Abstract
The neonatal immune system is believed to be biased towards T helper type 2 (Th2) immunity, but under certain conditions neonates can also develop Th1 immune responses. Neonatal Th2 immunity to myelin antigens is not pathogenic and can prevent induction of experimental autoimmune encephalomyelitis (EAE) in adulthood, but the consequences of neonatally induced Th1 immunity to self antigens have remained unresolved.
Here, we show that neonatal injection of mice with myelin antigens emulsified in complete Freunds’ adjuvant (CFA) induced vigorous production of IFN-γ and IL-17, but not IL-5, consistent with myelin-specific Th1/Th17 immunity. Importantly, the myelin-specific Th1/Th17 cells persisted in the mice until adulthood without causing symptoms of EAE. Intraperitoneal, but not subcutaneous injection of neonates with myelin antigens protected against induction of EAE as adults. Intraperitoneally injected neonates showed a substantial decrease of the number and avidity of myelin-reactive Th17 cells, suggesting a decrease in IL-17 producing precursor cells as the mechanism of protection from EAE upon reinjection with myelin antigens as adults. The results could provide a rationale for the presence of autoreactive T cells found in healthy human individuals without autoimmune disease.
Keywords: Multiple sclerosis, neonates, Th1, Th2, Th17
1. Introduction
Over half a century ago, Owens, Billingham, and Medawar discovered that tolerance to foreign tissues can be readily induced in neonatal animals, but not in adults (Billingham et al., 1953; Burnet and Fenner, 1949; Owen, 1945). Since then, the neonatal period has been viewed as a critical window in the development of the immature immune system during which tolerance to self or foreign tissues can be more easily induced than later in life (Billingham et al., 1953; Clayton et al., 1989; Paterson, 1958). The cellular basis for neonatal tolerance was initially interpreted to be the result of the deletion of particular clones of antigen-reactive lymphocytes, and, later ascribed to immune regulatory mechanisms (Gammon et al., 1986; McDonald and Swanborg, 1988; Swierkosz and Swanborg, 1977). Subsequently, it was recognized that the neonatal encounter with antigen may lead to the priming and expansion of antigen-specific T lymphocytes expressing anti-inflammatory cytokine profiles that favor acceptance of tissue grafts and oppose autoimmune tissue destruction (Fadel et al., 2002; Forsthuber et al., 1996; Ridge et al., 1996; Singh et al., 1996). Specifically, injection of protein antigens into neonates was shown to induce antigen-specific T helper type 2 (Th2)2 immunity to prototypic foreign or self antigens (Th2 immune deviation) (Forsthuber et al., 1996; Singh et al., 1996). This observation has since been confirmed in different systems and led to the view that the neonatal immune system is intrinsically biased towards anti-inflammatory Th2 immune responses (Adkins and Du, 1998; Chen and Field, 1995; Powell and Streilein, 1990).
However, the cytokine differentiation of T cells in neonates may be dependent on the type and status of the engaged APCs (Ridge et al., 1996), the dose and systemic availability of antigen (Fadel et al., 2002; Garza et al., 1997), and the adjuvant in which the antigens are injected (Forsthuber et al., 1996). Along these lines, it is known that most protein antigens need to be injected with adjuvant to induce immune responses (Janeway, 1989). In particular, complete Freund’s adjuvant (CFA), a mixture of mineral oil and heat inactivated mycobacteria, has been the adjuvant of choice for the induction of immune responses to protein antigens since its description by Freund and others in the 1930's (Freund et al., 1937; Freund, 1951). CFA is a potent Th1 adjuvant via signaling trough Toll- like receptors on innate immune cells (Yip et al., 1999), and it is required for the induction of autoimmune disease in most animal models (Freund et al., 1950; Lipton and Freund, 1953). In contrast, neonatal animals are usually injected with protein antigens emulsified in incomplete Freund’s adjuvant (IFA, mineral oil without mycobacteria) (Clayton et al., 1989; Forsthuber et al., 1996; Gammon et al., 1986). IFA, in contrast to CFA, is a potent Th2 immunity inducing adjuvant (Yip et al., 1999), which could contribute to the Th2 bias observed in neonates. Consistent with this view, injection of protein antigens in CFA into neonates can induceTh1 immunity (Adkins et al., 2000; Forsthuber et al., 1996).
Importantly, it has remained unresolved what the consequences are of neonatal Th1 immunity to self antigens. Obviously, neonatal induction of autoreactive Th1 cells could result in autoimmune pathology. However, neonatally-induced autoreactive Th1 cells could also be beneficial and aid in tissue repair after injury, as has been suggested for autoreactive T cells in healthy individuals (Hofstetter et al., 2003; Moalem et al., 1999). Finally, the neonatally induced Th1 cells could be pathogenic, but be kept under regulatory control. Such regulatory mechanisms may eventually fail in some individuals, leading to overt autoimmune disease. Since autoreactive T cells are frequently found in healthy individuals (Weissert et al., 2002), it is critical to understand when and how these cells are generated, and what is their function. In the present study, we show that neonatal induction of proinflammatory autoreactive T cells does not lead to autoimmune disease. Furthermore, we show that neonatally induced Th1 immunity can protect from EAE induced as adults. The data show that the tissue site where neonatal T cells encounter self antigen is critical for the protection from autoimmune disease, and for the pathogenic potential of the induced T cells. Moreover, the data suggest that protection from EAE correlates with a decrease in the number and avidity of myelin-reactive Th17 cells.
2. Materials and methods
2.1. Animals, antigens, and treatments
Mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained at CWRU under specific pathogen-free conditions. All animal procedures were conducted according to guidelines of the Institutional Animal Care and Use Committee (IACUC) of CWRU. SJL/J mice were injected as neonates (less than 48 hours old) or as adults (6–10 wk old) with the antigen in CFA. For the induction of EAE, pertussis toxin (PT, 200 ng, List Biological Laboratories, Campbell, CA) was injected i.p., in 500 μl saline, at 0 and 24 hours. PLP139-151 and MBPAc1-11 peptides were synthesized by Princeton Biomolecules (Langhorn, PA). Guinea pig myelin basic protein (gpMBP) was prepared as described (Rauch et al., 1968). IFA was purchased from Gibco BRL, Grand Island, NY, and CFA was made by mixing M. tuberculosis H37RA (Difco Laboratories, Detroit, MI) at 5 mg/ml into IFA. Antigens were mixed with the adjuvant to yield a 2 mg/ml emulsion, of which 50 μl was injected either i.p. or s.c. as specified.
