Abstract
Background:
Pathogenic bacteria colonize the airways of 30% to 40% of patients with COPD and cause approximately 50% of exacerbations. New strains of nontypeable Haemophilus influenzae (NTHI) and Moraxella catarrhalis are associated with exacerbations. Antimicrobial protein/peptides (AMPs) play important roles in innate lung defense against pathogens. To our knowledge, the changes in AMP baseline levels in respiratory secretions during bacterial colonization and exacerbation have not been described. The objective of this study was to elucidate the effects of the acquisition of a new strain of pathogenic bacteria on the airway levels of AMPs in patients with COPD.
Methods:
One hundred fifty-three samples from 11 patients were selected from COPD sputum samples collected prospectively over 6 years. Samples were grouped as culture-negative (no pathogenic bacteria), colonization, and exacerbation due to new strains of NTHI and M catarrhalis. Levels of lysozyme, lactoferrin, LL-37, and secretory leukocyte protease inhibitor (SLPI) were measured by enzyme-linked immunosorbent assay and compared among groups by paired analysis.
Results:
Compared with baseline, sputum lysozyme levels were significantly lower during colonization and exacerbation by NTHI (P = .001 and P = .013, respectively) and M catarrhalis (P = .007 and P = .018, respectively); SLPI levels were lower with exacerbation due to NTHI and M catarrhalis (P = .002 and P = .004, respectively), and during colonization by M catarrhalis (P = 032). Lactoferrin levels did not change significantly; LL-37 levels were higher during exacerbation by NTHI and M catarrhalis (P = .001 and P = .018, respectively).
Conclusions:
Acquisition of NTHI and M catarrhalis is associated with significant changes in airway levels of AMPs, with larger changes in exacerbation. Airway AMP levels are likely to be important in pathogen clearance and clinical outcomes of infection in COPD.
The innate immune system of the human respiratory tract is the first line of lung defense against bacterial pathogens and it functions to maintain sterile airways (by culture) in the healthy state.1 Altered innate immunity is thought to contribute to bacterial colonization and infection of the airways in COPD.2,3 The airways of most patients with COPD are intermittently colonized by pathogenic bacteria, mostly nontypeable Haemophilus influenzae (NTHI), Moraxella catarrhalis, Streptococcus pneumoniae, and Pseudomonas aeruginosa.4‐6 Approximately 50% of exacerbations are caused by the same species of bacteria that are found in lower airways during stable periods, especially when a new strain of these pathogenic species is acquired by a patient.7,8 We have demonstrated previously that new bacterial strain acquisition is accompanied by an airway and systemic inflammatory response and with the development of mucosal and systemic antibodies specific to the infecting strain.9‐11 Exploring the alterations of innate lung defense with the acquisition of a new pathogenic bacterial strain that results in colonization or exacerbation could advance our understanding of the mechanisms of infection, and elucidate more precisely the role of these infections in the pathology and progression of COPD.
In addition to the development of the mucosal barrier and the ciliary clearance of pathogens, secretion of antimicrobial protein/peptides (AMPs) plays an important role in innate lung defense.12‐14 Multiple AMPs, which include lysozyme, lactoferrin, secretory leukocyte protease inhibitor (SLPI), and the human cathelicidin LL-37, are present in respiratory secretions. Lysozyme, produced by respiratory epithelium and neutrophils, has antibacterial activity against gram-negative and gram-positive pathogens, and is the most abundant AMP in respiratory secretions.13,15,16 Lactoferrin is an iron-binding antibacterial AMP in respiratory secretions, produced by epithelial cells and neutrophils.16,17 The only known human cathelicidin, LL-37, has multiple functions, including microbial killing by pore formation, wound healing, angiogenesis, and activation of lymphocytes.18,19 It is produced by respiratory epithelial cells, as well as neutrophils.20,21 SLPI is one of the major antiproteases in human airways and has antimicrobial activity.22,23 It is produced by respiratory epithelial cells and inhibits the activity of proteases such as neutrophil elastase.
