Abstract
The biased competition theory proposes that items matching the contents of visual working memory will automatically have an advantage in the competition for attention. However, evidence for an automatic effect has been mixed, perhaps because the memory-driven attentional bias can be overcome by top-down suppression. To test this hypothesis, the Pd component of the event-related potential waveform was used as a marker of attentional suppression. While observers maintained a color in working memory, task-irrelevant probe arrays were presented that contained an item matching the color being held in memory. We found that the memory-matching probe elicited a Pd component, indicating that it was being actively suppressed. This result suggests that sensory inputs matching the information being held in visual working memory are automatically detected and generate an “attend-to-me” signal, but this signal can be overridden by an active suppression mechanism to prevent the actual capture of attention.
Keywords: visual working memory, attend-to-me signal, attentional suppression, event-related potential, Pd
The natural visual environment typically contains a large number of objects, and the human visual system must rapidly select important information from this complex input. According to the biased competition theory (Desimone, 1998; Desimone & Duncan, 1995), sensory inputs compete for neural representation in visual cortex, and this competition can be controlled by introducing biases that favor the processing of one stimulus at the expense of others. Two classes of biasing mechanisms have been proposed. One is a bottom-up mechanism in which the competition is biased in favor of physically salient stimuli. The other is a top-down mechanism in which the competition is biased in favor of current goals. Goals are thought to influence competition by means of working memory representations in visual cortex, which essentially pre-activate the representations of goal-relevant stimuli and therefore confer an advantage to these representations (Chelazzi, Duncan, Miller, & Desimone, 1998). To test this hypothesis, several studies have investigated whether attention is automatically guided toward items that match the contents being held in visual working memory (Downing, 2000; Downing & Dodds, 2004; Han & Kim, 2009; Hollingworth & Luck, 2009; Houtkamp & Roelfsema, 2006; Kumar, Soto, & Humphreys, 2009; Olivers, 2009; Olivers, Meijer, & Theeuwes, 2006; Pan, Xu, & Soto, 2009; Peters, Goebel, & Roelfsema, 2009; Soto, Heinke, Humphreys, & Blanco, 2005; Soto & Humphreys, 2006, 2008, 2009; Soto, Humphreys, & Heinke, 2006; Soto, Humphreys, & Rotshtein, 2007; Woodman & Luck, 2007; see review by Soto, Hodsoll, Rotshtein, & Humphreys, 2008).
Soto and colleagues have provided evidence of memory-driven attentional capture (Kumar et al, 2009; Pan et al, 2009; Soto et al, 2005, 2006, 2007; Soto & Humphreys, 2006, 2008, 2009). In their studies, a simple colored shape (e.g., a red square) was presented at the beginning of each trial and participants were required to remember this object until the end of the trial. A visual search array then appeared, containing a tilted target bar among vertical distractor bars. Each bar was surrounded by a colored shape, one of which could match the object being held in memory. Although the memory-matching item was unrelated to the location of the search target, search performance was impaired when the memory-matching item surrounded one of the non-target bars, and the memory-matching item tended to attract gaze more than items that did not match memory (Soto et al., 2005, 2006). This result suggests that the contents of visual working memory automatically guide attention.
In contrast, other studies reported no evidence of memory-driven attentional capture (Carlisle & Woodman, in press; Downing & Dodds, 2004; Houtkamp & Roelfsema, 2006; Woodman & Luck, 2007) or found that memory-driven attentional capture is eliminated under some situations (Han & Kim, 2009; Olivers, 2009). In Woodman and Luck (2007), a colored square was first presented for storage in working memory, followed by a search array that required a response to a target defined by its shape. On half of the trials, the colors of all the squares in the visual search array were different from the color of the memory object. In the other half, one of the distractors was drawn in the same color as the memory object. No evidence for memory-driven attentional capture was obtained. Indeed, the results indicated that attention could be directed away from the location of the memory-matching distractor, suggesting that the influence of visual working memory on selective attention is not automatic but controlled and flexible.
Here, we propose that an active suppression mechanism can be used to prevent attention from being captured by sensory inputs that match the contents of visual working memory. This hypothesis is based on previous research examining the capture of attention by stimuli having bottom-up salience. Specifically, Sawaki and Luck (2010) reported that salient singletons are detected and generate an attention capture signal (an attend-to-me signal), irrespective of attentional control settings, but that the actual deployment of attention to these singletons can be avoided by an active suppression process. This signal suppression hypothesis of controlled attention capture shares elements of the two main competing theories of attention capture. Like the bottom-up saliency hypothesis (Theeuwes, 1991, 2004, 2010; Theeuwes & Burger, 1998), the signal suppression hypothesis proposes that salient items are detected irrespective of top-down control settings, insofar as the attend-to-me signal is generated by salient items whether or not they match control settings. However, like the contingent involuntary orienting hypothesis (Bacon & Egeth, 1994; Folk & Remington, 1998; Folk, Remington, & Johnston, 1992; Folk, Remington, & Wright, 1994; Kiss, Jolicœur, Dell'acqua, & Eimer, 2008; Leber & Egeth, 2006), the signal suppression hypothesis proposes that top-down control settings can influence whether this attend-to-me signal actually leads to the allocation of attention.