2.2 Adoptive transfer of PLP139-151-specific T cells and evaluation of clinical disease
For adoptive transfer experiments, splenic mononuclear cells from mice immunized as indicated in the legend were prepared as described previously (Hofstetter et al., 2002). The cells were subsequently preactivated by incubation with PLP139-151 peptide at the previously established optimal stimulatory concentration (20 μg/ml) in complete DMEM for three days before ip. injection into the recipient animals. The recipient mice were monitored daily after injection, and clinical disease was reported according to a standard scale (Hofstetter et al., 2002). Neonatal mice were observed for clinical signs of EAE during the first week, and scored for clinical disease as outlined above thereafter. For the experiments shown in Fig. 4, the numbers of injected cells were standardized by measuring the frequencies of PLP139-151-specific cytokine producing T cells by ELISPOT prior to transfer, and adjusting the number of injected T cells according to the number of IFN-γ-producing T cells and the total number of cells recovered as previously described (Hofstetter et al., 2002).
Figure 4.
Subcutaneously induced neonatal Th1 cells are encephalitogenic. Groups of neonatal SJL mice (n=6 – 10 mice) were injected i.p. or s.c. with PLP 139-151:CFA (open versus filled symbols). Two months later spleen cells from both groups of mice were recovered and restimulated in vitro with PLP peptide. Subsequently 1 - 3 × 107 cells were adoptively transferred into naive adult recipient SJL mice as described in Materials and Methods. Shown are the means + SE of the clinical EAE scores for the recipient mice of cells from i.p. injected neonates (open circles, n=3 mice) or s.c. injected neonates (filled circles, n=7 mice) in one of two experiments with similar results.
2.3. Isolation of mononuclear cells from the CNS
After sacrificing the mice, brains were harvested, placed into DMEM, and disrupted with the back of a syringe and passed through a 45 μm cell strainer (BD Pharmingen, San Jose, CA). Cell suspensions from 3 – 4 animals were pooled, centrifuged at 1200 rpm for 10 min, resuspended in DMEM, and then layered on top of a 70%:37% Percoll (Sigma) gradient. After centrifugation at 3000 rpm for 20 min, the mononuclear cells were collected from the 70%:37% interface, washed three times with DMEM and resuspended in HL-1 medium. The cells were typically plated at 1 × 104 to 1 × 105 cells/well, together with 1 × 105 irradiated splenocytes.
2.4. Cytokine measurements by ELISPOT, computer-assisted ELISPOT image analysis
Cytokine ELISPOT assays were performed as previously described (Shive et al., 2000). ELISPOT plates (Multiscreen IP, Millipore) were coated overnight with specific cytokine capture antibody (IFN-γ, AN-18, 2 μg/ml; IL-5, TRFK5, 4μg/ml; all eBioscience; IL-17, TC11-18H10, 2 μg/ml; BD Pharmingen) diluted in 1× PBS. The plates were blocked with 1% BSA in PBS, for 1 h at room temperature, then washed 4× with PBS. Spleen cells were plated at 1 × 106 cells/well alone or in combination with antigen (7 μM) in serum free HL-1 medium supplemented with 1% L-glutamine and cultured for 24 h. Subsequently, the cells were removed by washing 4× with PBS and 4× with PBS/Tween, and the respective biotinylated detection antibody (IFN-γ, R4-6A2, 2 μg/ml; 2 μg/ml; IL-5, TRFK4; all eBioscience; IL-17, TC11-8H4, 0.125 μg/ml; BD Pharmingen) was added and incubated overnight. The plate-bound secondary antibody was visualized by adding streptavidin-alkaline phosphatase (SAV-AP, Dako, Carpenteria, CA) and NBT/BCIP substrate (Biorad, Hercules, CA/ Sigma, St. Louis, MO). Image analysis of ELISPOT assays was performed on a Series 2 ImmunoSpot™ Image Analyzer (Cellular Technology, Cleveland, OH) as previously described (Karulin et al., 2000; Shive et al., 2000). In brief, digitized images of individual wells of the ELISPOT plates were analyzed for cytokine spots based on the comparison of experimental wells (containing T cells and APC with antigen) and control wells (T cells and APC, no antigen). After separation of spots that touched or partially overlapped, non-specific noise was gated out by applying spot size and circularity analysis as additional criteria. Spots that fell within the accepted criteria were highlighted and counted. The spot number in unimmunized or control mice (irrelevant antigen) was in the same range as the medium control (usually < 5 spots). Statistical analysis was performed with the paired t-test or the Mann-Whitney rank sum test using SigmaStat® software.
2.5. Histopathology
At the time of the experiment, the brain and spinal cord of the mice were removed and either preserved in Z-Fix or snap-frozen in 2-methyl-butane. Thin slices of the CNS tissue were prepared and stained with H&E or monoclonal antibodies to the indicated surface markers. The tissue was then examined by light microscopy or immunofluorescence microscopy in a blinded fashion by a pathologist and evaluated for the extent of inflammation as previously described (Rocken et al., 1996).
3. Results
3.1. Induction of myelin-specific Th1 immunity in neonatal mice
Injection of adult mice with self antigens such as PLP and MBP emulsified in CFA induces vigorous Th1 immunity. However, it has remained unresolved whether induction of Th1 immunity to self antigens is similarly efficient in neonatal mice.
To begin to address this issue, we injected neonatal SJL and B10.PL mice 24 – 48 hours after birth intraperitoneally with PLP139-151 peptide, MBP protein, or MBPAc1-11 peptide emulsified in CFA. At 6 - 8 weeks of age, the mice were sacrificed and single cell suspensions of spleens tested by cytokine ELISPOT assay for Th1 and Th2 cytokines. As shown in Fig. 1, the injection of neonatal SJL mice with PLP139-151 in CFA induced vigorous antigen-specific production of IFN-γ (and IL-2, not shown), but only negligible amounts of IL-5, consistent with a Th1 type cytokine profile (Fig. 1, left panel). Similar results were obtained in B10.PL mice injected as neonates with MBP in CFA (Fig. 1, right panel) or with MBPAc1-11 (not shown). The magnitude of the neonatally induced, myelin antigen-specific Th1 response was somewhat lower as compared with mice immunized as adults (Fig. 1, center panel). However, this could be due to the fact that the neonatally injected mice were tested 6 – 8 weeks after injection of antigen for recall responses, whereas adult mice were tested 2 – 3 weeks after injection.