Levels of sputum lysozyme, lactoferrin, and LL-37 are elevated in stable COPD patients compared with healthy control patients.17,24 Sputum SLPI level decreases during acute exacerbations compared with stable baseline in patients with COPD.11,25 Changes in levels of AMPs from baseline stable states in response to the acquisition of new bacterial strains that result in colonization or exacerbation have not been reported.
The aims of the present study were to (1) establish baseline values for AMPs in sputum for a group of patients with COPD, and (2) assess the effects of the acquisition of a new strain of pathogenic bacteria, resulting in colonization or exacerbation, on airway levels of AMPs in these same patients. We focused on NTHI and M catarrhalis, two common causes of bacterial exacerbations of COPD,7,26 and on levels of lysozyme, lactoferrin, LL-37, and SLPI. Considering the well-described antimicrobial roles of these AMPs, we hypothesized that the levels of these AMPs would increase in respiratory secretions with the acquisition of a new strain when compared with baseline levels.
Materials and Methods
COPD Study Clinic
A prospective study of adults with COPD has been conducted at the VA Medical Center, Buffalo, New York, since 1994 under approval by the VA Western New York Healthcare System institutional review board committee (ID No. 0013), and has been described extensively in previous publications.9,26 Informed consent is obtained from each patient in a manner approved by the IRB committee. Patients are seen monthly and whenever increased respiratory symptoms suggestive of exacerbation occur. Clinical information, expectorated sputum, and blood samples are collected at each visit as described previously.26 At each visit, a determination as to whether the patient’s COPD is stable or in exacerbation is determined as described previously. Molecular characterization of bacterial isolates in sputum is performed as described previously.26 Strains of bacteria that had not been isolated previously from the sputum of patients since they enrolled in the clinic are characterized as new strains, whereas those that had been isolated previously are classified as preexisting strains.7,27
Sputum Samples
Spontaneously expectorated samples of sputum were homogenized by incubation with an equal volume of 10% dithiothreitol (Sputolysin; Calbiochem; Gibbstown, New Jersey). Serial dilutions of the homogenized sputum in phosphate-buffered saline (PBS) were plated on blood, chocolate, and MacConkey agar. Identification of bacteria was done using standard techniques. The sputum sample was centrifuged at 15,000 rpm for 20 min at 4°C and the supernatants stored at −70°C.
To study the effects of bacterial colonization and exacerbation on the levels of AMPs in respiratory secretions, samples meeting five defined criteria were collected prospectively from 11 patients. A total of 153 samples met the criteria for one of the following groups: (1) sputum samples in which no potential bacterial pathogen grew on culture and the patient was stable clinically (culture-negative samples); (2 and 3) samples in which a new strain of NTHI or M catarrhalis was isolated, respectively, and the patient was clinically stable (NTHI and M catarrhalis colonization samples); (4 and 5) samples in which a new strain of NTHI or M catarrhalis was isolated, respectively, and the patient was determined to have an exacerbation (NTHI and M catarrhalis exacerbation samples). In these episodes, NTHI or M catarrhalis was the sole bacterial pathogen that grew in culture. Samples collected during the period 1994 to 2000 were included in the analysis if the patient had a minimum of one sample in each of the five sample groups.
Analysis of Sputum Supernatants
The sputum supernatant samples were analyzed for levels of lactoferrin, lysozyme, and SLPI by sandwich enzyme-linked immunosorbent assay as described previously.11,28 LL-37 was measured by enzyme-linked immunosorbent assay as follows: 96 well plates were incubated with 100 μL per well of samples (diluted 1:1,000) and standards in sodium carbonate/0.1 M sodium bicarbonate buffer (pH 9.6) overnight at 4C. After being washed with PBS-Tween, the wells were blocked with 1% bovine serum albumin in PBS with 0.05% Tween-20 (Malinckrodt Baker; Phillipsburg, New Jersey) (PBS-Tween) for 2 h at room temperature. After washing, the first antibody (rabbit polyclonal to LL-37; Santa Cruz Biotechnology) diluted 1:800 in PBS-Tween was applied to the wells for 3 h. After further washing, the second antibody (horseradish peroxidase-labeled anti-rabbit IgG; [KPL; Gaithersburg, Maryland]) was added for 1 h at a dilution of 1:1000 in PBS-Tween. Color was developed using tetramethylbenzidine in dimethyl sulfoxide, and the reaction was stopped using 1N H2SO4. The plate was read at 450 nM, and sample levels were read off the standard curve that was determined simultaneously with known concentrations of LL-37 (Innovagen; Lund, Sweden). The lower limit of detection of this assay was 0.08 ng/mL, and the linear range was from 0.35 ng/mL to 22.5 ng/mL.
