Abstract
Objective
We hypothesized that cofilin activation by members of the slingshot (SSH) phosphatase family is a key mechanism regulating VSMC migration and neoinitima formation following vascular injury.
Methods and Results
Scratch wound and modified Boyden chamber assays were used to assess VSMC migration following downregulation of the expression of cofilin and each slingshot phosphatase isoforms (SSH1,-2,-3) by siRNA, respectively. Cofilin siRNA greatly attenuated the ability of VSMC migration into the “wound” and PDGF-induced migration was virtually eliminated versus a 3.5-fold increase in non-treated VSMCs, establishing a critical role for cofilin in VSMC migration. Cofilin activation (dephosphorylation) was increased in PDGF-stimulated VSMCs. Thus, we assessed the role of the SSH family of phosphatases on cofilin activation and VSMC migration. Treatment with either SSH1 or SSH2 siRNA attenuated cofilin activation, while SSH3 siRNA had no effect. Only SSH1 siRNA significantly reduced wound healing and PDGF-induced VSMC migration. Both SSH1 (4.7 fold) and cofilin (3.9 fold) expression were increased in balloon injured versus non-injured carotid arteries and expression was prevalent in the neointima.
Conclusions
These studies demonstrate that the regulation of VSMC migration by cofilin is SSH1 dependent, and that this mechanism potentially contributes to neointima formation following vascular injury in vivo.
Keywords: PDGF, VSMC migration, vascular injury, slingshot phosphatase, neointima
Vascular smooth muscle cell (VSMC) migration is a critical aspect of pathological remodeling in response to vascular injury (restenosis and neointima formation).1,2 Given the importance of cell chemotaxis in this processes, understanding the mechanisms which regulate VSMC migration are of high clinical relevance.
A key component of cell migration is the dynamic regulation of the actin cytoskeleton at the leading edge of the cell. This process is regulated by a family of actin-binding proteins, including actin-related protein 2/3 (Arp 2/3), which assembles new actin filaments (F-actin), and the actin depolymerizing factor (ADF) cofilin, which is responsible for depolymerization (severing) of existing F-actin to provide substrate for Arp 2/3.2–4 Thus, cofilin is likely to play a critical role in directional cell migration.5 In further support of the important role for cofilin in migration are findings that cells deficient in cofilin exhibit impaired motility, while those overexpressing cofilin are highly motile.6,7 It is however important to note that these facts may not translate to the unique physiology of smooth muscle, where, unlike in any other cell type, the actin cytoskeleton is anchored into dense bodies, structures characteristic to SMCs and, importantly, intimately related to VSMC function in intact vessels, including VSMC positioning with respect to blood flow, force transduction and maintenance of vessel diameter under varying blood pressures. In addition, this knowledge was accumulated in cell types which are far less phenotypically plastic than VSMCs, and, unlike VSMCs, do not acquire a migratory phenotype in either physiological or pathological situations in vivo.
Activity of cofilin is regulated by phosphorylation, so that the dephosphorylated form is the active form. Stimulation by PDGF (PDGF-BB), one of the most potent pro-migratory growth factors, has recently been reported to increase cofilin dephosphorylation, but the signaling pathways involved in the regulation of cofilin activity have not been fully identified. LIM-Kinase (LIMK) has been shown to inactivate cofilin by phosphorylation at Ser3, resulting in abolition of actin depolymerization in other cell types.4,8 Of several phosphatases that have been reported to activate cofilin by dephosphorylation, the slingshot family of phosphatases (SSH1, 2, and 3) has emerged as the primary mediator in restoring cofilin’s actin depolymerizing activity and inducing cell motility.9–11
Most studies have focused on the role of SSH1,6,8,12,13 but additional studies indicate a crucial role of the other SSH isoforms in mediating cell motility,12,14 suggesting that the regulation of cofilin during chemotaxis is highly complex. To date, there has been a single study which has examined the role of SSH1 in mediating VSMC migration.15 No studies have compared the influence of the other SSH isoforms (SSH2 and SSH3) during VSMC chemotaxis. Also, a role for cofilin in VSMC migration has not been definitively established.
As SSH-mediated cofilin activation potentially regulates VSMC migration, one physiologically relevant process where it could be seminal is during the neointimal formation in response to vascular injury. Following artery damage, such as that associated with percutaneous transluminal coronary angioplasty (PTCA), the injured vascular wall is repaired by cells derived from adjacent normal tissue. The initial injury to the artery results in destruction of the endothelium and extensive medial VSMC death. After this, both medial smooth muscle migration and proliferation result in neointima growth. While the SSH isoforms mediate cofilin activation and cell chemotaxis in vitro, to date there are no studies which have examined their contribution in vivo. In our current study, we examined changes in SSH and cofilin expression following balloon injury as a novel mechanism contributing to neointimal formation, presumably via mediating VSMC migration.
Thus, in these studies, we tested two hypotheses, one, that activation of cofilin by SSH phosphatase-mediated dephosphorylation is a key mediator of both non-directional VSMC migration and of PDGF-induced directional VSMC migration in vitro, and, two, that SSH1 and cofilin expression are increased during neointima formation following vascular injury in vivo. Our findings demonstrate that: 1) downregulation of cofilin expression by siRNA attenuates VSMC migration, 2) siRNA targeting specific SSH isoforms results in differential effects on the regulation of VSMC migration and cofilin dephosphorylation, and 3) SSH1 and cofilin are upregulated in the neointima following vascular injury in vivo. Our study is the first to compare the roles of the SSH isoforms in cofilin activation and chemotaxis in VSMCs, and demonstrate increased SSH1 and cofilin expression following vascular injury in vivo.
Methods
Cell culture
Male 8-week-old Sprague-Dawley rats were anesthetized by pentobarbital overdose (100 mg/kg intraperitoneal). Thoracic aortas were removed and VSMCs isolated by enzymatic digestion as described previously.16
siRNA transfection procedures
VSMCs in the log growth phase (~70–80% confluent) were trypsinized, collected by centrifugation (1000g, 10 min), resuspended in OPTIMEM media, counted and plated at a density of 5×105 cells/well in 6 well plates. Following 24 hrs in culture, OPTIMEM media was replaced with fresh media containing DharmaFECT 1 transfection reagent and either 100 nmol non-targeting siRNA or siRNA specifically targeting cofilin, SSH1, SSH2 or SSH3 (Dharmacon SMARTpool). Non-targeting siRNA pools, which have no gene targets in rat cells, serve as a control to discriminate sequence-specific silencing from non-specific effects. Transfection efficiency was confirmed by harvesting VSMCs 48–72 hours post transfection for determination of protein expression by immunoblotting or mRNA expression using Real-time PCR. All siRNA sequences used are included in Supplemental Table IV.
