Abstract
Ischemic kidney injury often occurs in the context of multiple organ failure and sepsis. Here, we review the major components of this dynamic process, which involves hemodynamic alterations, inflammation, and endothelial and epithelial cell injury, followed by repair that can be adaptive and restore epithelial integrity or maladaptive, leading to chronic kidney disease. Better understanding of the cellular pathophysiological processes underlying kidney injury and repair will hopefully result in the design of more targeted therapies to prevent the injury, hasten repair, and minimize chronic progressive kidney disease.
Introduction
Acute kidney injury (AKI), formerly known as “acute renal failure,” has been traditionally described as a rapid (ranging from hours to weeks, to less than 3 months) decrease in kidney function as measured by increases in serum creatinine. The Acute Kidney Injury Network (AKIN) defined it more precisely as “An abrupt (within 48 hours) reduction in kidney function,” and offered specific laboratory and clinical values to guide diagnosis (1). The incidence of AKI in hospitalized patients has generally been reported to be in the 2%–7% range, with an incidence of 5% to greater than 10% in the ICU population (2, 3), often in the context of multiorgan disease and sepsis, and is steadily increasing overall. Incidence rates are even higher when the AKIN definition is used (4). The incidence of AKI has grown steadily in many demographic groups, and the yearly community incidence of AKI was estimated to be 550 per 100,000 individuals in 2003 (5), higher than the yearly incidence of stroke (6). Despite advances in preventive strategies and support measures, AKI continues to be associated with high morbidity and mortality, particularly in those admitted to the ICU, where in-hospital mortality rates may exceed 50%. In addition to mortality rates — generally reported to be in the 30%–70% range — there are chronic consequences even if the patients survive their acute illness, with a high risk of developing or exacerbating chronic kidney disease (CKD) and hastened development of end-stage renal disease (ESRD) (7–9).
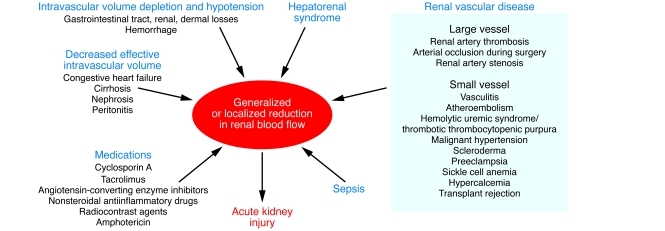
Renal ischemia/reperfusion injury (IRI), a common cause of AKI (10–12), results from a generalized or localized impairment of oxygen and nutrient delivery to, and waste product removal from, cells of the kidney (13). There is mismatch of local tissue oxygen supply and demand and accumulation of waste products of metabolism. As a result of this imbalance, the tubular epithelial cells undergo injury and, if it is severe, death by apoptosis and necrosis (acute tubular necrosis [ATN]), with organ functional impairment of water and electrolyte homeostasis and reduced excretion of waste products of metabolism. There are many pathophysiological states and medications that can contribute to generalized or localized ischemia (Figure 1).
Figure 1. Causes of reduction in generalized or regional renal blood flow (RBF).
Various pathophysiological states and medications can contribute to reduction of RBF, causing generalized or localized ischemia to the kidney leading to AKI. This figure represents a partial list and points to ischemia as being a common pathway in a variety of clinical states affecting the kidney.
In this review, we summarize the important components of the cellular pathophysiology in AKI associated with ischemia. We also indicate what is known about the repair process and how this process can be maladaptive, leading to fibrosis and CKD.
Endothelium and vascular components of injury
The endothelial and smooth muscle cells of the microcirculation play critical roles in the pathophysiology of AKI. While an overall decrease in renal blood flow (RBF) of approximately 40%–50% has been observed in poorly functioning kidney transplant allografts (14), in many cases in animals and humans a decrease in total RBF alone cannot entirely account for the reduction in glomerular filtration rate during an episode of AKI (15, 16). Of greater importance are the regional alterations in RBF that occur during AKI (13). Blood flow to the outer medulla is reduced disproportionately to the reduction in total kidney perfusion in animal models of AKI (17, 18) and likely in humans following ischemic injury to the kidney. Endothelial cells are important determinants of vascular tone, leukocyte function, and smooth muscle responsiveness (19). The endothelium is injured, and small arterioles in postischemic kidney vasoconstrict more than do vessels from normal kidney in response to increased tissue levels of endothelin-1, angiotensin II, thromboxane A2, prostaglandin H2, leukotrienes C4 and D4, and adenosine as well as sympathetic nerve stimulation (20–23). There is also decreased vasodilatation in response to acetylcholine, bradykinin, and nitric oxide (24, 25). Vasoconstriction is amplified due, in part, to reduced production of nitric oxide and other vasodilatory substances (25) by the damaged endothelial cell. These effects on the arterioles are augmented by vasoactive cytokines including TNF-α, IL-1β, IL-6, IL-12, IL-15, IL-18, IL-32, and endothelin, generated as a result of the enhanced leukocyte-endothelial adhesion and leukocyte activation that are characteristic of ischemic injury (26). Enhanced vasoconstriction together with small vessel occlusion due to endothelial-leukocyte interactions and activation of the coagulation system results in local compromise of the microcirculation and regional ischemia especially in the outer medulla. Tubulo-glomerular feedback also likely contributes to a functional pre-glomerular arteriolar vasoconstrictive response as a result of macula densa sensing of more solute delivery to the distal nephron due to inadequate sodium reabsorption in the injured, more proximal parts of the tubule (27). This feedback reduces glomerular forces for filtration.