Statistical Analysis
The data were entered in a Microsoft Excel spreadsheet and analyzed using Analyze-it (Analyze-it Software Ltd; Leeds, England). To minimize the confounding effects of interpatient variability in the AMP response to pathogens, we used a paired analytic approach to compare changes in the levels of AMPs within each patient. Culture-negative levels of each of the AMPs were calculated for each sample group by averaging the values of each individual’s samples. These baseline values were then used to determine the effects of bacterial colonization and exacerbation with Wilcoxon ranked pairs tests. A P value < .05 was considered statistically significant. The relationships between AMP levels and the clinical score of patients at their visits (calculated from symptoms and signs [e-Fig 1]) were analyzed using the Pearson method of correlation.
Results
Subjects and Sputum Samples
From a cohort of 50 patients followed during the period 1994 to 2000, samples representing each sample group defined previously were available from 11 patients, with a total of 153 samples being available for analysis. Of the 153 samples, 55 were in the culture-negative group, 24 in the NTHI colonization and 23 in the NTHI exacerbation groups, and 23 in the M catarrhalis colonization and 28 in the M catarrhalis exacerbation groups. Patient characteristics are shown in Table 1. A clinical score (variables in e-Fig 1) calculated using clinical symptoms and signs was significantly higher during exacerbations due to NTHI and M catarrhalis, compared with culture-negative and colonization visits (e-Fig 1).
Table 1.
—Patient Characteristics
| Total No. patients | 11 |
| Total No. samples | 153 |
| Mean samples per patient analyzed (range) | 13.9 (10-18) |
| Mean age, y (range) | 66.1 (50-81) |
| Mean FEV1, L | 1.6 |
| Mean FEV1, % predicted | 46.8 |
| GOLD severity, No. | |
| II | 3 |
| III | 5 |
| IV | 3 |
GOLD = Global Initiative for Chronic Obstructive Lung Disease.
Effect of Acquisition of a New Bacterial Strain on Sputum AMP Levels
Lysozyme and Lactoferrin:
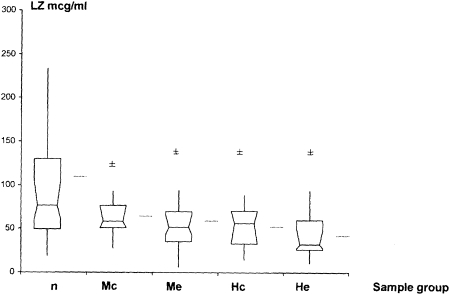
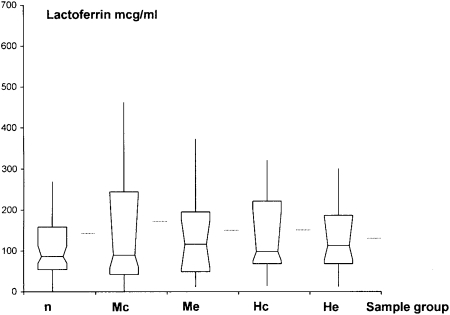
Sputum lysozyme levels at exacerbations due to NTHI and M catarrhalis were significantly lower compared with culture-negative samples (P = .013 and P = .018, respectively) (Fig 1, Table 2). NTHI and M catarrhalis colonization were also accompanied by significantly lower lysozyme sputum levels (P = .001 and P = .007, respectively), compared with culture-negative samples. There was no significant difference when levels in exacerbation samples were compared with those in colonization samples for NTHI (P = .24) or M catarrhalis (P = .97). No significant change in levels among baseline, colonization, and exacerbation samples was found for NTHI or M catarrhalis (Fig 2).