Figure 1.
Neonatal induction of Th1 immunity to neuroantigens. Newborn SJL or B10.PL mice (< 48 hours old) were injected i.p. with PLP139-151 or MBP Ac1-11 peptides emulsified in CFA, respectively. Adult 6 – 8 week old SJL mice were injected s.c. with PLP139-151 in CFA Antigen-induced recall responses were tested by cytokine ELISPOT assay in single cell suspensions of splenocytes after 6 weeks (neonatal injection) or 2 – 3 weeks (adult injection), respectively, in the presence of 25 μg/ml of the indicated antigen. Symbols represent the mean number of cytokine spots in triplicate wells for individual animals with the background subtracted. Bars represent the mean number of cytokine spots averaged for all mice tested.
Collectively, the results show that injection of neonatal mice with myelin antigens in CFA induced antigen-specific Th1 immunity, independent of the strain background or myelin antigen.
3.2. Neonatally induced myelin-specific Th1 immunity does not result in clinical EAE
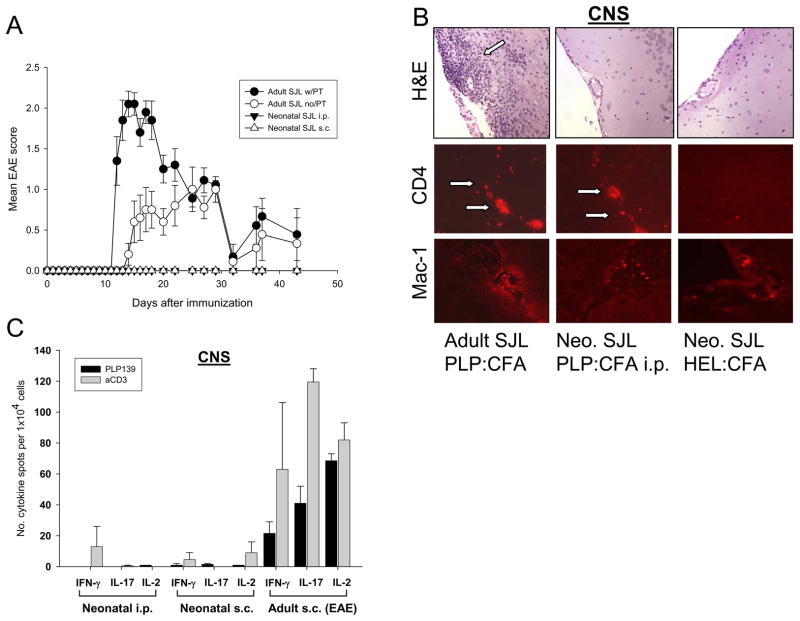
To determine the consequences of neonatally induced Th1 immunity for autoimmune disease, neonatal SJL or B10.PL mice were injected i.p. with PLP139-151 in CFA and observed for up to 6 months for clinical symptoms of EAE. As control, adult SJL mice were injected with PLP139-151 in CFA with or without pertussis toxin (PT). As expected, adult mice injected with PLP139-151:CFA and PT developed clinical EAE with an average disease onset on day 11, and disease peak around day 14 (Fig. 2A, closed circles). Adult SJL mice injected without PT also developed EAE, but with somewhat delayed onset (day 13), and the average disease score was lower as compared with PT injected animals (Fig.2A, open circles). Importantly, SJL mice injected i.p. as neonates with PLP139-151:CFA showed no clinical signs of EAE over the entire observation period (Fig. 2A, closed triangles). Similarly, B10.PL mice injected i.p. as neonates with gpMBP protein or MBPAc1-11 in CFA did not develop EAE (not shown).
Figure 2.
Neonatally induced Th1 autoimmunity does not result in clinical EAE. (A) EAE scores of SJL mice injected with PLP139-151 in CFA with or without pertussis toxin as adults (filled or open circles) or neonates (triangles). Shown are the means and SD of 6 – 10 mice per group. (B) H&E staining and immunofluorescence staining (for CD4, arrows, and Mac-1) of brain sections obtained from representative SJL mice injected as adults or neonates with PLP139-151:CFA or HEL:CFA. (C) PLP139-151 or anti-CD3 antibody-induced cytokine production by mononuclear cells isolated from the CNS of SJL mice injected as adults or neonates with PLP139-151:CFA and tested 2 – 3 weeks (adult injected) or 6 weeks (neonatal injection) later by cytokine ELISPOT assay in the presence of 20 ug/ml PLP139-151 or 1 ug/ml anti-CD3 antibody. Shown are means + SD of pooled cells from 2 – 3 mice per group repeated twice with similar results.
Finally, following the protocol for the injection of EAE in adult mice, neonatal injection of PLP139-151:CFA subcutaneously (s.c.) also did not result in clinical EAE over the entire observation period (Fig. 2A, open triangles).
As expected, histologic examination by H&E of the brains of SJL mice injected as adults with PLP139-151:CFA showed extensive perivascular and periventricular inflammatory infiltrates, corresponding with the severity of the clinical disease (Fig. 2B, left panels). The inflammatory infiltrates consisted predominantly of CD4+ T cells and Mac-1+ microglia (Fig. 2B, left lower panels). In strong contrast, no obvious inflammatory infiltrates were found in SJL mice injected i.p. or s.c. as neonates with PLP139-151:CFA, consistent with the finding that these mice did not develop clinical signs of EAE (Fig. 2B, middle and right upper panels) . Interestingly, however, by immunofluorescence staining we consistently detected a low but reproducible number of CD4+ T cells dispersed within the perivascular spaces and CNS parenchyma of SJL mice injected as neonates with PLP139-151:CFA, but not with the control antigen HEL (Fig. 2B, lower middle and right panels). These CD4+ cells were negative for CD25 staining (data not shown), suggesting that they were not classical regulatory T cells.
To begin to investigate the function of these T cells, we recovered mononuclear cells from the CNS of the mice injected with PLP139-151:CFA as neonates or adults and tested their proinflammatory cytokine production by cytokine ELISPOT recall assay at 4 - 6 weeks or 2 – 3 weeks after injection, respectively.