Transwell migration assay
VSMC migration was assayed 48–72 hours post siRNA transfection using collagen-coated (5 ng/mL) transwell dishes (Greiner Bio-One) containing 8 μm pores as described previously.16
Scratch wound assay
VSMCs were cultured to 80 to 90% confluence. Artificial wounds were created by scratching the monolayer with a 10 μL pipette tip, and media was replaced with DMEM containing 10% calf serum. Cells were incubated at 37°C in a 5% CO2 incubator for 12 hours. VSMCs were fixed in 5% formaldehyde, stained with coomassie blue, and images captured using phase-contrast microscopy.17 Open area was calculated using analysis algorithm-based T-scratch software.18
Immunoblotting
Quiescent VSMCs at 80–90% confluence were stimulated with PDGF (20 ng/mL) at 37 C in serum-free DMEM for specified durations. Following treatment, cells were washed with ice-cold PBS, and lysed with 500 μL ice-cold lysis buffer, containing 1% Triton X-100, as previously described.19 For carotid artery samples, equal protein loading was verified by immunoblotting for β-actin. The data were then normalized as the ratio of protein expression in the injured left carotid to protein expression the non-injured right carotid to obtain the LC/RC ratio within each animal. Antibody sources were as follows: phospho-cofilin (Ser3) and cofilin (Cell Signaling Technology), α-actin and β-actin (Sigma), GAPDH and SSH1 (ABR Affinity BioReagents), and SSH3 (Abcam).
Rat carotid injury
Sprague-Dawley rats were anesthetized with ketamine/xylazine (75/5 mg/kg, intraperitoneal) and the left external carotid artery isolated by blunt dissection. A Fogarty 2F arterial embolectomy catheter (Edwards Life Sciences) was inserted into the common carotid artery in the direction of the aortic arch via the external carotid and the balloon was inflated for 1 min three times. The external carotid branch was ligated following catheter removal.20,21 The right carotid artery served as a non-injured contralateral control in each animal. Carotid tissue was harvested from rats 4, 7 or 14 days following injury for immunoblotting or immunohistochemistry. All animal procedures were conducted with approval of the IACUC of the University of South Alabama and in accordance to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Immunohistochemistry (IHC)
For histological analysis, carotids were placed in OCT and cut in 7 μm frozen sections prior to staining with hematoxylin and eosin (H&E). Additional sections were fixed in methanol prior to blocking in 3% goat serum/TBS, rinsed with TBS and placed in primary antibody recognizing SSH1 or cofilin. Sections were then incubated with AlexaFluor 568 conjugated secondary antibody (Invitrogen), mounted on microscope slides using Vectashield and visualized by fluorescent microscopy.
Statistical Analysis
Summary data for all experiments are presented as mean ± SEM. Statistical significance was determined by either t-test or one-way ANOVA followed by a Newman-Keuls multiple-comparison post-test (Graph Pad Prism; San Diego, CA). p<0.05 denotes statistical significance.
Results
PDGF activates cofilin in VSMCs