Figure 1.
LZ levels in n, Mc, Me, Hc, and He samples. Mean, median, and interquartile ranges are shown. Horizontal lines outside box-whisker plots represent means. ±P ≤ .05 compared with culture-negative samples by Wilcoxon matched pairs analysis. Hc = nontypeable Haemophilus influenzae colonization; He = nontypeable Haemophilus influenzae exacerbation; LZ = lysozyme; Mc = Moraxella catarrhalis colonization; Me = Moraxella catarrhalis exacerbation; n = culture negative.
Table 2.
—Median (95% CI) Values in the Five Sample Groups
|
Moraxella catarrhalis Colonization (n = 23) |
M catarrhalis Exacerbation(n = 28) |
NTHI Colonization (n = 24) |
NTHI Exacerbation (n = 23) |
||||||
| Antimicrobial Protein/Peptide | Culture-Negative (n = 55), μg/mL | μg/mL | P Value | μg/mL | P Value | μg/mL | P Value | μg/mL | P Value |
| Lysozyme | 76.80 (60.56-103.98) | 58.23 (51.27-76.59) | .007 | 51.90 (43.17-65.91) | .018 | 60.16 (33.08-71) | .001 | 35.10 (26.02-62.3) | .013 |
| Lactoferrin | 85.20 (70.62-111.6) | 89.97 (59.46-240.18) | … | 116.10 (53.38-174.66) | … | 97.69 (63.86-270.02) | … | 102.35 (57.69-185.98) | … |
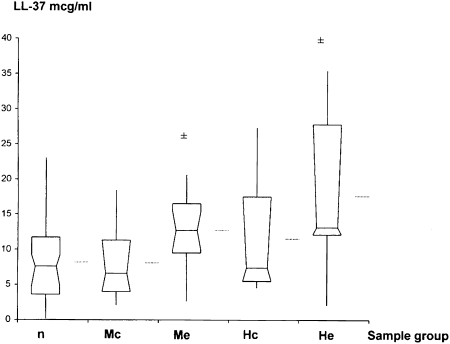
| LL-37 | 7.57 (4.75-9.23) | 6.63 (4.03-11.18) | … | 12.72 (9.88-15.38) | .018 | 7.01 (5.37-17.48) | … | 13.12 (12.11-27.85) | .001 |
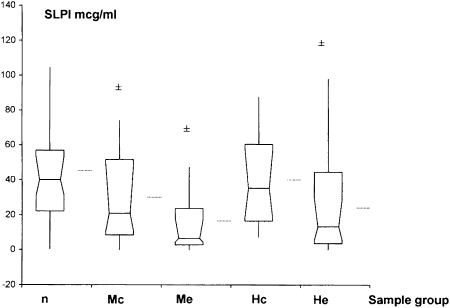
| SLPI | 39.98 (31.11-53.10) | 21.00 (10.44-48.81) | .032 | 6.58 (4.53-17.79) | .004 | 38.84 (18.26-64.06) | … | 8.16 (2.06-25.63) | .002 |
P values were derived by Wilcoxon matched pairs test in comparison with culture-negative samples from the same patient. NTHI = nontypeable Haemophilus influenzae; SLPI = secretory leukocyte protease inhibitor.
Figure 2.
Lactoferrin levels in n, Mc, Me, Hc, and He samples. Mean, median, and interquartile ranges are shown. Horizontal lines outside box-whisker plots represent means. See Figure 1 legend for expansion of abbreviations.
LL-37:
On paired analysis, exacerbations due to new strains of M catarrhalis (P = .018) and NTHI (P = .001) were associated with significantly higher sputum levels of LL-37 compared with culture-negative samples (Fig 3). Moreover, exacerbation levels were also significantly higher than levels during colonization by NTHI (P = .001) and M catarrhalis (P = .009). Levels during colonization were not significantly different from culture-negative levels.
Figure 3.