The results show that T cells isolated from the CNS of mice injected as adults which developed EAE, mounted vigorous PLP139-151-specific IFN-γ, IL-2, and IL-17 cytokine responses (Fig. 2C, right group of bars). In strong contrast, T cells isolated from the CNS of neonatally injected mice did not produce IFN-γ or IL-17 upon recall with PLP139-151, and the response to mitogen also was substantially decreased (Fig. 2C, left and middle group of bars). Since we also did not detect significant production of IL-2 by these T cells, it is conceivable that these cells were anergic (Jenkins and Schwartz, 1987). The paucity of T cells in the CNS of neonatally injected mice precluded testing for a shift towards Th2 type cytokine production, i.e. Th2 immune deviation. Nevertheless, since we did not detect substantial Th2 type cytokine production in the periphery in these animals, it seems unlikely that Th2 type immune deviation occurred in this model. Finally, the lack of proinflammatory cytokine production by CNS T cells in neonatally injected mice was independent of whether the animals were injected s.c. or i.p (Fig. 2C, left and middle bars).
Overall, the results clearly show that neonatally induced myelin-specific Th1 immunity did not result in clinical EAE.
3.3. Intraperitoneal, but not subcutaneous neonatal induction of neuroantigen-specific Th1 immunity protects from EAE
Neuroantigen-specific Th1 cells were present in high frequencies in neonatally injected mice, yet the animals did not develop EAE. To begin to address the mechanism of why the animals did not develop disease, we asked whether these T cells were immunologically "ignorant", as has previously been reported in other autoimmune disease models (Ohashi et al., 1991), or if T cell regulation took place.
In models of immunologic ignorance, autoreactive T cells can be induced to mediate autoimmune disease upon reactivation by antigen (Ohashi et al., 1991). To test this question in our model, mice injected as neonates i.p. or. s.c. with PLP139-151:CFA or MBP1-11:CFA were re-immunized as adults with myelin antigens as outlined in Methods. For a control, mice injected as neonates i.p. with the non-self antigen hen eggwhite lysozyme (HEL) in CFA (HEL:CFA) were re-immunized as adults with the respective neuroantigen.
As expected, the control mice (neonatally injected i.p. with HEL:CFA) developed EAE upon immunization with PLP139-151 or MBPAc1-11 in CFA and PT (Fig. 3A & B, open symbols). Importantly, mice injected i.p. as neonates with PLP139-151:CFA or MBPAc1-11:CFA were protected from disease and did not develop EAE upon re-injection with the respective myelin antigens (Fig. 3A & B, closed circles). In contrast, mice injected as neonates s.c. were not protected (Fig. 3B, closed triangles). In fact, B10.PL mice injected s.c. with MBPAc1-11:CFA as neonates showed accelerated disease onset and enhanced disease severity as compared with HEL:CFA injected control mice (Fig. 3B, closed triangles). Of note, we found that adult mice injected with PLP139-151:CFA also did not develop EAE, and were protected from the disease upon re-injection with PLP139-151:CFA (data not shown). In contrast, HEL:CFA i.p. injected adult mice were not protected and developed severe EAE upon re-injection with PLP139-151:CFA.
Figure 3.
Neonatal Th1 immunity to neuroantigens protects from EAE. (A) Neonatal SJL mice were injected i.p. with PLP 139-151:CFA (closed symbols, n=11 mice) or HEL:CFA (open symbols, n=5 mice). Two months later, the animals were re-injected s.c. as adults with PLP 139-151:CFA and PT. Shown is the mean + SD of the clinical EAE scores for both groups in one of three experiments with similar results. (B) Groups of neonatal B10.PL mice (n= 8 – 10 mice/group) were injected either i.p. or s.c. with MBP Ac1-11:CFA i.p. (filled circles versus filled triangles), or with HEL:CFA i.p. (open circles). Two months later, the animals were reinjected s.c. with MBP Ac1-11:CFA s.c. and PT. Shown is the mean + SE of the clinical EAE scores for both groups in one of two experiments with similar results.
To directly address whether neonatally s.c. induced Th1 cells were encephalitogenic we asked whether these cells could adoptively transfer EAE to naive recipients. Splenic T cells were recovered from mice injected s.c. or i.p. as neonates with PLP139-151:CFA, or, as a control, from adult mice injected s.c. with PLP139-151:CFA, and restimulated in vitro with PLP peptide. After three days of culture, the cells were adoptively transferred into naive adult recipient mice and the animals were observed for EAE.
As expected, adoptive transfer of T cells from control mice primed as adults with PLP139-151 s.c. resulted in EAE (data not shown). Importantly, adoptive transfer of spleen T cells from neonatally s.c. injected mice also induced EAE (Fig. 4, filled symbols), thereby clearly establishing that these cells were encephalitogenic. However, the disease was less severe than EAE induced by adoptive transfer of T cells derived from mice immunized as adults (data not shown). In contrast, T cells derived from PLP139-151:CFA i.p. injected neonates did not transfer EAE (Fig. 4, open symbols).
Collectively, the data show that neonatally s.c. induced Th1 T cells were encephalitogenic. The results suggested that the lack of autoimmune pathology upon neonatal injection of autoantigen s.c. was due to immunologic ignorance, which could be broken after re-injection with autoantigen as adults. In contrast, the data indicated that neonatal i.p. injection resulted in non-encephalitogenic T cells, and that protection from autoimmune disease in this system was due to a different mechanism.
3.4. Neonatally-induced Th1 autoimmunity protects from EAE via passive tolerance
Next, we asked whether neonatally-induced Th1 autoimmunity protected from EAE via an active tolerance mechanism, such as regulatory cells, or whether the protection was a passive process, based, for example, on anergy or clonal deletion.
To begin to address this issue we first determined whether spleen cells from mice injected as neonates i.p. with myelin antigen could confer protection against EAE in naive adult recipient mice. Neonatal SJL mice were injected i.p. or s.c. with PLP139-151:CFA, and at 6 weeks of age, spleen cells from both groups of animals were recovered and adoptively transferred into naive adult recipient SJL mice. The spleen cells were not restimulated in vitro in this model since we reasoned that regulatory cells should have been reactivated by myelin antigen released by the adjuvant in the donor mice. One day after adoptive transfer, the recipient mice were immunized with PLP139-151:CFA and PT and observed for EAE.