The present study extends the signal suppression hypothesis to signals that are based on the match between a sensory input and a working memory representation. Specifically, we propose that memory-matching items automatically generate an attend-to-me signal, but this signal can be overridden by top-down suppression under some conditions.
Memory-driven attentional capture has been typically measured by the effects of memory-matching items on reaction time (RT). However, RT measures have some key shortcomings in addressing the control of attention. First, it is difficult to determine whether the absence of capture reflects the active suppression of a bias signal or simply the lack of any bias signal. Second, RT tasks typically create situations in which the observers are trying to allocate attention to a target item while simultaneously dealing with bias signals arising from the distractors, and the RT effects may reflect an interaction between these factors rather than the pure impact of the bias signals.
The present study investigated this issue by using the Pd component of the event-related potential (ERP) waveform, which appears to reflect attentional suppression of distractors. The Pd component is observed in the ERP waveform as a more positive voltage at contralateral scalp sites than at ipsilateral scalp sites relative to the position of the to-be-suppressed item, with maximal voltage at lateral occipito-temporal electrode sites. This component typically begins 150-250 ms post-stimulus, depending on stimulus salience. Hickey, Di Lollo, and McDonald (2009) first described this component in detail. In their study, a target item and a non-salient distractor item were presented in the display, and participants were asked to discriminate the identity of the target. They showed that the distractor item elicited the Pd component. It is assumed that the distractor was actively suppressed because the target and the distractor were the only items in the display, and task-irrelevant distractors can provide strong competition for attention under such low perceptual load conditions (Lavie, 1995, 2005; Lavie, Hirst, de Fockert, & Viding, 2004; Lavie & Tsal, 1994). Furthermore, Sawaki and Luck (2010) showed that salient distractors elicit the Pd component under conditions in which no behavioral capture would be expected. Similar results were observed by Eimer and Kiss (2008), although the relationship between Pd and distractor suppression was not known when that study was published.
In contrast to the suppression-related Pd component, the N2pc component reflects the allocation of attention to an item (Eimer, 1996; Eimer & Kiss, 2008; Hickey, McDonald, & Theeuwes, 2006; Kiss et al., 2008; Leblanc, Prime, & Jolicoeur, 2008; Lien, Ruthruff, Goodin, & Remington, 2008; Luck & Hillyard, 1994a,b; Rodríguez Holguín, Doallo, Vizoso, & Cadaveira, 2009; Woodman & Luck, 1999, 2003; see review by Luck, in press). Whereas Pd is positive contralateral to a suppressed item, N2pc is negative contralateral to an attended item. Together, these two components make it possible to determine whether a given item is attended or suppressed.
Recently, Kumar, Soto, and Humphreys (2009) used ERP recordings to investigate whether attention is automatically deployed toward items that match the contents of visual working memory. They found that the N2pc component elicited by a visual search target was larger when a memory-matching distractor was in the same visual field as the target and smaller when the distractor was in the opposite field, which is the pattern that would be expected if the memory-matching distractor elicited an N2pc component. That is, the N2pc to the target and memory-matching distractor would add together when they appeared on the same side, leading to a large N2pc contralateral to this side, but they would cancel when on opposite sides, leading to a small N2pc contralateral to the target side. However, because they never presented a memory-matching distractor without a target, it is impossible to know whether this distractor actually captured attention or whether it indirectly modulated the allocation of attention to the target. In addition, when the memory-matching distractor was presented on the same visual field as the target, the distance between the distractor and the target was closer than when it was presented on the opposite side. Therefore, it is possible that the large N2pc was observed when the memory-matching distractor appeared on the target side because more attention was allocated to the target to resolve the competition and not because attention was captured by the memory-matching distractor.
In the present study, we instead measured ERPs elicited by task-irrelevant probe arrays consisting of a memory-matching item and a completely irrelevant non-matching item. The absence of a task for the probe array makes it possible to examine the pure effects of a working memory match, unconfounded by the presence of a target.
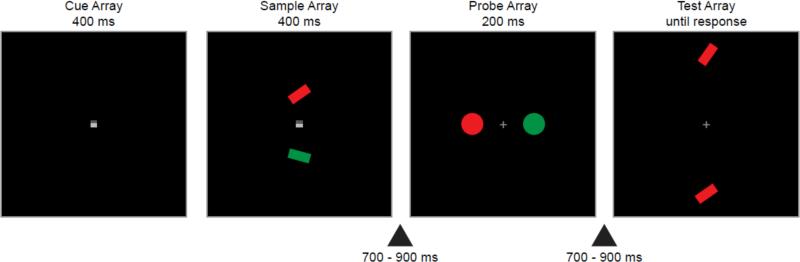
The basic task is illustrated in Figure 1. ERPs are highly sensitive to the physical features of stimulus sequence, and this makes special control procedures necessary. Thus, rather than simply presenting a single object and asking observers to store this object in working memory, two objects were presented, and a spatial cue indicated that one of these two objects should be stored in memory. In addition, to avoid any possible strategic effects, observers were asked to store the orientation of the cued item in memory to perform an orientation-matching task at the end of the trial, and the color was task-irrelevant. Previous studies indicate that observers will automatically store all features of an object in working memory, even irrelevant features (Hollingworth & Luck, 2009; Hyun, Woodman, Vogel, Hollingworth, & Luck, 2009), so we assumed that the task-irrelevant color of the cued rectangle would be stored in working memory in the present study.