Human cathelicidin LL-37 levels in n, Mc, Me, Hc, and He samples. Mean, median, and interquartile ranges are shown. Horizontal lines outside box-whisker plots represent means. ±P ≤ .05 compared with culture-negative samples by Wilcoxon paired sample analysis. See Figure 1 legend for expansion of abbreviations.
SLPI Levels:
Levels were significantly lower in exacerbations due to M catarrhalis (P = .004) and NTHI (P = .002) and during colonization by M catarrhalis (P = .032), but not during colonization by NTHI (Fig 4). Levels during NTHI exacerbation were significantly lower than during colonization (P = .024). However, this difference was not seen with M catarrhalis.
Figure 4.
SLPI levels in n, Mc, Me, Hc, and He samples. Mean, median, and interquartile ranges are shown. Horizontal lines outside box-whisker plots represent means. ±P ≤ .05 compared with culture-negative samples by Wilcoxon paired sample analysis. SLPI = secretory leukocyte protease inhibitor. See Figure 1 legend for expansion of other abbreviations.
There were no significant differences in levels of these AMPs when preacquisition culture-negative samples were compared with culture-negative levels after resolution of an exacerbation or colonization. Charts showing the longitudinal trends of each of these AMPs in an individual patient through episodes of culture-negative, colonization, and exacerbation, representative of the group of patients studied, are shown in e-Figures 2 to 5. When AMP levels were correlated with the severity of exacerbation, as calculated by a clinical score of symptoms and signs, LL-37 levels were higher with increasing clinical score (R2 = 0.34, P ≤ .001) (e-Fig 6) and SLPI levels were lower with increasing clinical score (R2 = −0.34, P ≤ .001) (e-Fig 7). There were no significant correlations for lysozyme and lactoferrin levels with the clinical scores.
Discussion
We found that, contrary to our hypothesis of an increase in the various AMPs with airway infection in COPD, airway AMP levels change in complex ways. LL-37 levels do increase with bacterial infection; however lysozyme and SLPI levels decrease and lactoferrin levels remain unchanged. Interestingly, the changes in AMP levels, irrespective of direction, are of a greater magnitude in exacerbation than in colonization. This mirrors previous observations regarding the inflammatory response to bacterial infection in COPD, which is also more intense during exacerbation compared with colonization. Except for the observation regarding the decrease in SLPI levels with exacerbations, all these observed AMP changes are novel observations and could be of substantial importance in the host-pathogen interaction in COPD.
The decrease in lysozyme levels during colonization and exacerbation is an intriguing observation, although it is as yet unexplained. The usual sources of lysozyme in the lower airway are the respiratory epithelium, especially submucosal glands, and infiltrating neutrophils. NTHI and M catarrhalis may decrease transcription and/or secretion of lysozyme from airway epithelial cells. Such immune-evasion strategies have been described for other bacterial pathogens, such as Klebsiella pneumoniae, which inhibits secretion of β defensins from respiratory epithelial cells.29 Taylor et al30 found that patients with COPD with lower salivary lysozyme in the stable state were more prone to exacerbations. However, in that study, dynamic changes in sputum lysozyme with the acquisition of bacteria were not determined. Cleavage of lysozyme by proteolytic enzymes in the respiratory tract is another possible explanation of the low levels observed. Although an attractive hypothesis because free sputum neutrophil elastase levels increase with bacterial exacerbations of COPD, neutrophil elastase does not cleave lysozyme.31 Cleavage by other proteases, such as cathepsins32 or proteases secreted by bacterial pathogens, might cause this effect,31 but has not been reported for NTHI or M catarrhalis. Lysozyme has antibacterial activity against gram-positive and gram-negative pathogens.16,33 Therefore, decreased lysozyme during bacterial exacerbation and colonization in COPD could contribute to bacterial persistence and its attendant consequences. The mechanisms underlying this observation need to be elucidated in future studies.