The data show that the recipients of spleen cells from either i.p. or s.c. injected neonates developed EAE and were not protected from disease (Fig. 5A, open and closed symbols). Furthermore, EAE onset was slightly accelerated in recipients of cells from neonatally s.c. injected mice (Fig. 5A, open symbols), suggesting that neonatally s.c. induced T cells may have contributed to the disease.
Figure 5.
EAE protection mediated by neonatally induced Th1 immunity cannot be transferred (A) Adult SJL mice were adoptively transferred with splenocytes from 6 week old SJL mice injected either s.c. or i.p. as neonates with PLP139-151 in CFA. Subsequently, active EAE was induced by immunization with PLP139-151:CFA and PT at 0 and 24 hours as outlined in Materials and Methods. Shown is the mean + SE of the clinical EAE scores for the recipient mice of cells from i.p. injected neonates (closed circles, n=8 mice) or s.c. injected neonates (open circles, n=9 mice) in one of two experiments with similar results. (B) Neonatal SJL mice were injected either s.c. or i.p. with PLP139-151:CFA as outlined above. At 6 - 8 weeks of age, the animals were adoptively transferred with 3 × 107 spleen cells from PLP139-151:CFA primed adult SJL mice stimulated in vitro for 3 days in the presence of 20 μg/ml peptide. Shown is one of two experiments with similar results.
To further investigate the role of T cell regulation, we asked whether neonatal mice injected with myelin antigen i.p. or s.c. were protected against EAE induced by adoptive transfer of encephalitogenic T cells.
Neonatal mice were injected i.p. or s.c. with PLP139-151:CFA, and at 6 - 8 weeks of age, short-term PLP139-151-reactive T cell lines generated from adult SJL mice were adoptively transferred into these animals or age matched naive adult control mice.
As expected, naive adult recipient mice developed EAE upon adoptive transfer of 3 × 107 PLP139-151-specific T cells, starting on day 6 after adoptive transfer (Fig. 5B, closed circles). However, neonatally PLP139-151:CFA-injected recipient mice also developed EAE upon adoptive transfer of PLP139-151-specific T cells, irrespective of whether the mice were injected s.c. or i.p. as neonates (Fig. 5B, open circles and filled triangles). Disease onset and severity was similar in both groups, and comparable with EAE in the control group.
Taken together, the results suggested that neonatally induced Th1 immunity did not protect from EAE via the induction of regulatory cells, or was not sufficient to prevent EAE in adoptive transfer models in our studies.
3.5. Decreased frequencies of neuroantigen-specific Th1/Th17 cells in mice injected as neonates intraperitoneally
A number of studies recently showed the importance of IL-17 for the induction of EAE. Therefore, we asked whether the difference in encephalitogenicity and protection from EAE between neonatally s.c. versus i.p. injected mice was reflected by an altered production of IL-17. Neonatal SJL mice were injected s.c. or i.p. with PLP139-151:CFA, and splenic T cells were recovered at 6 weeks of age and tested by cytokine ELISPOT assay for peptide-induced production of the proinflammatory cytokines IL-17 and IFN-γ, and the Th2 cytokine IL-5. The results show that T cells recovered from mice injected s.c. with PLP139-151:CFA as neonates vigorously produced antigen-specific IFN-γ and IL-17, but little IL-5 (Fig. 6A, closed symbols), consistent with their encephalitogenic potential and ability to induce EAE upon adoptive transfer (Fig. 4). The frequencies of IFN-γ and IL-5 producing T cells were similar to those observed in mice injected as adults, whereas the numbers of IL-17 producing cells were about one third of those observed upon injection of adults (Fig. 6A). In contrast, neonatal i.p. injection induced considerably fewer IL-17 and IFN-γ producing T cells as compared with adult and neonatally s.c. injected animals (Fig. 6A, open symbols), consistent with the observation that these cells were not encephalitogenic. Similar to our earlier results, relatively few myelin-reactive T cells producing IL-5 were detected, and the frequencies were similarly low between neonatally s.c. and i.p. injected mice. Furthermore, we found that the functional avidity (activation threshold) (Targoni and Lehmann, 1998) of IL-17 producing T cells was increased in mice injected i.p. as neonates, as compared with mice injected s.c. as neonates or adults (Fig. 6B). The decreased frequencies and increased avidity of neuroantigen-specific T cells in neonatally i.p. injected mice could explain the inability of these cells to mediated EAE by adoptive transfer (Fig. 4). In contrast, vigorous production of IL-17 and IFN-γ by neonatally s.c. induced T cells is consistent with the potential of these cells to induce EAE upon adoptive transfer. While the data were not corrected for CD4+ T cells, we have not observed significant differences in T cell percentage between neonatally and adult injected mice (data not shown). We found that peritoneal macrophages produced less IL-12 and IL-23 as compared with dendritic cells (data not shown), which could account for the decreased production of IL-17 and IFN-γ by i.p. injected neonates.
Figure 6.
Decreased frequencies and increased activation threshold of IL-17 producing T cells in mice injected i.p. as neonates. SJL mice were injected as neonates s.c. or i.p. with PLP139-151:CFA, or as adults with PLP139-151:CFA, respectively. Frequencies of antigen-induced cytokine-producing T cells were measured 6 - 8 weeks later in neonatally injected mice, or after 3 weeks in adult injected mice, respectively, by cytokine ELISPOT assay, as outlined in Materials and Methods, in single-cell suspensions of spleen cells. Symbols represent the mean number of cytokine spots in triplicate wells per million cells with the background subtracted for individual animals. Bars represent the mean number of cytokine spots averaged for all mice tested. (A) Frequencies of PLP139-151-reactive T cells producing IL-17, IFN-γ, and IL-5 in the presence of 25 μg/ml peptide. (B) Frequencies of IL-17 producing T cells with the PLP139-151 peptide titrated as indicated.
Irrespective, the results clearly show that the site of neonatal antigen encounter is important for the encephalitogenic potential of the induced autoimmune T cells. Furthermore, the data suggest that intraperitoneal induction of neonatal Th1 immunity protected from EAE via shaping of the neuroantigen-specific precursor pool towards a decreased frequency of neuroantigen-reactive Th17 cells with lower avidity.