Figure 1.
Example sequence of events in a trial of Experiment 1. Half of the participants were instructed to attend to the region indicated by the dark half of the cue. In this example trial, these participants would store the upper rectangle in memory and compare it with the two rectangles shown in the test array, and the red circle would be the memory-matching probe item. For the other participants, who were instructed to attend to the region indicated by the light half of the cue, the lower rectangle would be stored in memory and the green circle would be the memory-matching probe item.
While the cued rectangle was being maintained in working memory, two task-irrelevant probe circles were briefly presented, one on each side of fixation. One circle had the same color as the memory rectangle (memory-matching probe), allowing us to determine whether the memory match leads to capture of attention (N2pc) or suppression (Pd). Observers performed no task with these circles, but the biased competition theory assumes that the biasing effects of the working memory representation will have the same effects on task-relevant and task-irrelevant stimuli.
We anticipated three possible outcomes for the probe stimuli. If memory-matching items automatically receive priority for attention, and this inevitably results in deployment of attention, then an N2pc component should be observed contralateral to the memory-matching probe circle. Alternatively, if memory-matching items automatically generate an attend-to-me signal, but an active suppression mechanism is used to prevent the actual deployment of attention, then the memory-matching probe should elicit a Pd component. A third possibility is that memory-matching items do not automatically receive priority for attention, in which case the memory-matching and non-memory-matching probe items would elicit equivalent neural responses, and no significant lateralization of ERP activity would be observed relative to the location of the matching item.
It should be noted that capture (N2pc) may occur on some trials, and suppression (Pd) may occur on others. Because N2pc and Pd have opposite polarities and similar scalp distributions, they will cancel each other, and the averaged data will reflect the balance of capture and suppression. Thus, the above predictions reflect this balance across many trials and not necessarily the brain activity on any given single trial.
Method
Participants
The participants were 12 neurologically normal volunteers between 18 and 30 years old who were paid for their participation. All participants had normal or corrected-to-normal vision. Informed consent was obtained at the beginning of the experiment.
Stimuli and Procedure
The stimuli were presented on a video monitor with a black background at a distance of 70 cm. The sequence of events on a typical trial is illustrated in Figure 1. Following a blank intertrial interval (700–900 ms, rectangular distribution), a central square cue (0.4° × 0.4°) was presented for 400 ms (cue array). On half of the trials, the upper half of the cue was dark gray (8.86 cd/ m2) and the lower half was light gray (17.76 cd/ m2). The luminances were reversed for the remaining trials, selected at random. Half of the participants were instructed to direct attention to the region indicated by dark gray side, and the other half were instructed to direct attention to the region indicated by light gray side.
While the cue stimulus remained visible, two rectangles (0.6° x 1.4°) were presented for 400 ms, each centered 2.0° above or below the fixation point (sample array). One rectangle was red (u’ = .47, v’ = .51, 20 cd/m2) and the other was green (u’ = .14, v’ = .54, 20 cd/m2), with the color at each location varied randomly across trials. The orientation of each rectangle was chosen randomly within a range between 25° and 65° or between 115° and 155° (with 0° being vertical). The values were used to minimize categorical, verbal representations. Participants were instructed to remember the orientation of the rectangle in the cued region and to ignore the orientation of the rectangle in the uncued region.
After a blank interval (700–900 ms, rectangular distribution), two task-irrelevant probe circles (1.4° in diameter) were presented for 200 ms, each centered 2.0° to the left or right of fixation. One circle was red and the other was green, and the color at each stimulus location varied randomly across trials. Therefore, one of the circle probes matched the color of the to-be-remembered rectangle for that trial (memory-matching probe) and the other did not (non-matching probe). Participants were explicitly instructed that the probe circles were not task-relevant.
Next, after a blank interval (700–900 ms, rectangular distribution), a test array was presented. This array consisted of two rectangles, each centered 4.5° above or below the horizontal meridian. One rectangle was in the same orientation as the memory rectangle that the participant was asked to remember. The other was randomly chosen to be +20 or -20 degrees tilted relative to the memory rectangle. Both rectangles had the same color as the memory rectangle. The location of the orientation-matching and orientation-mismatching rectangles varied randomly across trials. Participants responded on a game pad, pressing the upper button with their right index finger or the lower button with their right middle finger to indicate whether the upper or lower rectangle in the test array matched the orientation of the memory rectangle. The test array was extinguished by the participant's response or after 3000 ms, whichever came sooner.
It should be emphasized that stimuli in the sample and test arrays were always presented at the vertical locations, whereas stimuli in the probe array were always presented at the horizontal locations. Therefore, we assumed that there would be no confusion between the probe array and the sample and test arrays.