Initial studies were completed to determine if cofilin activation and/or expression were regulated by PDGF in VSMCs. Quiescent VSMCs were treated with PDGF for increasing times and cofilin activity was assessed using a phospho-specific antibody recognizing cofilin phosphorylated at Ser3. PDGF stimulation of VSMCs resulted in time-dependent dephosphorylation of cofilin, without changes in cofilin expression (Supplemental Figure I).
Treatment with cofilin siRNA attenuates VSMC migration
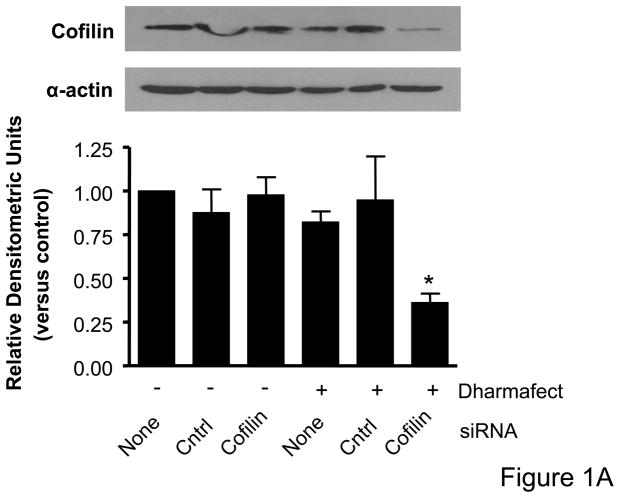
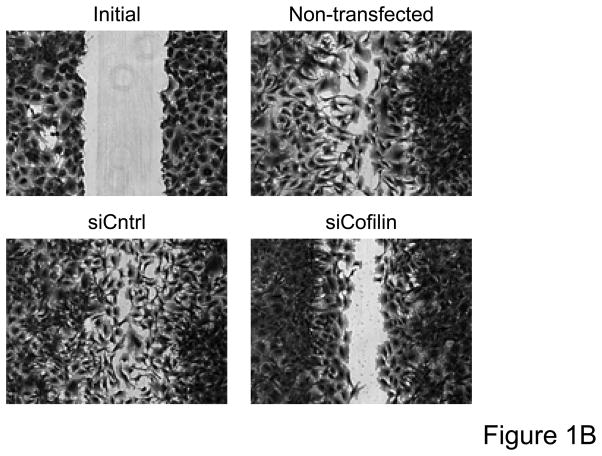
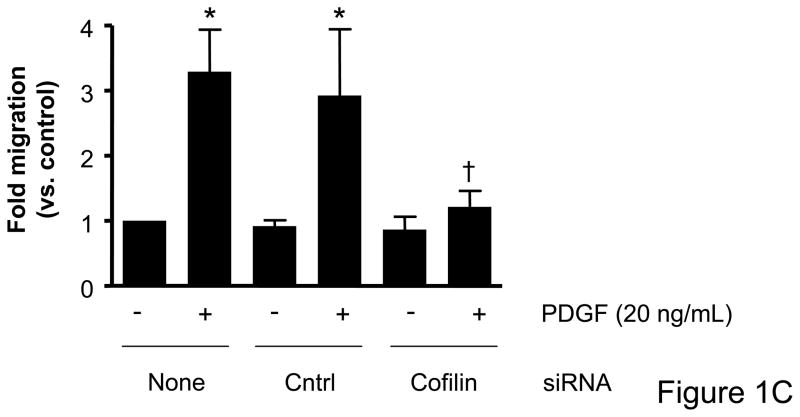
Immunoblot analysis for total cofilin expression indicated that transfection of VSMCs with siRNA targeting cofilin were effective at decreasing protein expression (Figure 1A). No change in cofilin expression was observed as a result of DharmaFECT treatment alone or when VSMCs were transfected with the control non-targeting siRNA. Real time PCR also confirmed decreased cofilin mRNA following siRNA treatment targeting cofilin (data not shown). For initial assessment of the importance of cofilin in serum-induced, non-directional cell migration, prior to scratch wound VSMCs were treated with siRNA targeting cofilin, which greatly impaired migration of cells into the “wounded” area as compared to non-transfected or control siRNA treated cells (Figure 1B, Supplemental Table V). To further analyze and, importantly, quantify the effect of siRNA targeting cofilin, we next examined their effect in agonist (PDGF)-mediated directional migration in a transwell migration assay. In non-transfected VSMCs, PDGF stimulation resulted in a 3.5 fold increase in migration, consistent with our previous studies.16 Neither DharmaFECT alone nor transfection with control non-targeting siRNA had any effect on VSMC migration. In contrast, transfection with siRNA targeting cofilin resulted in a nearly complete abolishment of PDGF-induced VSMC migration (Figure 1C). Taken together, results in Figure 1 demonstrate that cofilin is critically important for the regulation of VSMC migration.
Figure 1. Cofilin siRNA treatment inhibits VSMC migration.
A. Treatment with cofilin siRNA suppresses endogenous protein expression. VSMCs were transfected with either control siRNA (non-targeting) or cofilin siRNA as indicated. After 48 hours, cell lysates were analyzed by immunoblotting with anti-cofilin antibody (top) or α-actin antibody (bottom) which served as a loading control. Summary data are shown in bar graphs (n=3). * indicates a significant decease in cofilin expression vs. non-transfected control VSMCs (p<0.05). B. Phase-contrast images illustrate that cofilin siRNA treatment diminished VSMC movement in response to a scratch wound 12 hours post-injury as compared to non-transfected control and control siRNA treated cells. C. Migration in response to PDGF (20 ng/mL, 4 hrs) was measured using a modified Boyden chamber transwell migration assay (n=5). * indicates a significant increase in migration vs. non-transfected non-stimulated control VSMCs (p<0.01), † indicates a significant decrease in migration vs. non-transfected cells stimulated with PDGF (p<0.001).