4. Discussion
Myelin-reactive T cells can be found in healthy individuals without evidence for autoimmune disease (Pette et al., 1990; Sospedra and Martin, 2005; Wucherpfennig et al., 1994). Frequently, the autoreactive T cells show an effector/memory phenotype and express proinflammatory T cell cytokines (Weissert et al., 2002). In the present study, we provide a rational for this observation by showing that the encounter of self-antigens during the neonatal period can result in the priming and clonal expansion of autoreactive T cells with a proinflammatory cytokine profile in the absence of autoimmune pathology. The neonatally induced autoreactive T cells produced high levels of IFN-γ, IL-17, and IL-2, but not IL-5, consistent with a Th1/Th17 cytokine profile, and were not Th2 immune deviated (Mosmann et al., 1986). Importantly, the results show that neonatal induction of myelin-specific Th1 immunity can confer passive protection against EAE in adulthood. The data show that the site where neonatal T cells encounter self antigen is a critical factor. Intraperitoneal injection of neuroantigens in CFA protected from EAE and generated non-pathogenic Th1 cells, whereas subcutaneous injection was not protective and induced T cells that could mediated EAE upon adoptive transfer.
Interestingly, we observed that i.p. injection of adult mice with PLP139-151:CFA also did not induce EAE, and protected the animals against subsequent induction of EAE upon injection with PLP139-151:CFA via the s.c. route (data not shown). In contrast, HEL:CFA i.p. injected adult mice were not protected and developed severe EAE upon re-injection with PLP139-151:CFA. While it is conceivable that similar mechanisms of protection are operational in neonates and adult mice, we have not tested the underlying mechanism.
Neonatally i.p. and s.c. induced T cells showed similar overall cytokine profiles. Moreover, both i.p. and s.c. induced T cells appeared to migrate to the CNS (Fig. 2B). However, production of IL-17 and IFN-γ by i.p. induced T cells was considerably lower when compared with s.c. induced T cells (Fig. 6). The finding that IL-17 production by i.p. induced T cells was decreased is of particular interest, because IL-17 has recently been implicated as a signature cytokine for encephalitogenic T cells in mice (Cua et al., 2003; Langrish et al., 2005; Park et al., 2005). Specifically, IL-23 knockout mice are resistant to EAE despite normal priming of myelin-specific naive T cells in peripheral lymphatic tissues (Cua et al., 2003). Furthermore, intracerebral, but not systemic reconstitution of IL-23, with a retroviral vector, restored EAE. Importantly, the potential of T cells to induce EAE is correlated with the production of IL-17 (Chen et al., 2006; Harrington et al., 2005; Langrish et al., 2005; Park et al., 2005). Finally, RNA message for IL-17 is prominently detected in CNS lesions in human MS patients (Lock et al., 2002), corroborating an important role for this cytokine in MS and EAE.
Our results extend these observations and show that i.p. injection of neonatal mice with myelin antigens resulted in the priming of T cells in vivo that produced IFN-γ, and relatively low levels of IL-17, and did not mediate EAE. The primed myelin-specific T cells persisted until adulthood, and the mice were protected from induction of EAE upon re-immunization.
Interestingly, while IFN-γ has been viewed in the past as a major cytokine that promotes MS and EAE, it has recently been implicated in protection from the disease, possibly by regulating the expansion and survival of encephalitogenic T cells (Chu et al., 2000; Ferber et al., 1996; Willenborg et al., 1996). Moreover, IFN-γ antagonizes the differentiation of Th17 cells (Bettelli et al., 2006; Harrington et al., 2005; Park et al., 2005). Thus, it is conceivable that neonatally-induced IFN-γ producing Th1 cells participated in the counter-regulation of pathogenic Th17 cells.
We did not find direct evidence for active tolerance mechanisms involved in this protection, such as regulatory T cells. Adoptive transfer of spleen cells from neonatally injected mice failed to protect naive recipients from induction of EAE, and disease could be induced in the neonatally treated mice by transfer of encephalitogenic T cells. Therefore, we propose that neonatal i.p. injection of myelin antigens in CFA resulted in the differentiation of autoreactive T cell precursors towards cells with a non-pathogenic cytokine profile, typified by lower frequencies and higher activation threshold of IL-17 producing T cells. Upon re-immunization as adults with myelin antigens, these cells were less likely to induce EAE. However, the mice remained susceptible to induction of passive EAE by adoptive transfer of encephalitogenic T cells, consistent with a lack of protection mediated by regulatory T cells.
We are currently investigating why i.p. injection of myelin antigen in CFA was inefficient at inducing IL-17 differentiation in T cells. We found that in adult mice, peritoneal macrophages secreted very low levels of IL-12 and IL-23 upon LPS stimulation, as compared with bone marrow-derived dendritic cells (data not shown), and confirming similar observations by others (Goriely et al., 2001). In contrast, subcutaneous neonatal injection of myelin antigens resulted in high frequencies of encephalitogenic T cells producing IL-17, which could be due to the substantially higher number of DCs residing in the skin. An important related question is why mice injected s.c. as neonates did not develop EAE despite harboring a large population of encephalitogenic T cells. The encephalitogenic potential of neonatally s.c. primed T cells was evidenced by their ability to induce EAE upon adoptive transfer to naive adult recipients (Fig. 4). Regulatory T cells were either not involved, or, alternatively, they were overwhelmed by the subsequent induction of EAE via re-immunization or adoptive transfer of encephalitogenic T cells.
Thus, we propose that the neonatally s.c. primed T cells remained immunologically ignorant until they were reactivated by re-immunization or in vitro stimulation, similar to the findings reported by Ohashi and colleagues in a model of murine autoimmune diabetes (Ohashi et al., 1991). It is likely that the lack of myelination in the CNS of neonates during the first two weeks after birth plays a role underlying the mechanism of immunologic ignorance in this model. Conceivably, neonatally-induced Th1/Th17 cells migrate to peripheral tissues upon priming, including the CNS. In the absence of myelin in the CNS of neonates, these T cells are not activated and return to the immune periphery without causing pathology. There, the T cells continue to persist until adulthood. Consistent with this view, EAE onset was accelerated and disease severity enhanced when mice injected s.c. with neuroantigens as neonates were re-injected with neuroantigen as adults (Fig. 3).