It is also important to note that all aspects of the physical stimuli were completely counterbalanced to eliminate any possible sensory confounds. First, the data from each participant were collapsed across stimulus colors and locations. Second, a given sequence of stimuli might have the memory-matching probe on the left side and the non-matching probe on the right side for participants who were instructed to remember the item indicated by the light gray portion of the cue stimulus, and this same stimulus would have the memory-matching probe on the right side and the non-matching probe on the left side for participants who were instructed to remember the item indicated by the dark gray portion of the cue stimulus. Once the data are averaged across these two groups of participants, all physical stimulus factors are completely controlled.
A gray fixation cross (0.4° × 0.4°, 11.55 cd/ m2) was continuously visible at the center of the display, except when occluded by the cue, and participants were instructed to maintain fixation on the central location throughout each trial.
Each participant performed 30–60 practice trials, followed by 20 blocks of 32 trials during which ERPs were recorded. This yielded 320 trials on which the memory-matching probe was in the left visual field and 320 trials on which this probe was in the right visual field.
Recording and Analysis
The EEG was recorded using active Ag/AgCl electrodes (BioSemi ActiveTwo) placed at the left and right mastoids and 32 scalp sites (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P9, P7, P5, P3, P1, Pz, P2, P4, P6, P8, P10, PO7, PO3, POz, PO4, PO8, O1, Oz, O2, and Iz, according to the modified 10-20 System; American Electroencephalographic Society, 1994). To detect eye movements and blinks, the electrooculogram (EOG) was recorded from electrodes placed at the outer canthi of each eye, and above and below the right eye. All signals were recorded in single-ended mode. The EEG and EOG were low-pass filtered with a 5th-order sinc filter (half-power cutoff at 208 Hz) and digitized at 1024 Hz.
All data analyses were conducted using ERPLAB Toolbox (http://www.erpinfo.org/erplab/) and EEGLAB Toolbox (Delorme & Makeig, 2004; http://sccn.ucsd.edu/eeglab/), which are freely available, open source, Matlab-based packages for EEG/ERP data analysis. The EEG signals were referenced offline to the average of the left and right mastoids, and the four EOG signals were referenced into bipolar vertical and horizontal EOG derivations. These signals were low-pass filtered offline using a noncausal Butterworth infinite impulse response filter with a half-power cutoff at 30 Hz and a roll-off of 12 dB/octave, and then down-sampled to 256 Hz. Averaged ERP waveforms were computed with a 600-ms epoch, beginning 100 ms before the onset of the probe array.
Trials were automatically excluded if they contained an incorrect behavioral response, if the RT was shorter than 100 ms or longer than 3000 ms, if the EEG exceeded ±100 μV in any channel, or if either EOG signal exceeded ±80 μV. To assess residual eye movements, we computed separate averaged horizontal EOG waveforms for trials with the memory-matching item on the left and right sides. Any consistent eye deflections toward or away from the memory-matching item would lead to a difference in HEOG voltage when this item appeared on the left versus right side, even if the deflections were small or infrequent. We routinely replace any participants for whom the residual HEOG activity is more than 3.2 μV, which means that the residual eye movements in the remaining participants were less than 0.2° with a propagated voltage of less than 0.1 μV at the posterior scalp sites (Lins, Picton, Berg, & Scherg, 1993). We also routinely replace participants for whom more than 25% of trials are rejected because of EEG/EOG artifacts. One participant was replaced for these reasons in the present experiment. Among the final set of twelve participants, artifacts led to the rejection of an average of 11.0% of trials (range 2.1 – 24.5%).
Results
For the memory task, mean reaction time was 996 ms and mean memory accuracy was 78.3% correct. Every trial contained a probe array with one memory-matching probe and one non-matching probe, so there was no way to determine whether the presence of a memory-matching probe influenced task performance.
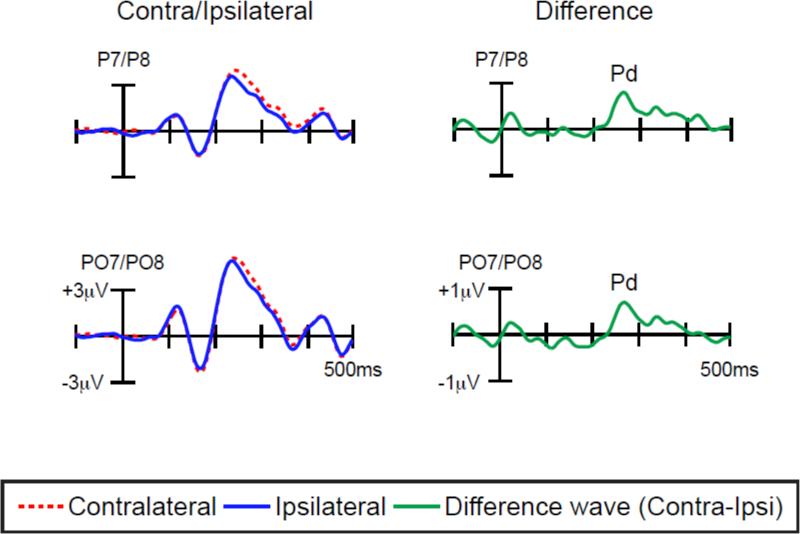
Figure 2 shows the ERP waveforms from lateral parietal-occipital scalp sites (P7/P8 and PO7/PO8), time-locked to the probe array. Separate waveforms are shown for contralateral and ipsilateral sites, relative to the location of the memory-matching probe circle (i.e., the contralateral waveform was the average of the left-hemisphere electrode when the memory-matching probe was in the right visual field and the right-hemisphere electrode when the memory-matching probe was in the left visual field; the ipsilateral waveform was the average of the left-hemisphere electrode when the memory-matching probe was in the left visual field and the right-hemisphere electrode when the memory-matching probe was in the right visual field). A Pd component—a more positive voltage contralateral to the location of the memory-matching item—was visible in the ERP waveform beginning approximately 220 ms post-stimulus.