Our observation of decreased SLPI levels in exacerbations confirms previous reports that have shown that SLPI levels decrease during exacerbations and that sputum SLPI levels are in inverse relation to levels of neutrophil elastase.11 Interestingly, elastase increases SLPI gene expression in respiratory epithelial cells.34 However, extracellular levels in tissue cultures and in respiratory secretions are low.35 SLPI forms complexes with elastase, and such complexes are bound to negatively charged cell membranes, decreasing levels in free secretions.35 Bacterial infection in COPD is a strong stimulus for increased proteolytic activity in the lower respiratory tract, which is the most likely mechanism of the decreased SLPI levels seen in this study.
In addition to its antimicrobial activity, LL-37 induces secretion of IL-836 and is a chemoattractant for neutrophils.37 LL-37 can, therefore, enhance the host inflammatory response and clearance of bacterial infection. LL-37 also promotes angiogenesis.38 Higher levels of LL-37 have been found in malignancies,39,40 including lung carcinomas,41 and have been associated with increased metastasis in breast cancer.42 The possible roles of elevated LL-37 levels in lungs, due to persistent bacterial colonization and infection, in tumor genesis, and the abnormal angiogenesis seen in COPD are intriguing hypotheses and areas for future studies.
Lactoferrin levels were unchanged with colonization and exacerbation by NTHI and M catarrhalis, compared with baseline levels. This was surprising, considering that lactoferrin has antibacterial activity16 and is produced by respiratory epithelial cells and neutrophils. Previous authors have reported a lack of increase in respiratory lactoferrin levels with NTHI infection in COPD.43 A clear explanation for this observation is lacking and needs further investigation.
Colonization is defined as the presence of bacteria in a host without eliciting a host response or causing damaging effects to the host. However, in COPD, the term colonization is used to mean presence of bacteria in the lower airway without clinical symptoms of exacerbation. This characterization of the abnormal bacterial presence in the lower airways in clinically stable COPD as innocuous may not be appropriate, based on evidence of increased inflammation, structural damage, and host response with bacterial colonization in COPD. This work adds to the body of evidence that colonization is not an inert event in COPD, and changes in sputum AMP levels are clearly associated with it. The changes in AMP levels with colonization mirror those seen during exacerbation, although they are of a smaller magnitude.
The strengths of this study include its longitudinal design, with the use of multiple samples to establish a stable baseline. Comparing within patients and using molecular epidemiology to clearly define new strain acquisition are additional strengths of this study. However, it does have some limitations. We have not examined the levels during infection by other pathogenic bacteria such as S pneumoniae or P aeruginosa and therefore cannot extend the findings to these infections. Sputum samples may reflect large-airway rather than small-airway phenomena and can be contaminated by saliva. However, an analysis of multiple longitudinally collected samples, as is necessary to answer the questions in this study, precludes the repeated use of BAL. Expectorated sputum, if collected and processed in a standardized manner as was done in this study, has been used by us and others to obtain important information about the airway milieu in COPD.9,26,44,45 The mechanisms underlying the changes in the AMPs seen are also not elucidated in this study, but are the focus of our current research efforts. Whether concomitant viral infection or environmental changes could have contributed to the observed changes in AMP levels is also not determined in this study. Considering the sample size of 11 patients, we have not attempted to analyze the relationships between changes in AMP levels and rate of recovery from exacerbations or changes in lung function.
Conclusions
In conclusion, we show that infection by NTHI and M catarrhalis in patients with COPD causes significant changes in the levels of AMPs in respiratory secretions. The patterns of change for AMP levels are distinct for each AMP, and the changes differ in magnitude between colonization and exacerbation. These observations suggest that such innate host defense mechanisms may play a role in determining the microbiologic and clinical outcomes of infection in COPD, but further work is necessary to confirm these hypotheses. The mechanisms underlying these changes and their implications for host immunity, inflammation, and angiogenesis are areas worthy of further study.
Supplementary Material
Acknowledgments
Author contributions: Dr Parameswaran: contributed to measurements, analysis, and drafting of the manuscript.
Dr Sethi: contributed to planning and supervision of the clinical study and writing of the manuscript.
Dr Murphy: contributed to planning and supervision of the clinical study, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, the decision to publish, or in the preparation of the manuscript.
