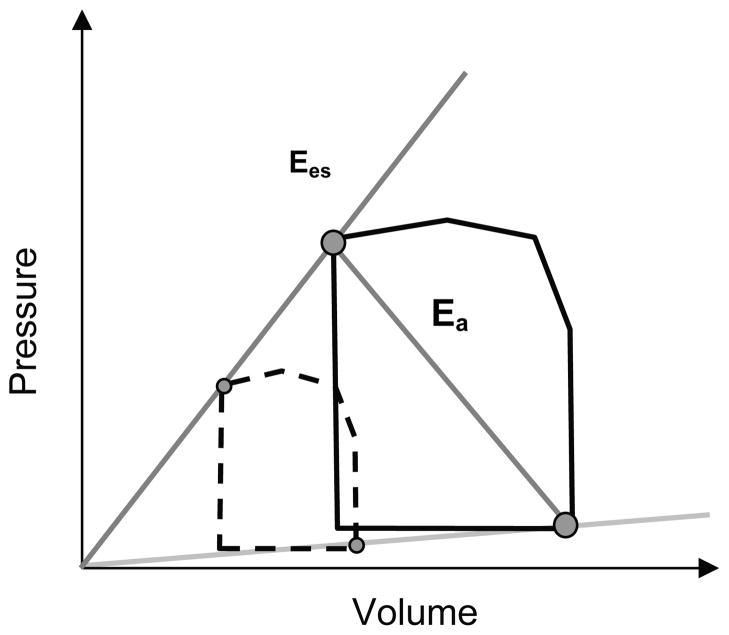
To understand the dependency of ventricular function on arterial function, the concept of ventricular-vascular coupling was developed by Sagawa and coworkers [26]. In their approach, ventricular end-systolic elastance (Ees) and arterial effective elastance (Ea) are calculated from ventricular pressure-volume loops (Figure 2). Pressure-volume loops at different levels of preload are required for this technique, which can be difficult to achieve clinically. Recently, the so-called single beat method was developed to enable calculation of Ees and Ea from only RV and pulmonary artery pressure waveforms [27].
Fig. 2.
Schematic of right ventricular pressure-volume (PV) loops showing calculation of Ees and Ea. The normal PV loop is shown with a solid black line. Preload is reduced by vena caval occlusion, which yields the dashed PV loop. The line connecting end systole for these loops approximates Ees. Ea is the absolute value of the slope of the line end systole to end diastole.
Ees provides insight into the ventricular function, Ea provides insight into vascular function, and their ratio measures the efficiency of ventricular-vascular hemodynamic interactions [28, 29]. When the ventricle and vasculature are efficiently coupled, minimal energy is wasted in the pulse pressure and maximal energy is transmitted in the mean pressure [30]. In this case, the ventricle operates at a maximum efficiency and submaximal stroke work such that Ees/Ea > 1.5. By contrast, for a poorly performing ventricle or high impedance vasculature, energy may be wasted through a variety of mechanisms including overly rapid pulse wave reflections as occur with age [31, 32] increased arteriolar resistance as occurs in hypertension [33], and ventricular dilation as occurs with heart failure [33]. In these cases, Ees/Ea < 1 and ventricular-vascular uncoupling occurs [34, 35].
IV. Conclusion
Pulmonary vascular resistance is well known to increase with pulmonary arterial hypertension, but prolonged periods of high pressure may also decrease pulmonary vascular compliance. Thus, measures such as PVZ that capture changes in both resistance and compliance are critical to understanding changes in pulmonary vascular function. Right ventricular function is also dynamic; nearly 25% of total work required of the right ventricle goes into creating the pulmonary artery pulse pressure. The concept of ventricular-vascular coupling efficiency is a useful tool for understanding dynamic right ventricular function and impairments in function that are caused by ventricular and/or vascular disease.
Contributor Information
Naomi C. Chesler, Email: chesler@engr.wisc.edu, Department of Biomedical Engineering at the University of Wisconsin, Madison WI 53706 USA. (608-265-8929; fax: 608-265-9239)
Alejandro Roldan, Email: roldan@wisc.edu, Department of Biomedical Engineering at the University of Wisconsin, Madison WI 53706 USA.