Multiple SSH isoforms attenuate PDGF-induced cofilin dephosphorylation
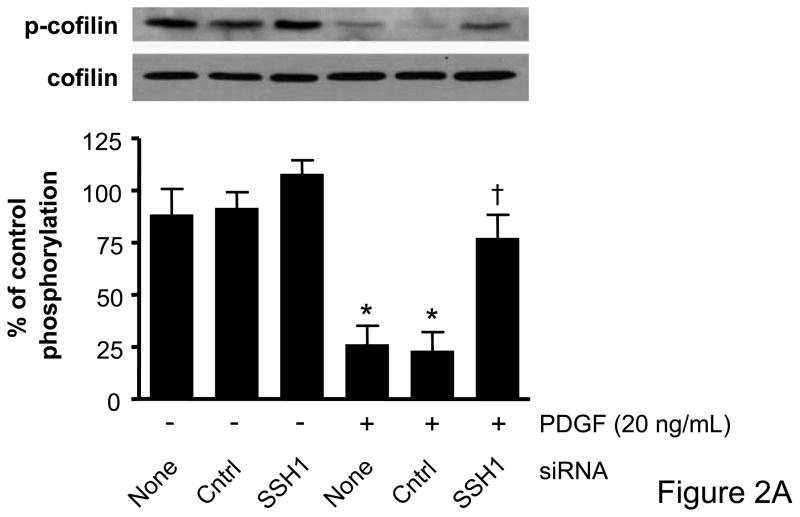
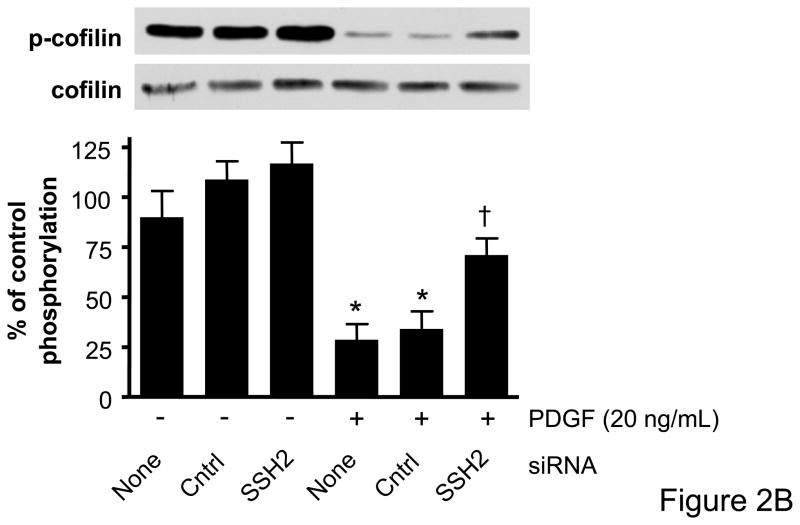
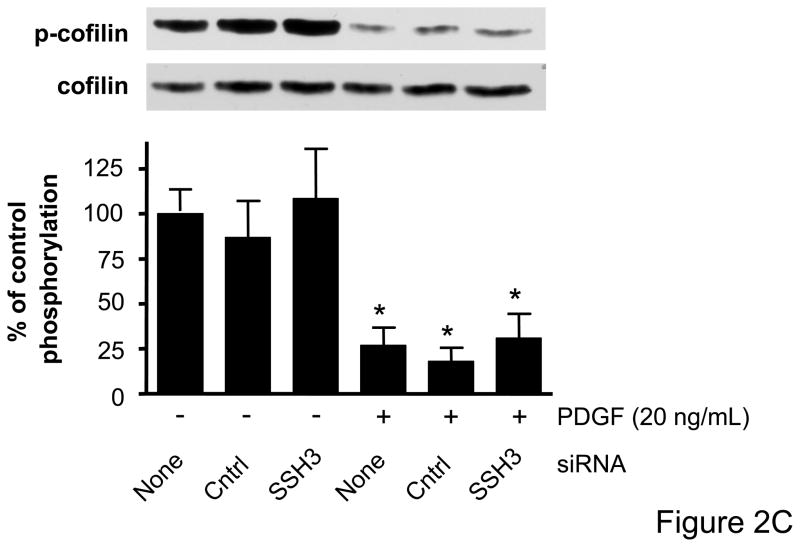
Cofilin has recently been identified as a target of the SSH1 phosphatase in VSMCs,15 but to date there has been no assessment of the role of other SSH isoforms on cofilin activity and ultimately VSMC migration. The effectiveness of VSMC transfection with siRNA targeting SSH1, SSH2, or SSH3, respectively, was confirmed by real-time PCR and Western blotting (Supplemental Data Figure II). Each of the SSH siRNAs were specific to the isoform which they targeted and did not change mRNA levels of other non-targeted SSH isoforms, cofilin, or GAPDH (data not shown). We also confirmed that cofilin protein expression was unaffected by any of the siRNA targeting the SSH isoforms (Figure 2). To determine if PDGF indeed regulates cofilin dephosphorylation via the SSH phosphatases, VSMCs were transfected with siRNA targeting SSH1, 2, or 3, respectively, prior to stimulation with PDGF. As expected, PDGF induced a significant dephosphorylation of cofilin at Ser3. Decreasing the expression of either SSH1 or SSH2 was effective in attenuating PDGF mediated cofilin activation (Figures 2A–B, Supplemental Figure III). In contrast, siRNA targeting SSH3 had no effect upon PDGF-induced dephosphorylation (Figure 2C). Thus, while each of the SSH isoforms are expressed in VSMCs, these findings suggest that their ability to regulate cofilin in response to PDGF activation is varied.
Figure 2. SSH1 and SSH2 siRNA treatments prevent dephosphorylation of cofilin in PDGF-stimulated VSMCs.
Following 48–72 hours of transfection with control siRNA (non-targeting), SSH1 siRNA (n=6) (A), SSH2 siRNA (n=5) (B) or SSH3 siRNA (n=5) (C) cells were stimulated with PDGF for 15 minutes (20 ng/mL). Immunoblot analysis was performed with anti-phospho-specific (Ser3) cofilin antibody (top). Total cofilin was used as a loading control (bottom). Summary data are shown in bar graphs. * indicates a significant decrease in phosphorylation vs. non-transfected non-stimulated VSMC (p<0.05), † indicates a significant increase in phosphorylation vs. non-transfected cells stimulated with PDGF (p<0.01).
VSMC migration is attenuated following treatment with SSH1 but not SSH2 or SSH3 siRNA
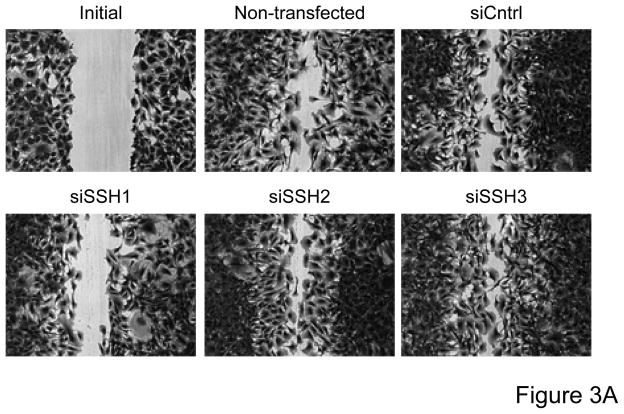
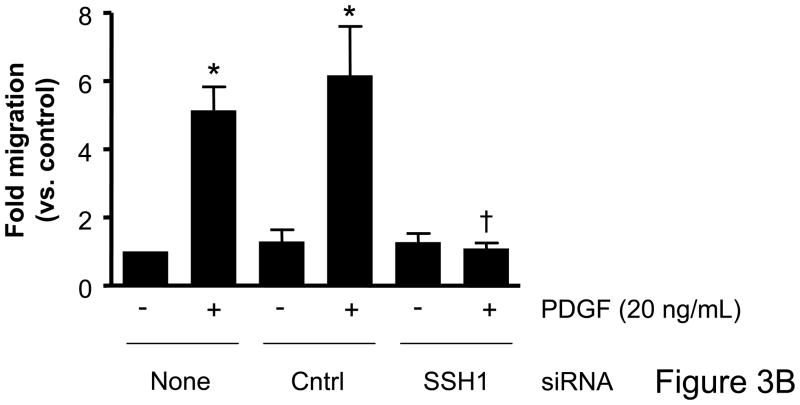
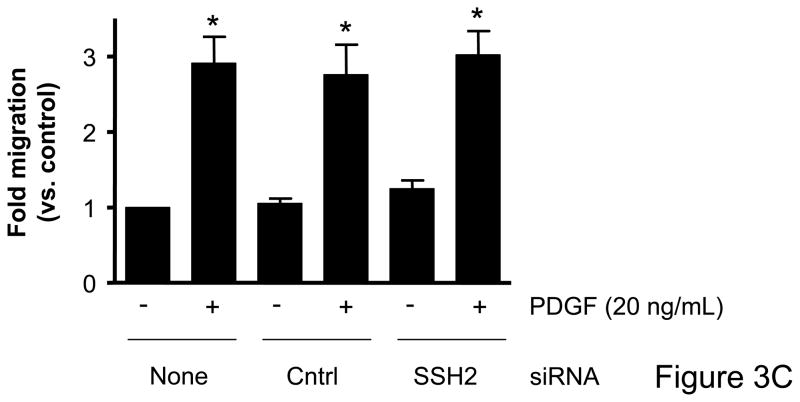
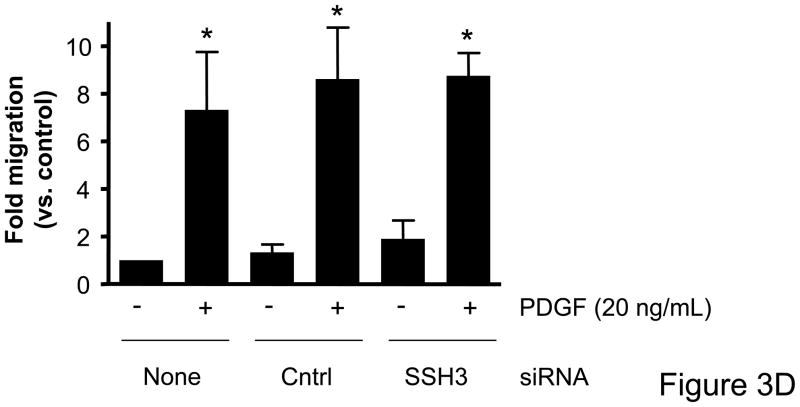
Based on the differential regulation of the SSH isoforms in mediating cofilin dephosphorylation we wanted to determine the role of each isoform as a mechanism mediating VSMC migration. VSMCs were transfected with siRNA targeting the SSH isoforms and migration studies were conducted. In the absence of non-specific effects of the transfection procedure, siRNA targeting SSH1 significantly attenuated wound healing (Figure 3A, Supplemental Table V) as well as PDGF-induced directional VSMC migration quantified in the transwell assay (Figure 3B). Somewhat surprisingly, siRNA targeting SSH2 had virtually no effect on wound healing (Figure 3A) or PDGF-induced transwell migration (Figure 