Figure 2.
Grand average waveforms for memory-matching probe at contralateral versus ipsilateral electrode sites, along with the difference between the contralateral and ipsilateral waveforms.
Pd amplitude was measured as the mean voltage between 250 and 300 ms relative to the mean voltage during the 100-ms pre-stimulus baseline period at the P7 and P8 electrode sites and PO7 and PO8 electrode sites. These sites were chosen because the Pd component was largest at these sites in the present study and in previous studies (Hickey et al., 2009; Sawaki & Luck, 2010). This time window was chosen based on the latency of the peak in the grand average waveform, but the pattern of statistical significance did not depend on the specific time window used for measuring the Pd.
A one-sample t-test of the contralateral-ipsilateral difference versus zero revealed that the voltage was significantly more positive over the contralateral hemisphere than over the ipsilateral hemisphere, relative to the location of the memory-matching probe [P7/P8: t (11) = 3.4, p < .01, PO7/PO8: t (11) = 4.0, p < .005]. Therefore, the memory-matching probe did not elicit an N2pc but instead elicited a Pd.
Discussion
In this study, we found that task-irrelevant probes matching the contents of visual working memory elicited a Pd component (indexing attentional suppression) rather than an N2pc component (indexing attentional allocation). Thus, these results indicate that attention is not inevitably captured by an item that matches the contents of visual working memory. Instead, the finding of the Pd effect suggests that the memory-matching item was actively suppressed. This is the same pattern we observed for task-irrelevant stimuli with high bottom-up salience (color singletons) in a previous study (Sawaki & Luck, 2010).
It can be challenging to link a physiological measure with a specific neurocognitive process (see Kappenman & Luck, in press), and it can be difficult to make inferences about the presence or absence of a neurocognitive process on the basis of a physiological measure (Poldrack, 2006). It is therefore important to consider exactly what conclusions can be drawn with certainty from these results. The mere fact that the voltage varied according to the location of the memory-matching item indicates that this item and the non-matching item were processed differently. Thus, we can conclude with certainty that the brain detected the fact that one of the two probe items matched memory. In addition, this difference did not take the form of a negative potential contralateral to the memory-matching item (N2pc), whereas dozens of previous studies have shown that an N2pc is observed when attention is directed to an item in a bilateral stimulus array (reviewed by Luck, in press). Thus, we can conclude with considerable confidence that attention was not reliably directed to the location of the memory-matching item. Finally, we observed a positive potential contralateral to the memory-matching item, and this potential at least coarsely resembles the Pd component previously observed contralateral to to-be-suppressed distractor items (Hickey et al., 2009; Sawaki & Luck, 2010). Although the Pd component has received much less study than the N2pc component, this result gives us some confidence that an active suppression process was applied to the memory-matching item.
These results are consistent with the signal suppression hypothesis that we developed to explain previous ERP and behavioral studies of attentional capture (Sawaki & Luck, 2010). This hypothesis proposes that stimuli with a competitive advantage—based on either bottom-up sensory factors or top-down factors such as a match with working memory—automatically generate an attend-to-me signal. In the absence of strong top-down control, this attend-to-me signal will lead to a shift of attention (indexed by the N2pc component). However, it is possible for top-down control systems to veto this shift of attention by means of active suppression of attentional priority at the location of the attend-to-me signal (indexed by the Pd component). Therefore, the actual deployment of attention is determined by the relative strengths of the attend-to-me signal and top-down control signals. This hypothesis predicts that suppression will fail and capture will occur when the attend-to-me signal is stronger than the top-down control signal. Indeed, previous studies have observed trial-by-trial variations in attentional capture (Hickey, van Zoest, & Theeuwes, 2010; Leber, 2010; Mazaheri, DiQuattro, Bengson, & Geng, 2011), and we have similarly found trial-by-trial variations in whether an N2pc or Pd will be elicited by an irrelevant distractor item (Sawaki, Geng, & Luck, in preparation). Thus, the fact that memory-matching stimuli led to capture of attention in some previous studies but not in others may reflect differences across studies in the relative strengths of the attend-to-me signal and the top-down control signal.