The data show that priming of proinflammatory autoreactive T cells during the neonatal period does not result in autoimmune pathology, and, under certain conditions, can confer protection against induction of autoimmune disease during adulthood. This observation may have important implications for human autoimmune diseases, and the significance of the presence of autoreactive T cells in healthy humans may have to be re-evaluated. First, autoreactive Th1 cells may be beneficial and could participate in CNS tissue repair after injury. This view is consistent with reports that autoreactive T cells can promote tissue repair after CNS and spinal cord injury (Hofstetter et al., 2003; Moalem et al., 1999). Secondly, the differentiation of autoreactive precursor T cells towards a non-pathogenic cytokine profile may be a feasible strategy to prevent autoimmune disease in individuals with a known genetic bias for development of autoimmune diseases (Aaltonen et al., 1993; Sospedra and Martin, 2005). To gain a better understanding of the role of autoreactive T cells in humans, it will be of interest to determine whether these cells produce IL-17 in healthy individuals as compared with T cells in MS or rheumatoid arthritis patients. Moreover, production of IL-17 by myelin-reactive T cells may be relevant as a prognostic clinical marker. Along these lines, it will be important to learn whether IL-17 contributes directly to disease pathology, or whether it is a surrogate marker for pathogenic T cells. For example, IL-17 could promote autoimmune pathology by collaborating with TNF-α to induce IL-6 (Ruddy et al., 2004), a cytokine that has been shown to be critical in the pathogenesis of EAE (Eugster et al., 1998).
Recently, evidence has emerged supporting an important role for myelin-reactive CD8 T cells in EAE and MS (Ford and Evavold, 2005; Huseby et al., 2001; Sun et al., 2001). While we have not investigated the role of myelin-reactive CD8 T cells in neonatal Th1 immunity, this is an important question to be addressed in future studies.
Finally, our results show that T cell responses can be polarized towards a Th1 cytokine profile in neonatal mice with adjuvants, such as CFA. Therefore, while the neonatal immune system may be intrinsically biased towards anti-inflammatory Th2 responses, it is clearly capable of mounting Th1 cytokine responses if the adequate signals are provided, such as Toll-like receptor signaling. Since the induction of neonatal Th1 immunity is apparently not detrimental for the host, even if directed towards self antigens, neonatal induction of Th1 immunity may therefore be a feasible vaccination strategy against infectious microorganisms that require Th1 cytokines (e.g. IFN-γ) for clearance, such as Mycobacterium tuberculosis. While CFA itself is not a suitable vaccine adjuvant for humans, alternative Th1 adjuvants, such as CpGs, are being developed (Chu et al., 1997) that may find application for the immunization of human neonates. An alternative approach could be the administration of myelin antigens via the oral route into neonates. Oral administration of myelin antigens has been shown to be highly efficacious in preventing EAE in adult mice (Bitar and Whitacre, 1988). Interestingly, oral administration of myelin antigens into neonates has been shown in some models to accelerate and substantially exacerbate EAE severity (Melo et al., 2004). The underlying mechanisms have not been fully elucidated, but one consideration is that the mucosal application of myelin antigen may result in enhanced B cell priming and antibody formation, which may contribute during adulthood to disease exacerbation.
In conclusion, our data show that neonatal induction of autoreactive Th1/Th17 cells does not result in autoimmune pathology. Furthermore, the results show that the age and tissue site at which T cells encounter autoantigen are critical factors for the induction of pathogenic T cells or protection from autoimmune disease.
Acknowledgments
We thank Dr. Neal Guentzel for critically reading the manuscript. This work was supported by grants of the National Institutes of Health (NIH AI-41609) and the National Multiple Sclerosis Society (Harry Weaver Neuroscience Scholarship JF-2092-A-1) to TGF, and a fellowship of the Studienstiftung des Deutschen Volkes to H.H.H.
Footnotes
Abbreviations: Th1, T helper type 1; Th2, T helper type 2; EAE, experimental autoimmune encephalomyelitis; CFA, complete Freund’s adjuvant; APC, antigen presenting cell;
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaltonen J, Komulainen J, Vikman A, Palotie A, Wadelius C, Perheentupa J, Peltonen L. Autoimmune polyglandular disease type I. Exclusion map using amplifiable multiallelic markers in a microtiter well format. Eur J Hum Genet. 1993;1:164–171. [PubMed] [Google Scholar]
- Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–2353. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- Adkins B, Du RQ. Newborn mice develop balanced Th1/Th2 primary effector responses in vivo but are biased to Th2 secondary responses. J Immunol. 1998;160:4217–4224. [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Billingham RE, Brent L, Medawar PB. Activity acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bitar DM, Whitacre CC. Suppression of experimental autoimmune encephalomyelitis by the oral administration of myelin basic protein. Cell Immunol. 1988;112:364–370. doi: 10.1016/0008-8749(88)90305-x. [DOI] [PubMed] [Google Scholar]
- Burnet FM, Fenner F. The Production of Antibodies. Melbourne: Macmillan; 1949. pp. 102–105. [Google Scholar]
- Chen N, Field EH. Enhanced type 2 and diminished type 1 cytokines in neonatal tolerance. Transplantation. 1995;59:933–941. doi: 10.1097/00007890-199504150-00002. [DOI] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JP, Gammon GM, Ando DG, Kono DH, Hood L, Sercarz EE. Peptide-specific prevention of experimental allergic encephalomyelitis. Neonatal tolerance induced to the dominant T cell determinant of myelin basic protein. J Exp Med. 1989;169:1681–1691. doi: 10.1084/jem.169.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Eugster HP, Frei K, Kopf M, Lassmann H, Fontana A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur J Immunol. 1998;28:2178–2187. doi: 10.1002/(SICI)1521-4141(199807)28:07<2178::AID-IMMU2178>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Fadel SA, Ozaki DA, Sarzotti M. Enhanced type 1 immunity after secondary viral challenge in mice primed as neonates. J Immunol. 2002;169:3293–3300. doi: 10.4049/jimmunol.169.6.3293. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Ford ML, Evavold BD. Specificity, magnitude, and kinetics of MOG-specific CD8+ T cell responses during experimental autoimmune encephalomyelitis. Eur J Immunol. 2005;35:76–85. doi: 10.1002/eji.200425660. [DOI] [PubMed] [Google Scholar]