3C). Similar to decreasing SSH2 expression, siRNA targeting SSH3 had no effect on wound healing (Figure 3A) or transwell migration (Figure 3D). As the results with siRNA targeting SSH1 were similar to those of transfection with siRNA targeting cofilin, this further supports the notion that both cofilin and SSH1 are mediators of VSMC migration under varying conditions. But our findings also suggest a potentially complex regulation of cofilin during migration in that while both the SSH1 and SSH2 isoforms regulate cofilin activity, clearly their function deviates when examining their role in mediating cell chemotaxis.
Figure 3. Transfection with SSH1 siRNA reduces VSMC migration; however treatment with SSH2 or SSH3 siRNA had no effect.
Both a scratch wound assay and a modified Boyden chamber transwell migration assay were used to determine VSMC migration following 48–72 hours of transfection with a control siRNA (non-targeting), SSH1, SSH2, or SSH3 siRNA. A. Phase-contrast images were used to demonstrate VSMC movement in response to a scratch wound 12 hours post injury. B. VSMCs were transfected with either control siRNA or SSH1 siRNA for 72 hours. PDGF-induced migration (20 ng/mL, 4 hrs) was measured using a modified Boyden chamber transwell migration assay (n=4). C. Same as B, except VSMCs were transfected with control siRNA or SSH2 siRNA for 48 hours (n=5). D. Same as B, except VSMCs were transfected with control siRNA or SSH3 siRNA for 48 hours (n=5). * indicates a significant increase in migration vs. non-transfected non-stimulated control VSMCs (p<0.05), † indicates a significant decrease in migration vs. non-transfected cells stimulated with PDGF (p<0.05).
Cofilin and SSH1 expression are increased following balloon injury
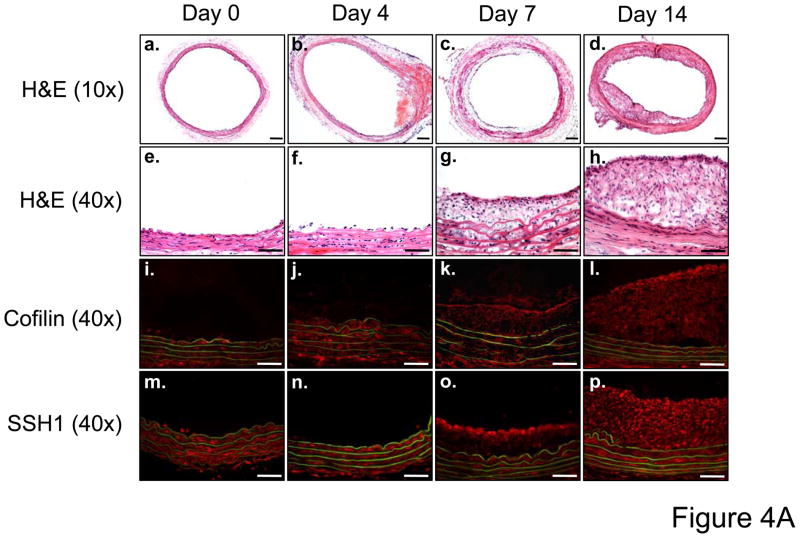
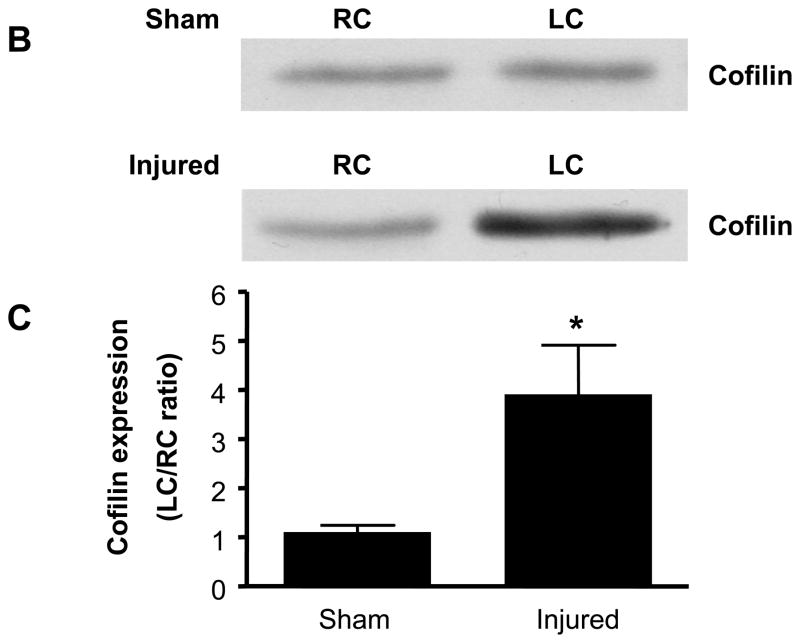
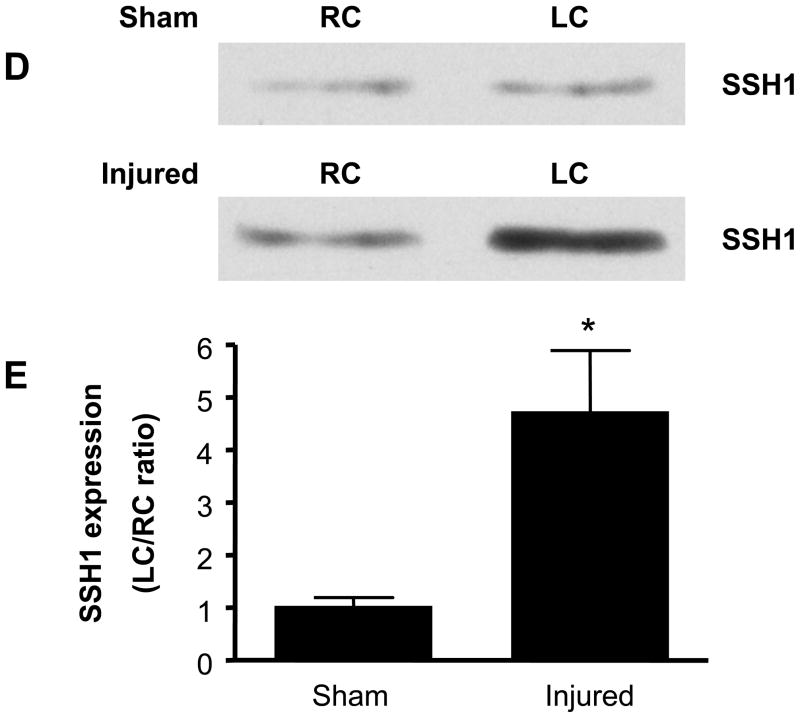
While cofilin and each of the three SSH isoforms is expressed basally in rat carotid artery (data not shown), in vitro studies implicate SSH1 regulation of cofilin as a key mediator of VSMC migration. Thus, a major goal of these studies was to assess if SSH1 and cofilin are physiologically relevant regulators of neointima formation following balloon injury. IHC demonstrated neointima formation at both 7 and 14 days following balloon injury (Figure 4A, a–h). At these time points, both cofilin and SSH1 are expressed in the neointima of the artery (Figure 4A, i–p), which is consistent with their potential function in vascular repair following injury. To quantify changes in expression, 14 days post procedure, both injured and non-injured contralateral carotid arteries were harvested. Balloon injury resulted in approximately a four-fold increase in cofilin expression (Figures 4B and C) and a five-fold increase in SSH1 expression (Figures 4D and E) compared to non-injured control arteries at the time point tested. Immunoblotting detected no change in the expression of either SSH2 or SSH3 at any time point following the balloon injury (data not shown).
Figure 4. Both cofilin and SSH1 expression are increased after carotid injury.