Because the Pd and N2pc components have opposite polarities and similar scalp distributions, they will cancel each other if they are both equally strong at a given moment in time. The average ERP waveform therefore indicates the relative balance of N2pc and Pd. Thus, it is possible that both N2pc and Pd were present initially in the present study, but they cancelled each other until the time at which a clear Pd was visible (ca. 220 ms poststimulus). This could explain why the apparent onset latency of the Pd effect is later in this experiment than it was for salient irrelevant singletons in the study of Sawaki and Luck (2010), for which the Pd effect began at approximately 120 ms. However, these differences in Pd onset latency between studies could instead reflect differences in the amount of time needed by the visual system to determine that an item should be suppressed because the item is task-irrelevant (which may be faster for salient singletons that share no features with the target than for memory-matching items). In either scenario, however, the present results demonstrate that the memory-matching probe item ultimately triggered a Pd component, with no evidence of an initial period during which this item out-competed the non-matching item for control over attention. Thus, under these conditions, the visual system was able to overcome the biasing effects of a match between a sensory input and a working memory representation.
In addition, our reliance on contralateral-minus-ipsilateral difference waves makes it possible that the ERP lateralization observed here is not a Pd to the memory-matching item but is instead an N2pc to the non-matching item on the opposite side of the display. Indeed, Woodman and Luck (2007) demonstrated that attention can sometimes be biased away from memory-matching items. In that study, however, there was a benefit to strategically deploying attention toward a non-memory-matching stimulus, because the search target was always a non-matching item. In the present study, in contrast, there was no such benefit, and it is unlikely that observers strategically shifted attention to the non-matching item.
One might suppose that no suppression should have been needed for memory-matching probe items in the present study because memory targets were not presented together with memory-matching probe items. However, because the memory task was relatively difficult (memory accuracy rate was 78.3%), the participants needed to maintain their focus on maintaining the orientation representation in visual working memory. If attention were captured by the memory-matching probe, this may have made it difficult for participants to retain the precise orientation of the target rectangle in memory. Therefore, suppression of the memory-matching probe item may have been helpful to prevent degradation of the representation of the task-relevant information in working memory. In addition, memory-matching items might elicit eye movements (Olivers et al., 2006), and suppression may have been useful in avoiding unwanted eye movements that would lead to automatic working memory encoding (Hollingworth, Richard, & Luck, 2008).
In the present study, the uncued item in the sample array had the same color as the non-matching probe in the probe array (which was necessary to avoid sensory confounds). One might argue that the uncued item was actively inhibited to successfully remember the orientation of the cued item, as in negative priming experiments (Fox, 1995) and that the Pd therefore reflects suppression of the non-matching probe, primed by the active suppression of the uncued item. However, this is very unlikely. First, if the non-matching probe had been actively inhibited, an opposite pattern of ERP lateralization would have been observed. That is, this hypothesis would predict a more positive voltage contralateral to the location of the non-matching item, whereas we observed a more positive voltage contralateral to the location of the matching item. In addition, observers were asked to store the orientation of the cued item in memory, and the color of the item was task-irrelevant, so there would be no need to suppress the color of the uncued item.
It should also be noted that the items in the test array had the same color as the to-be-remembered rectangle. It is therefore possible, in principle, that the observers actively searched for this color. This is also very unlikely, because actively attending to the color of the cued item would have led to a more negative voltage (i.e., N2pc) rather than a more positive voltage (i.e., Pd) to the memory-matching item. Moreover, the color of the test display was irrelevant to the task, and no obvious benefit would be gained by retaining the color of the sample item in memory. In addition, because we are not trying to draw any conclusions about the automaticity of the color encoding, the possibility that subjects may have voluntarily attended to the color does not impact our conclusions.
Some previous studies reported that attention is automatically captured by items that match the contents of visual working memory (Downing, 2000; Kumar et al, 2009; Pan et al, 2009; Soto et al, 2005, 2006, 2007; Soto & Humphreys, 2006, 2008, 2009), whereas other studies obtained no evidence of memory-driven attentional capture (Downing & Dodds, 2004; Houtkamp & Roelfsema, 2006; Woodman & Luck, 2007). These studies focused primarily on RT measures, making it difficult to know whether the lack of evidence for memory-driven attentional capture in some studies occurred because the memory match was not detected, because the memory match did not lead to increased salience, or because the salience was overridden by active suppression. Kumar et al., (2009) used ERP recordings to investigate this issue, and they showed that the N2pc response to a visual search target was modulated by a memory-matching distractor. However, this study could not isolate the ERP response to the memory-matching distractor because the visual search target and the memory-matching distractor were always presented simultaneously. Consequently, it is impossible to know whether attention was directed to the memory-matching distractor or whether the presence of this distractor simply modulated the allocation of attention to the target. In addition, the memory-matching item was closer to the target when it was on the same side as the target, which may have necessitated additional filtering that would not have been needed in the present study. Nonetheless, given that several behavioral studies have found that memory-matching items capture attention (e.g., Soto et al., 2008), it is possible that attention was indeed captured by the memory-matching item in the Kumar et al. study, producing an N2pc. If so, the difference between that study and the present study could be explained by the relative balance between the attend-to-me signal and top-down control signals. Additional research is needed to understand the factors that control this balance, both from trial to trial and from experiment to experiment.