Rebecca R. Vanderpool, Email: beccav@cae.wisc.edu, Department of Biomedical Engineering at the University of Wisconsin, Madison WI 53706 USA
Robert Naeije, Email: rnaeije@ulb.ac.be, Director of the Department of Pathophysiology of the Erasme University Hospital, Brussels, Belgium.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert Consensus Document on Pulmonary Hypertension. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association. Circulation. 2009;119 doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.Milnor WR. Hemodynamics. 2. Baltimore: Williams & Wilkins; 1989. p. xii.p. 419. [Google Scholar]
- 3.Grant BJ, Lieber BB. Clinical significance of pulmonary arterial input impedance. Eur Respir J. 1996;9:2196–2199. doi: 10.1183/09031936.96.09112196. [DOI] [PubMed] [Google Scholar]
- 4.Caro CG, McDonald DD. The relation of pulsatile pressure and flow in the pulmonary vascular bed. J Physiol. 1961;157:426–53. doi: 10.1113/jphysiol.1961.sp006734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milnor WR, Conti CR, Lewis KB, O’Rourke MF. Pulmonary arterial pulse wave velocity and impedance in man. Circ Res. 1969;25:637–649. doi: 10.1161/01.res.25.6.637. [DOI] [PubMed] [Google Scholar]
- 6.Westerhof N, Lankhaar JW, Westerhof BE. The Arterial Windkessel. Med Biol Eng Comput. 2008 doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 7.Laskey W, Ferrari V, Palevsky H, Kussmaul W. Pulmonary artery hemodynamics in primary pulmonary hypertension. J Am Coll Cardiol. 1993;21:406–412. doi: 10.1016/0735-1097(93)90682-q. [DOI] [PubMed] [Google Scholar]
- 8.Syyed R, Reeves JT, Welsh D, et al. The relationship between the components of pulmonary artery pressure remains constant under all conditions in both health and disease. Chest. 2008;133(3):633–639. doi: 10.1378/chest.07-1367. [DOI] [PubMed] [Google Scholar]
- 9.Nakayama Y, Nakanishi N, Sugimachi M, et al. Characteristics of pulmonary artery pressure waveform for differential diagnosis of chronic pulmonary thromboembolism and primary pulmonary hypertension. Journal Of The American College Of Cardiology. 1997;29(6):1311–1316. doi: 10.1016/s0735-1097(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 10.Huez S, Brimioulle S, Naeije R, Vachiery JL. Feasibility of routine pulmonary arterial impedance measurements in pulmonary hypertension. Chest. 2004;125:2121–2128. doi: 10.1378/chest.125.6.2121. [DOI] [PubMed] [Google Scholar]
- 11.Murgo JP, Westerhof N. Input impedance of the pulmonary arterial system in normal man. Effects of respiration and comparison to systemic impedance. Circ Res. 1984;54:666–673. doi: 10.1161/01.res.54.6.666. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki T. Pulmonary vascular input impedance in patients with atrial septal defect. Acta Medica Nagasakiensia. 1994;39:107–113. [Google Scholar]
- 13.Elkins RC, Peyton MD, Greenfield LJ. Pulmonary vascular impedance in chronic pulmonary hypertension. Surgery. 1974;76(1):57–64. [PubMed] [Google Scholar]
- 14.Kussmaul WG, Noordergraaf A, Laskey WK. Right ventricular pulmonary arterial interactions. Ann Biomed Eng. 1992;20:63–80. doi: 10.1007/BF02368506. [DOI] [PubMed] [Google Scholar]
- 15.Ewalenko P, Brimioulle S, Delcroix M, Lejeune P, Naeije R. Comparison of the effects of isoflurane with those of propofol on pulmonary vascular impedance in experimental embolic pulmonary hypertension. Br J Anaesth. 1997;79:625–30. doi: 10.1093/bja/79.5.625. [DOI] [PubMed] [Google Scholar]
- 16.Maggiorini M, Brimioulle S, De Canniere D, Delcroix M, Naeije R. Effects of pulmonary embolism on pulmonary vascular impedance in dogs and minipigs. J Appl Physiol. 1998;84:815–21. doi: 10.1152/jappl.1998.84.3.815. [DOI] [PubMed] [Google Scholar]