A. Immunohistological analysis in sequential carotid artery cross-sections of neointimal formation stained with H&E (10X, panels a–d and 40X, panels e–h), cofilin expression (40X, panels i–l) and SSH1 expression (40X, panels m–p) at days 0, 4, 7, and 14 post balloon injury. Scale bar is equal to 50 μm in all panels. B. Representative immunoblots for cofilin expression in carotid arteries from sham operated and balloon-injured rats 14 days post-surgery. C. Summary data reported as the ratio of cofilin expression in the left carotid (LC) to right carotid (RC) within each animal in rats following either a sham surgery or balloon injury (n=6). D. Representative immunoblots for SSH1 expression in sham-operated and injured carotid arteries 14 days post-surgery. E. Summary data reported as the ratio of SSH1 expression in the left carotid (LC) to right carotid (RC) within each animal in rats following either a sham surgery or balloon injury (n=6). * indicates a significant increase in expression following carotid injury relative to non-injured control arteries (p<0.01).
Discussion
In response to vascular injury, VSMC migration is in part, critical for neointima formation. Several signaling pathways in migrating VSMCs are clearly defined; however, the mechanisms regulating changes in the actin cytoskeleton during VSMC migration are poorly understood. These studies establish cofilin and its activation by SSH1 as a key regulatory mechanism of VSMC migration, presumably through the regulation of the actin cytoskeleton of migrating VSMCs. Downregulation of cofilin expression by siRNA attenuated VSMC migration during in vitro wound healing and completely blocked directional PDGF-induced migration. Furthermore, we demonstrate that, while all the SSH isoforms are present in VSMCs, only SSH1 mediates VSMC migration, via control of cofilin. Finally, we demonstrate, for the first time that both cofilin and SSH1 expression are found within the neointima of carotid arteries following vascular injury at both 7 and 14 days post-injury, suggesting that both cofilin and SSH1 may be key mediators of neointima formation in vivo.
The active/dephosphorylated form of cofilin has been reported to localize to structures that require high turnover of actin filaments, such as the lamellipodia, filopodia and invadopodia.22 In the lamellipodia of migrating fibroblasts and in neuronal growth cones7,9,23, cofilin may be responsible for the polarization of lamellipodia.5 In essence, active cofilin establishes the directional regulation of migration. Our findings in this study demonstrate that the highly potent pro-migratory stimulus PDGF facilitates cofilin dephosphorylation suggesting that PDGF-mediated VSMC migration is dependent upon cofilin activation, and this may be the potential mechanism by which PDGF regulates the actin cytoskeleton. This is strongly supported by our current results that demonstrate that ~80% downregulation in cofilin expression results in a large decrease in the ability of VSMCs to migrate into the “wounded” area in vitro and in complete blockade of directional migration in response to PDGF (Figure 1), suggesting that cofilin is a critical regulator of both types of VSMC migration.