Acknowledgments
This study was supported by grant R01MH076226 to S.J.L. from the National Institute of Mental Health and by a postdoctoral fellowship to R.S. from the Japan Society for the Promotion of Science.
References
- American Electroencephalographic Society Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Bacon WF, Egeth HE. Overriding stimulus-driven attentional capture. Perception & Psychophysics. 1994;55:485–496. doi: 10.3758/bf03205306. [DOI] [PubMed] [Google Scholar]
- Carlisle NB, Woodman GF. When Memory Is Not Enough: Electrophysiological Evidence for Goal-dependent Use of Working Memory Representations in Guiding Visual Attention. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2011.21602. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. Journal of Neurophysiology. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Downing PE. Interactions between visual working memory and selective attention. Psychological Science. 2000;11:467–473. doi: 10.1111/1467-9280.00290. [DOI] [PubMed] [Google Scholar]
- Downing PE, Dodds CM. Competition in visual working memory for control of search. Visual Cognition. 2004;11:689–703. [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Involuntary attentional capture is determined by task set: evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 2008;20:1423–1433. doi: 10.1162/jocn.2008.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk CL, Remington R. Selectivity in distraction by irrelevant featural singletons: Evidence for two forms of attentional capture. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:847–858. doi: 10.1037//0096-1523.24.3.847. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Folk CL, Remington RW, Wright JH. The structure of attentional control: Contingent attentional capture by apparent motion, abrupt onset, and color. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:317–329. doi: 10.1037//0096-1523.20.2.317. [DOI] [PubMed] [Google Scholar]
- Fox E. Negative priming from ignored distractors in visual selection: A review. Psychonomic Bulletin & Review. 1995;2:145–173. doi: 10.3758/BF03210958. [DOI] [PubMed] [Google Scholar]
- Han SW, Kim M. Do the contents of working memory capture attention? Yes, but cognitive control matters. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1292–1302. doi: 10.1037/a0016452. [DOI] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. Electrophysiological Indices of Target and Distractor Processing in Visual Search. Journal of Cognitive Neuroscience. 2009;21:760–775. doi: 10.1162/jocn.2009.21039. [DOI] [PubMed] [Google Scholar]
- Hickey C, McDonald JJ, Theeuwes J. Electrophysiological evidence of the capture of visual attention. Journal of Cognitive Neuroscience. 2006;18:604–613. doi: 10.1162/jocn.2006.18.4.604. [DOI] [PubMed] [Google Scholar]
- Hickey C, van Zoest W, Theeuwes J. The time course of exogenous and endogenous control of covert attention. Experimental Brain Research. 2010;201:789–796. doi: 10.1007/s00221-009-2094-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth A, Luck SJ. The role of visual working memory (VWM) in the control of gaze during visual search. Attention, Perception & Psychophysics. 2009;71:936–949. doi: 10.3758/APP.71.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth A, Richard AM, Luck SJ. Understanding the function of visual short-term memory: Transsaccadic memory, object correspondence, and gaze correction. Journal of Experimental Psychology: General. 2008;137:163–181. doi: 10.1037/0096-3445.137.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkamp R, Roelfsema PR. The effect of items in working memory on the deployment of attention and the eyes during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:423–442. doi: 10.1037/0096-1523.32.2.423. [DOI] [PubMed] [Google Scholar]
- Hyun JS, Woodman GF, Vogel EK, Hollingworth A, Luck SJ. The comparison of visual working memory representations with perceptual inputs. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1140–1160. doi: 10.1037/a0015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappenman ES, Luck SJ. ERP components: The ups and downs of brainwave recordings. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. Oxford University Press; New York: in press. [Google Scholar]
- Kiss M, Jolicœur P, Dell'acqua R, Eimer M. Attentional capture by visual singletons is mediated by top-down task set: New evidence from the N2pc component. Psychophysiology. 2008;45:1013–1024. doi: 10.1111/j.1469-8986.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Soto D, Humphreys GW. Electrophysiological evidence for attentional guidance by the contents of working memory. The European Journal of Neuroscience. 2009;30:307–317. doi: 10.1111/j.1460-9568.2009.06805.x. [DOI] [PubMed] [Google Scholar]
- Lavie N. Perceptual load as a necessary condition for selective attention. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:451–468. doi: 10.1037//0096-1523.21.3.451. [DOI] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused? : Selective attention under load. Trends in Cognitive Sciences. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Lavie N, Tsal Y. Perceptual load as a major determinant of the locus of selection in visual attention. Perception & Psychophysics. 1994;56:183–197. doi: 10.3758/bf03213897. [DOI] [PubMed] [Google Scholar]
- Leber AB. Neural predictors of within-subject fluctuations in attentional control. Journal of Neuroscience. 2010;30:11458–11465. doi: 10.1523/JNEUROSCI.0809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber AB, Egeth HE. It's under control: Top-down search strategies can override attentional capture. Psychonomic Bulletin & Review. 2006;13:132–138. doi: 10.3758/bf03193824. [DOI] [PubMed] [Google Scholar]
- Leblanc E, Prime DJ, Jolicoeur P. Tracking the location of visuospatial attention in a contingent capture paradigm. Journal of Cognitive Neuroscience. 2008;20:657–671. doi: 10.1162/jocn.2008.20051. [DOI] [PubMed] [Google Scholar]