- Forsthuber T, Yip HC, Lehmann PV. Induction of TH1 and TH2 immunity in neonatal mice [see comments] Science. 1996;271:1728–1730. doi: 10.1126/science.271.5256.1728. [DOI] [PubMed] [Google Scholar]
- Freund J. The effect of paraffin oil and mycobacteria on antibody formation and sensitization; a review. Am J Clin Pathol. 1951;21:645–656. doi: 10.1093/ajcp/21.7.645. [DOI] [PubMed] [Google Scholar]
- Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Proc Soc Exp Biol Med. 1937;37:509–513. [Google Scholar]
- Freund J, Lipton MM, Morrison LR. Demyelination in the guinea pig in chronic allergic encephalomyelitis produced by injecting guinea pig brain in oil emulsion containing a variant of mycobacterium butyricum. Arch Pathol (Chic ) 1950;50:108–121. [PubMed] [Google Scholar]
- Gammon G, Dunn K, Shastri N, Oki A, Wilbur S, Sercarz EE. Neonatal T-cell tolerance to minimal immunogenic peptides is caused by clonal inactivation. Nature. 1986;319:413. doi: 10.1038/319413a0. [DOI] [PubMed] [Google Scholar]
- Garza KM, Griggs ND, Tung KS. Neonatal injection of an ovarian peptide induces autoimmune ovarian disease in female mice: requirement of endogenous neonatal ovaries. Immunity. 1997;6:89–96. doi: 10.1016/s1074-7613(00)80245-9. [DOI] [PubMed] [Google Scholar]
- Goriely S, Vincart B, Stordeur P, Vekemans J, Willems F, Goldman M, De Wit D. Deficient IL–12(p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–2146. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann PV, Fabry Z. Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol. 2003;134:25–34. doi: 10.1016/s0165-5728(02)00358-2. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Shive CL, Forsthuber TG. Pertussis Toxin Modulates the Immune Response to Neuroantigens Injected in Incomplete Freund's Adjuvant: Induction of Th1 Cells and Experimental Autoimmune Encephalomyelitis in the Presence of High Frequencies of Th2 Cells. J Immunol. 2002;169:117–125. doi: 10.4049/jimmunol.169.1.117. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CAJ. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen- specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karulin AY, Hesse MD, Tary-Lehmann M, Lehmann PV. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J Immunol. 2000;164:1862–1872. doi: 10.4049/jimmunol.164.4.1862. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton MM, Freund J. Allergic encephalomyelitis in the rat induced by the intracutaneous injection of central nervous system tissue and adjuvants. J Immunol. 1953;71:98–109. [PubMed] [Google Scholar]
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- McDonald AH, Swanborg RH. Antigen-specific inhibition of immune interferon production by suppressor cells of autoimmune encephalomyelitis. J Immunol. 1988;140:1132–1138. [PubMed] [Google Scholar]
- Melo ME, Stevens DB, Sercarz EE, Gabaglia CR. Nasal instillation of gpMBP can exacerbate murine EAE: effect of mucosal priming is an age-dependent phenomenon. J Autoimmun. 2004;22:13–20. doi: 10.1016/j.jaut.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Moalem G, Leibowitz-Amit R, Yoles E, Mor F, Cohen IR, Schwartz M. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5:49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW. Two types of murine helper T cell clone. I Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348. [PubMed] [Google Scholar]
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson PY. Studies of immunological tolerance to nervous tissue in rats. Ann N Y Acad Sci. 1958;73:811–818. doi: 10.1111/j.1749-6632.1959.tb40860.x. [DOI] [PubMed] [Google Scholar]
- Pette M, Fujita K, Kitze B, Whitaker JN, Albert E, Kappos L, Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–1776. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- Powell TJJ, Streilein JW. Neonatal tolerance induction by class II alloantigens activates IL-4- secreting, tolerogen-responsive T cells. J Immunol. 1990;144:854–859. [PubMed] [Google Scholar]
- Rauch HC, Einstein ER, Csejtey J, Davis WJ. Protective action of the encephalitogen and other basic proteins in experimental allergic encephalomyelitis. Immunochemistry. 1968;5:567–575. doi: 10.1016/0019-2791(68)90092-x. [DOI] [PubMed] [Google Scholar]
- Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells [see comments] Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- Rocken M, Racke M, Shevach EM. IL-4 induced immune deviation as antigen-specific therapy for inflammatory autoimmune disease. Immunol Today. 1996;17:275. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- Shive CL, Hofstetter H, Arredondo L, Shaw C, Forsthuber TG. The enhanced antigen-specific production of cytokines induced by pertussis toxin is due to clonal expansion of T cells and not to altered effector functions of long-term memory cells. Eur J Immunol. 2000;30:2422–2431. doi: 10.1002/1521-4141(2000)30:8<2422::AID-IMMU2422>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Singh RR, Hahn BH, Sercarz EE. Neonatal peptide exposure can prime T cells and, upon subsequent immunization, induce their immune deviation: implications for antibody vs. T cell-mediated autoimmunity. J Exp Med. 1996;183:1613–1621. doi: 10.1084/jem.183.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of Multiple Sclerosis. Ann Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Swierkosz JE, Swanborg RH. Immunoregulation of experimental allergic encephalomyelitis: conditions for induction of suppressor cells and analysis of mechanism. J Immunol. 1977;119:1501–1506. [PubMed] [Google Scholar]
- Targoni OS, Lehmann PV. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J Exp Med. 1998;187:2055–2063. doi: 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissert R, Kuhle J, de Graaf KL, Wienhold W, Herrmann MM, Muller C, Forsthuber TG, Wiesmuller KH, Melms A. High immunogenicity of intracellular myelin oligodendrocyte glycoprotein epitopes. J Immunol. 2002;169:548–556. doi: 10.4049/jimmunol.169.1.548. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Wucherpfennig KW, Zhang J, Witek C, Matsui M, Modabber Y, Ota K, Hafler DA. Clonal expansion and persistence of human T cells specific for an immunodominant myelin basic protein peptide. J Immunol. 1994;152:5581–5592. [PubMed] [Google Scholar]
- Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, Trezza RP, Heinzel FP, Forsthuber T, Lehmann PV. Adjuvant-guided type-1 and type-2 Immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–3949. [PubMed] [Google Scholar]