Signaling mechanisms that regulate cofilin activity are complex and have not been fully characterized in VSMCs. In other cell types, potential regulators include changes in pH, the binding of polyphosphoinositides and phosphorylation, which is by large, the most potent regulator. Both LIMK and TESK can inactivate cofilin via phosphorylation at Ser3, while several protein phosphatases including the SSH family, Chronophin (CIN), PP1, PP2A, and PP2B activate cofilin by dephosphorylation.4,8,10,24 Of these, the SSH family and CIN have been shown to be specific to cofilin, but the CIN phosphatase appears to play a more significant role in processes such as cell division where no directional cellular protrusion or movement is required. In contrast, SSH1 activity is specifically associated with directional formation of both lamellipodia4 and actin cytoskeleton-mediated chemotaxis.6,25
Much of the function of SSH phosphatases has been examined in neuronal or immortalized cell lines and is not fully understood. Downstream targets of SSH1 include cofilin and LIMK; both of which have been reported to be mediators of cell migration.8 Our observation that SSH1 siRNA significantly attenuated cofilin dephosphorylation in response to PDGF (Figure 2A) is in agreement with the findings of San Martin.15 This previous study, however, falls short of definitively linking SSH1-mediated cofilin dephosphorylation to cofilin-dependent VSMC migration. In combination with our current data demonstrating the crucial role of cofilin in mediating PDGF-induced VSMC migration as well as in vitro wound healing (Figure 1), these results conclusively link SSH1-mediated regulation of cofilin activity with VSMC migration. It remains to be determined whether this effect of SSH1 on cofilin is mediated exclusively via direct dephosphorylation, or whether SSH1 also targets LIMK in VSMCs.
In human keratinocytes, expression of phosphatase-dead versions of the SSH isoforms singularly (SSH1, 2 or 3) increased cofilin phosphorylation while decreasing lamellipodia formation and directional motility.12 To date, no study has examined SSH2 or SSH3 regulation of cofilin phosphorylation in VSMCs or the potential contribution of these SSH isoforms in mediating VSMC migration, and thus a complete assessment of SSH isoform participation in VSMC migration is seminal to fully understanding cofilin regulation of VSMC chemotaxis. Similar to the downregulation of SSH1, decreasing SSH2 expression via siRNA almost completely blocked cofilin dephosphorylation (Figure 2). This somewhat unexpected finding suggested some redundancy between the isoforms in VSMCs. Basically, both isoforms are capable of inducing cofilin dephosphorylation, but in the absence of one isoform, the expression of the remaining isoform is sufficient to almost entirely dephosphorylate and activate cofilin. Our observation that decreasing SSH1 expression virtually eliminates PDGF-induced VSMC migration, as well as severely impairing in vitro wound healing (Figure 3), is consistent with the previously reported role of SSH1 in directional actin cytoskeleton-mediated chemotaxis.6,15,25 Interestingly, downregulation of neither SSH2 nor SSH3 had any effect on wound healing or PDGF-induced VSMC migration. These data suggest an isoform specific effect by the SSH phosphatase family on VSMC chemotaxis. Thus, while either SSH1 or SSH2 is capable of almost complete dephosphorylation of cofilin, the distinct subcellular localization of these isoforms may determine their differential effects on migration.
The lack of participation by SSH2 in cell migration in our studies is in contrast to recent findings where knockdown of SSH2 by siRNA was more efficacious than SSH1 siRNA at attenuating tumor cell infiltration into a mesothelial cell monolayer.14 One plausible explanation for this discrepancy in findings could be due to cell specific functions of the SSH family of phosphatases during dynamic cellular processes that require changes in the actin cytoskeleton. Overexpression of either wild type or phosphatase inactive forms of mouse SSH (mSSH) isoforms in HeLa cells revealed interesting findings in regard to the cellular location of these proteins; both mSSH1 and mSSH2 co-localized with F-actin fibers, but only SSH2 demonstrated a propensity to localize at focal adhesions at the terminal end of stress fibers, further confirmed by vinculin co-localization.26 Taken together, these findings suggest differences in the specific intracellular localization of versus SSH2 and/or differential effects of SSH1 versus SSH2 on other signaling pathways involved in migration. Furthermore, cofilin has been identified as a mediator of invadopodia formation, which is a specialized cell protrusion rich in actin filaments and characteristic of cells with a high propensity for invasive behavior such as tumor cells. Given the differences observed between SSH1 and SSH2 in both cellular localization and cell invasion it would be interesting to determine whether SSH2 is more critical to maintaining cofilin functionality in distinct subcellular locations.
Accumulated data suggest that LIMK is a crucial mediator necessary for cell migration, albeit that the mechanism by which it regulates cell movement is not yet clear.6,7,27,28 To date, SSH1 is the only phosphatase which has been shown to dephosphorylate LIMK.8 One plausible explanation for these seemingly disparate findings between SSH1 and SSH2 may be that while SSH2 can dephosphorylate cofilin, it may not dephosphorylate LIMK, thus being unable to create a positive feedback loop fully allowing for cell migration.
SSH3 siRNA treatment had minimal effect on either cofilin dephosphorylation or VSMC migration (Figures 2 and 3). SSH3, the shortest isoform of the SSH family, lacks expression of the region which allows for F-actin interaction potentially reducing 1) its propensity to localize in cellular regions of increased actin density such as the leading edge during migration, and 2) may explain why it is thought to have the weakest phosphatase activity of the isoforms for cofilin.11,26,29
As our in vitro data suggest that SSH1 isoform is crucial in mediating both cofilin activity and VSMC migration, to determine the physiological relevance of these findings we turned to in vivo studies. Following balloon injury, mimicking PTCA, VSMCs contribute to neointimal formation via both proliferation and migration. We examined both cofilin and SSH1 expression at three different time points throughout the process of healing. Our findings showed an increased expression of both cofilin and SSH1 following injury (Figure 4). It is thought that proliferation occurs early during the repair process while migration is likely playing a key role in neointima formation during the later stages.30,31 The localization of cofilin and SSH1 in cells of the neointima (associated with a high propensity for migration) further supports our theory that SSH1 and cofilin may play a critical role during the in vivo repair process by mediating VSMC migration. One limitation of the current study is that it does not fully establish a cause-effect relationship between cofilin, SSH1 and neointima formation following vascular injury. Additional studies in which these proteins are either inhibited or overexpressed in animal models are required to definitively establish this mechanistic link in vivo.