- Lien M, Ruthruff E, Goodin Z, Remington RW. Contingent attentional capture by top-down control settings: converging evidence from event-related potentials. Journal of Experimental Psychology: Human Perception and Performance. 2008;34:509–530. doi: 10.1037/0096-1523.34.3.509. [DOI] [PubMed] [Google Scholar]
- Lins OG, Picton TW, Berg P, Scherg M. Ocular artifacts in EEG and event-related potentials. I: Scalp topography. Brain Topography. 1993;6:51–63. doi: 10.1007/BF01234127. [DOI] [PubMed] [Google Scholar]
- Luck SJ. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components. In: Luck SJ, Kappenman ES, editors. Oxford Handbook of ERP Components. Oxford University Press; New York: in press. [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994a;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994b;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: Psychophysical and electrophysiological evidence for early selection. Journal of Experimental Psychology: Human Perception and Performance. 1994;20:887–904. doi: 10.1037//0096-1523.20.4.887. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, DiQuattro NE, Bengson J, Geng JJ. Pre-stimulus activity predicts the winner of top-down vs. bottom-up attentional selection. PLoS One. 2011;6:e16243. doi: 10.1371/journal.pone.0016243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivers CNL. What drives memory-driven attentional capture? The effects of memory type, display type, and search type. Journal of Experimental Psychology: Human Perception and Performance. 2009;35:1275–1291. doi: 10.1037/a0013896. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Meijer F, Theeuwes J. Feature-based memory-driven attentional capture: visual working memory content affects visual attention. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:1243–1265. doi: 10.1037/0096-1523.32.5.1243. [DOI] [PubMed] [Google Scholar]
- Pan Y, Xu B, Soto D. Dimension-based working memory-driven capture of visual selection. Quarterly Journal of Experimental Psychology. 2009;62:1123–1131. doi: 10.1080/17470210802624353. [DOI] [PubMed] [Google Scholar]
- Peters JC, Goebel R, Roelfsema PR. Remembered but unused: the accessory items in working memory that do not guide attention. Journal of Cognitive Neuroscience. 2009;21:1081–1091. doi: 10.1162/jocn.2009.21083. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rodríguez Holguín S, Doallo S, Vizoso C, Cadaveira F. N2pc and attentional capture by colour and orientation-singletons in pure and mixed visual search tasks. International Journal of Psychophysiology. 2009;73:279–286. doi: 10.1016/j.ijpsycho.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Driver J. Attentional preparation for a lateralized visual distractor: behavioral and fMRI evidence. Journal of Cognitive Neuroscience. 2006;18:522–538. doi: 10.1162/jocn.2006.18.4.522. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, Luck SJ. A common neural mechanism for preventing and terminating attention. in preparation. [DOI] [PMC free article] [PubMed]
- Sawaki R, Luck SJ. Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend-to-me signal. Attention, Perception & Psychophysics. 2010;72:1455–1470. doi: 10.3758/APP.72.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. NeuroImage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Soto D, Heinke D, Humphreys GW, Blanco MJ. Early, involuntary top-down guidance of attention from working memory. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:248–261. doi: 10.1037/0096-1523.31.2.248. [DOI] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. Automatic guidance of attention from working memory. Trends in Cognitive Sciences. 2008;12:342–348. doi: 10.1016/j.tics.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Soto D, Humphreys GW. Seeing the content of the mind: enhanced awareness through working memory in patients with visual extinction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4789–4792. doi: 10.1073/pnas.0510718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Humphreys GW. Stressing the mind: the effect of cognitive load and articulatory suppression on attentional guidance from working memory. Perception & Psychophysics. 2008;70:924–934. doi: 10.3758/pp.70.5.924. [DOI] [PubMed] [Google Scholar]
- Soto D, Humphreys GW. Automatic selection of irrelevant object features through working memory. Experimental Psychology. 2009;56:165–172. doi: 10.1027/1618-3169.56.3.165. [DOI] [PubMed] [Google Scholar]
- Soto D, Humphreys GW, Heinke D. Working memory can guide pop-out search. Vision Research. 2006;46:1010–1018. doi: 10.1016/j.visres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Soto D, Humphreys GW, Rotshtein P. Dissociating the neural mechanisms of memory-based guidance of visual selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17186–17191. doi: 10.1073/pnas.0703706104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J. Exogenous and endogenous control of attention: The effect of visual onsets and offsets. Perception & Psychophysics. 1991;49:83–90. doi: 10.3758/bf03211619. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down search strategies cannot override attentional capture. Psychonomic Bulletin & Review. 2004;11:65–70. doi: 10.3758/bf03206462. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Top-down and bottom-up control of visual selection. Acta psychologica. 2010;135:77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Burger R. Attentional control during visual search: The effect of irrelevant singletons. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1342–1353. doi: 10.1037//0096-1523.24.5.1342. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Do the contents of visual working memory automatically influence attentional selection during visual search? Journal of Experimental Psychology: Human Perception and Performance. 2007;33:363–377. doi: 10.1037/0096-1523.33.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]