Other contributions: The authors acknowledge the help of Catherine T. Wrona, BA, with standardization of enzyme-linked immunosorbent assay tests.
Additional information: The e-Figures can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/140/3/611/suppl/DC1.
Abbreviations
- AMP
antimicrobial protein/peptide
- NTHI
nontypeable Haemophilus influenzae
- PBS
phosphate-buffered saline
- SLPI
secretory leukocyte protease inhibitor
Funding/Support: This work was supported by a New York State Empire Clinical Research Award (G. I. P.); National Institutes of Health [Grants A119641 and A128304] (T. F. M.); and a VA Merit Review Award (S. S. and T. F. M.).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23(2):327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1(1):59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 3.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(23):2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 4.Monsó E, Ruiz J, Rosell A, et al. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152(4 Pt 1):1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 5.Soler N, Ewig S, Torres A, Filella X, Gonzalez J, Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 6.Zalacain R, Sobradillo V, Amilibia J, et al. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):343–348. doi: 10.1034/j.1399-3003.1999.13b21.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy TF, Brauer AL, Grant BJ, Sethi S. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am J Respir Crit Care Med. 2005;172(2):195–199. doi: 10.1164/rccm.200412-1747OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 9.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(5):491–497. doi: 10.1164/rccm.200708-1234OC. [DOI] [PubMed] [Google Scholar]
- 10.Sethi S, Wrona C, Grant BJ, Murphy TF. Strain-specific immune response to Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;169(4):448–453. doi: 10.1164/rccm.200308-1181OC. [DOI] [PubMed] [Google Scholar]
- 11.Parameswaran GI, Wrona CT, Murphy TF, Sethi S. Moraxella catarrhalis acquisition, airway inflammation and protease-antiprotease balance in chronic obstructive pulmonary disease. BMC Infect Dis. 2009;9:178. doi: 10.1186/1471-2334-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1(3):141–150. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75(1):34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- 14.Travis SM, Conway BA, Zabner J, et al. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20(5):872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 15.Dajani R, Zhang Y, Taft PJ, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol. 2005;32(6):548–552. doi: 10.1165/rcmb.2005-0059OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellison RT, III, Giehl TJ. Killing of gram-negative bacteria by lactoferrin and lysozyme. J Clin Invest. 1991;88(4):1080–1091. doi: 10.1172/JCI115407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson AB, Bohling T, Payvandi F, Rennard SI. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990;115(2):148–158. [PubMed] [Google Scholar]
- 18.Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120(3):379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 19.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 20.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci U S A. 1998;95(16):9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bals R, Wilson JM. Cathelicidins—a family of multifunctional antimicrobial peptides. Cell Mol Life Sci. 2003;60(4):711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitch PM, Roghanian A, Howie SEM, Sallenave JM. Human neutrophil elastase inhibitors in innate and adaptive immunity. Biochem Soc Trans. 2006;34(Pt 2):279–282. doi: 10.1042/BST20060279. [DOI] [PubMed] [Google Scholar]
- 23.Vogelmeier C, Hubbard RC, Fells GA, et al. Anti-neutrophil elastase defense of the normal human respiratory epithelial surface provided by the secretory leukoprotease inhibitor. J Clin Invest. 1991;87(2):482–488. doi: 10.1172/JCI115021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128(4):2316–2326. doi: 10.1378/chest.128.4.2316. [DOI] [PubMed] [Google Scholar]
- 25.Hill AT, Campbell EJ, Bayley DL, Hill SL, Stockley RA. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ) Am J Respir Crit Care Med. 1999;160(6):1968–1975. doi: 10.1164/ajrccm.160.6.9904097. [DOI] [PubMed] [Google Scholar]