- 17.Naeije R, Maarek JM, Chang HK. Pulmonary vascular impedance in microembolic pulmonary hypertension: Effects of synchronous high-frequency jet ventilation. Respir Physiol. 1990;79:205–17. doi: 10.1016/0034-5687(90)90127-k. [DOI] [PubMed] [Google Scholar]
- 18.Pagnamenta A, Fesler P, Vandinivit A, Brimioulle S, Naeije R. Pulmonary vascular effects of dobutamine in experimental pulmonary hypertension. Crit Care Med. 2003;31:1140–6. doi: 10.1097/01.CCM.0000060126.75746.32. [DOI] [PubMed] [Google Scholar]
- 19.Wauthy P, Pagnamenta A, Vassalli F, Naeije R, Brimioulle S. Right ventricular adaptation to pulmonary hypertension: An interspecies comparison. Am J Physiol Heart Circ Physiol. 2004;286:H1441–7. doi: 10.1152/ajpheart.00640.2003. [DOI] [PubMed] [Google Scholar]
- 20.Tuchscherer HA, Webster E, Chesler NC. Pulmonary vascular resistance and impedance in isolated mouse lungs: Effects of pulmonary emboli. Annals of Biomedical Engineering. 2006;34(4):660–668. doi: 10.1007/s10439-005-9050-z. [DOI] [PubMed] [Google Scholar]
- 21.Westerhof N, Lankhaar JW, Westerhof BE. The Arterial Windkessel. Med Biol Eng Comput. 2008 doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- 22.Grant BJ, Paradowski LJ. Characterization of pulmonary arterial input impedance with lumped parameter models. Am J Physiol. 1987;252:H585–93. doi: 10.1152/ajpheart.1987.252.3.H585. [DOI] [PubMed] [Google Scholar]
- 23.Spilker RL, Feinstein JA, Parker DW, Reddy VM, Taylor CA. Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann Biomed Eng. 2007;35:546–59. doi: 10.1007/s10439-006-9240-3. [DOI] [PubMed] [Google Scholar]
- 24.Carabello BA. Evolution of the study of left ventricular function: everything old is new again. Circulation. 2002;105:2701–2703. doi: 10.1161/01.cir.0000021240.86593.9d. [DOI] [PubMed] [Google Scholar]
- 25.Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res. 1974;35:117–126. doi: 10.1161/01.res.35.1.117. [DOI] [PubMed] [Google Scholar]
- 26.Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. Oxford University Press; New York: 1988. [Google Scholar]
- 27.Brimioulle S, Wauthy P, Ewalenko P, Rondelet B, Vermeulen F, Kerbaul F, Naeije R. Single-beat estimation of right ventricular end-systolic pressure-volume relationship. Am J Physiol Heart Circ Physiol. 2003;284:H1625–1630. doi: 10.1152/ajpheart.01023.2002. [DOI] [PubMed] [Google Scholar]
- 28.Asanoi H, Sasayama S, Kameyama T. Ventriculoarterial coupling in normal and failing heart in humans. Circ Res. 1989;65:483–493. doi: 10.1161/01.res.65.2.483. [DOI] [PubMed] [Google Scholar]
- 29.Burkhoff D, Sagawa K. Ventricular efficiency predicted by an analytical model. Am J Physiol. 1986;250:R1021–1027. doi: 10.1152/ajpregu.1986.250.6.R1021. [DOI] [PubMed] [Google Scholar]
- 30.O’Rourke MF, Avolio AP, Nichols WW. Left Ventricular-Systemic Arterial Coupling in Humans and Strategies to Improve Coupling in Disease States. New York: Springer-Verlag; 1987. [Google Scholar]
- 31.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. doi: 10.1161/01.cir.68.1.50. [DOI] [PubMed] [Google Scholar]
- 32.Nichols WW, O’Rourke MF, Avolio AP, Yaginuma T, Murgo JP, Pepine CJ, Conti CR. Effects of age on ventricular-vascular coupling. Am J Cardiol. 1985;55:1179–1184. doi: 10.1016/0002-9149(85)90659-9. [DOI] [PubMed] [Google Scholar]
- 33.O’Rourke MF. Arterial Function in Health and Disease. Edinburgh: Churchill Livingstone; 1982. [Google Scholar]
- 34.Fourie PR, Coetzee AR, Bolliger CT. Pulmonary artery compliance: its role in right ventricular-arterial coupling. Cardiovasc Res. 1992;26:839–844. doi: 10.1093/cvr/26.9.839. [DOI] [PubMed] [Google Scholar]
- 35.Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng. 1992;20:41–62. doi: 10.1007/BF02368505. [DOI] [PubMed] [Google Scholar]