In summary, our study is the first to definitively test the contribution of each of the SSH isoforms in the control of cofilin activity and migration in VSMCs. Our findings demonstrate a critical role for cofilin and the control of its phosphorylation state by SSH1 in two distinct settings, during in vitro wound healing, involving non-directional VSMC migration and during PDGF-induced directional VSMC migration. Our study also demonstrate differential function for SSH family of phosphatases in VSMC chemotaxis. Finally, we identify SSH1 and cofilin as a potential mediators of neointimal formation. Thus, our study provides an important step in furthering the understanding of the mechanisms which underlie VSMC migration both in vitro and in vivo.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the National Institute of Health [HL084159] and the American Heart Association [0330019N].
Footnotes
Disclosures
None declared.
References
- 1.Schwartz SM. Smooth muscle migration in atherosclerosis and restenosis. J Clin Invest. 1997;100:S87–9. [PubMed] [Google Scholar]
- 2.Gerthoffer WT. Mechanisms of vascular smooth muscle cell migration. Circ Res. 2007;100:607–21. doi: 10.1161/01.RES.0000258492.96097.47. [DOI] [PubMed] [Google Scholar]
- 3.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–9. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 4.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Cofilin promotes actin polymerization and defines the direction of cell motility. Science. 2004;304:743–6. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]
- 6.Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K. Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol. 2005;171:349–59. doi: 10.1083/jcb.200504029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawe HR, Minamide LS, Bamburg JR, Cramer LP. ADF/cofilin controls cell polarity during fibroblast migration. Curr Biol. 2003;13:252–7. doi: 10.1016/s0960-9822(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 8.Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. Embo J. 2005;24:473–86. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gungabissoon RA, Bamburg JR. Regulation of growth cone actin dynamics by ADF/cofilin. J Histochem Cytochem. 2003;51:411–20. doi: 10.1177/002215540305100402. [DOI] [PubMed] [Google Scholar]
- 10.Ambach A, Saunus J, Konstandin M, Wesselborg S, Meuer SC, Samstag Y. The serine phosphatases PP1 and PP2A associate with and activate the actin-binding protein cofilin in human T lymphocytes. Eur J Immunol. 2000;30:3422–31. doi: 10.1002/1521-4141(2000012)30:12<3422::AID-IMMU3422>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Van Troys M, Huyck L, Leyman S, Dhaese S, Vandekerkhove J, Ampe C. Ins and outs of ADF/cofilin activity and regulation. Eur J Cell Biol. 2008;87:649–67. doi: 10.1016/j.ejcb.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Kligys K, Claiborne JN, DeBiase PJ, Hopkinson SB, Wu Y, Mizuno K, Jones JC. The slingshot family of phosphatases mediates Rac1 regulation of cofilin phosphorylation, laminin-332 organization, and motility behavior of keratinocytes. J Biol Chem. 2007;282:32520–8. doi: 10.1074/jbc.M707041200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaji N, Ohashi K, Shuin M, Niwa R, Uemura T, Mizuno K. Cell cycle-associated changes in Slingshot phosphatase activity and roles in cytokinesis in animal cells. J Biol Chem. 2003;278:33450–5. doi: 10.1074/jbc.M305802200. [DOI] [PubMed] [Google Scholar]
- 14.Horita Y, Ohashi K, Mukai M, Inoue M, Mizuno K. Suppression of the invasive capacity of rat ascites hepatoma cells by knockdown of Slingshot or LIM kinase. J Biol Chem. 2008;283:6013–21. doi: 10.1074/jbc.M706538200. [DOI] [PubMed] [Google Scholar]
- 15.San Martin A, Lee MY, Williams HC, Mizuno K, Lassegue B, Griendling KK. Dual regulation of cofilin activity by LIM kinase and Slingshot-1L phosphatase controls platelet-derived growth factor-induced migration of human aortic smooth muscle cells. Circ Res. 2008;102:432–8. doi: 10.1161/CIRCRESAHA.107.158923. [DOI] [PubMed] [Google Scholar]
- 16.Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK. Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res. 2004;94:1219–26. doi: 10.1161/01.RES.0000126848.54740.4A. [DOI] [PubMed] [Google Scholar]
- 17.Mercure MZ, Ginnan R, Singer HA. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol. 2008;294:C1465–75. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geback T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46:265–74. doi: 10.2144/000113083. [DOI] [PubMed] [Google Scholar]
- 19.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23:8019–29. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clowes AW, Reidy MA, Clowes MM. Mechanisms of stenosis after arterial injury. Lab Invest. 1983;49:208–15. [PubMed] [Google Scholar]
- 21.Panek RL, Dahring TK, Olszewski BJ, Keiser JA. PDGF receptor protein tyrosine kinase expression in the balloon-injured rat carotid artery. Arterioscler Thromb Vasc Biol. 1997;17:1283–8. doi: 10.1161/01.atv.17.7.1283. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endo M, Ohashi K, Sasaki Y, Goshima Y, Niwa R, Uemura T, Mizuno K. Control of growth cone motility and morphology by LIM kinase and Slingshot via phosphorylation and dephosphorylation of cofilin. J Neurosci. 2003;23:2527–37. doi: 10.1523/JNEUROSCI.23-07-02527.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem. 2005;280:12683–9. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- 25.Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R, Uemura T, Mizuno K. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J Cell Biol. 2004;165:465–71. doi: 10.1083/jcb.200401136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta Y, Kousaka K, Nagata-Ohashi K, Ohashi K, Muramoto A, Shima Y, Niwa R, Uemura T, Mizuno K. Differential activities, subcellular distribution and tissue expression patterns of three members of Slingshot family phosphatases that dephosphorylate cofilin. Genes Cells. 2003;8:811–24. doi: 10.1046/j.1365-2443.2003.00678.x. [DOI] [PubMed] [Google Scholar]
- 27.Bernard O. Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol. 2007;39:1071–6. doi: 10.1016/j.biocel.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka K, Foletta V, Bernard O, Itoh K. A role for LIM kinase in cancer invasion. Proc Natl Acad Sci U S A. 2003;100:7247–52. doi: 10.1073/pnas.1232344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto M, Nagata-Ohashi K, Ohta Y, Ohashi K, Mizuno K. Identification of multiple actin-binding sites in cofilin-phosphatase Slingshot-1L. FEBS Lett. 2006;580:1789–94. doi: 10.1016/j.febslet.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–33. [PubMed] [Google Scholar]
- 31.Fingerle J, Johnson R, Clowes AW, Majesky MW, Reidy MA. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989;86:8412–6. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.