- 26.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 27.Murphy TF, Brauer AL, Schiffmacher AT, Sethi S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(3):266–272. doi: 10.1164/rccm.200403-354OC. [DOI] [PubMed] [Google Scholar]
- 28.Dubin RF, Robinson SK, Widdicombe JH. Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L750–L755. doi: 10.1152/ajplung.00326.2003. [DOI] [PubMed] [Google Scholar]
- 29.Moranta D, Regueiro V, March C, et al. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells [published correction appears in Infect Immun. 2010;78(12):5352] Infect Immun. 2010;78(3):1135–1146. doi: 10.1128/IAI.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor DC, Cripps AW, Clancy RL. A possible role for lysozyme in determining acute exacerbation in chronic bronchitis. Clin Exp Immunol. 1995;102(2):406–416. doi: 10.1111/j.1365-2249.1995.tb03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacquot J, Tournier JM, Puchelle E. In vitro evidence that human airway lysozyme is cleaved and inactivated by Pseudomonas aeruginosa elastase and not by human leukocyte elastase. Infect Immun. 1985;47(2):555–560. doi: 10.1128/iai.47.2.555-560.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taggart CC, Lowe GJ, Greene CM, et al. Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor. J Biol Chem. 2001;276(36):33345–33352. doi: 10.1074/jbc.M103220200. [DOI] [PubMed] [Google Scholar]
- 33.Lee HY, Andalibi A, Webster P, et al. Antimicrobial activity of innate immune molecules against Streptococcus pneumoniaeMoraxella catarrhalis and nontypeable Haemophilus influenzae. BMC Infect Dis. 2004;4:12. doi: 10.1186/1471-2334-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbinante-Nissen JM, Simpson LG, Leikauf GD. Neutrophil elastase increases secretory leukocyte protease inhibitor transcript levels in airway epithelial cells. Am J Physiol. 1993;265(3 Pt 1):L286–L292. doi: 10.1152/ajplung.1993.265.3.L286. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan AL, Dafforn T, Hiemstra PS, Stockley RA. Neutrophil elastase reduces secretion of secretory leukoproteinase inhibitor (SLPI) by lung epithelial cells: role of charge of the proteinase-inhibitor complex. Respir Res. 2008;9:60. doi: 10.1186/1465-9921-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuyderduyn S, Ninaber DK, Hiemstra PS, Rabe KF. The antimicrobial peptide LL-37 enhances IL-8 release by human airway smooth muscle cells. J Allergy Clin Immunol. 2006;117(6):1328–1335. doi: 10.1016/j.jaci.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Tjabringa GS, Ninaber DK, Drijfhout JW, Rabe KF, Hiemstra PS. Human cathelicidin LL-37 is a chemoattractant for eosinophils and neutrophils that acts via formyl-peptide receptors. Int Arch Allergy Immunol. 2006;140(2):103–112. doi: 10.1159/000092305. [DOI] [PubMed] [Google Scholar]
- 38.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111(11):1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffelt SB, Marini FC, Watson K, et al. The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A. 2009;106(10):3806–3811. doi: 10.1073/pnas.0900244106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heilborn JD, Nilsson MF, Jimenez CI, et al. Antimicrobial protein hCAP18/LL-37 is highly expressed in breast cancer and is a putative growth factor for epithelial cells. Int J Cancer. 2005;114(5):713–719. doi: 10.1002/ijc.20795. [DOI] [PubMed] [Google Scholar]
- 41.von Haussen J, Koczulla R, Shaykhiev R, et al. The host defence peptide LL-37/hCAP-18 is a growth factor for lung cancer cells. Lung Cancer. 2008;59(1):12–23. doi: 10.1016/j.lungcan.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Weber G, Chamorro CI, Granath F, et al. Human antimicrobial protein hCAP18/LL-37 promotes a metastatic phenotype in breast cancer. Breast Cancer Res. 2009;11(1):R6. doi: 10.1186/bcr2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogel L, Schoonbrood D, Geluk F, et al. Iron-binding proteins in sputum of chronic bronchitis patients with Haemophilus influenzae infections. Eur Respir J. 1997;10(10):2327–2333. doi: 10.1183/09031936.97.10102327. [DOI] [PubMed] [Google Scholar]
- 44.Crooks SW, Bayley DL, Hill SL, Stockley RA. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J. 2000;15(2):274–280. doi: 10.1034/j.1399-3003.2000.15b09.x. [DOI] [PubMed] [Google Scholar]
- 45.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109(4):288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.