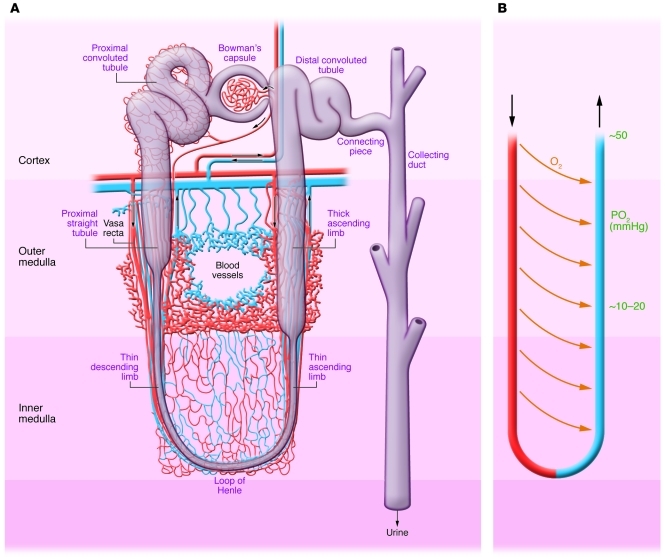
Local blood flow to the outer medulla, reduced due to arteriolar vasoconstriction, is further compromised by local edema. This results in interference with flow to the pars recta of the proximal tubule and the thick ascending limb, which are already normally hypoxic due to the countercurrent exchange properties of the vasa recta (Figure 2A and ref. 28). The anatomy of the capillaries in the outer medulla makes them very vulnerable to occlusion (Figure 2A and ref. 29). The resultant effects on oxygen and nutrient delivery to the epithelial cells results in damage particularly to the pars recta, whose cells cannot convert from oxidative to glycolytic metabolism (30).
Figure 2. Normal nephron, corticomedullary oxygen gradient, and outer medullary microvascular anatomy.
(A) Anatomy of nephron with regions identified. Outer medulla vasculature is shown with capillaries in red and venous system in blue. (B) The vasa recta with countercurrent exchange of oxygen resulting in a gradient of decreasing oxygen tension.
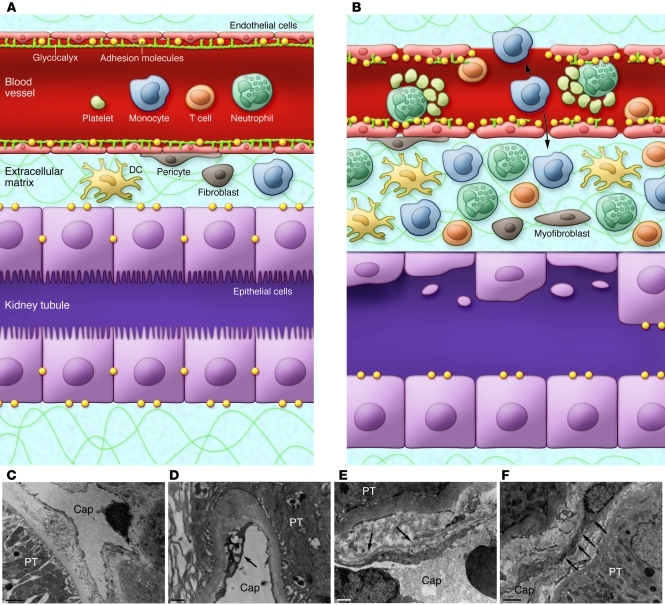
The endothelial cell contributes to the pathology of ischemic AKI in many additional ways (refs. 31, 32, and Figure 3). There are enhanced endothelium-leukocyte interactions due to increased expression of cell adhesion molecules such as ICAM-1 on damaged endothelial cells and increased expression of counterreceptors on leukocytes (33). This results in activation of the leukocytes, obstruction of capillaries and postcapillary venules, further activation and transmigration of leukocytes, production of cytokines, and a vigorous proinflammatory state (Figure 3, A and B, and ref. 26). Damage to the endothelium results in loss of the glycocalyx, disruption of the actin cytoskeleton, alteration of endothelial cell-cell contacts, and breakdown of the perivascular matrix, all of which culminate in increased microvascular permeability during AKI and loss of fluid into the interstitium (31, 32). Two-photon microscopy has revealed loss of endothelial barrier function in the cortex within two hours of reperfusion in the rodent (34). With reperfusion, a partial transient compromise of the patency of the peritubular capillaries can also be seen. Abnormalities in the capillaries of the human postischemic kidney are visible by transmission electron microcopy (Figure 3, C–F).
Figure 3. Endothelial injury in ischemia/reperfusion AKI.
(A) Normal epithelium and endothelium separated by a small interstitial compartment. A glycocalyx coats the endothelium. (B) Ischemia/reperfusion causes swelling of endothelial cells; disruptions of the glycocalyx and endothelial monolayer; and upregulation of adhesion molecules such as ICAMs, VCAMs, and selectins, resulting in enhanced leukocyte-endothelium interactions. There is formation of microthrombi, and some leukocytes migrate through the endothelial cells into the interstitial compartment. The interstitial compartment is expanded with enhanced numbers of inflammatory cells and interstitial edema forms. (C) Transmission electron microscopy of normal human peritubular capillary (Cap). (D–F) Acute tubular necrosis. The peritubular capillaries (PT) show vacuolar degeneration of the endothelial cell (arrow in D), thickening and multilayer basement membrane formation (arrows in E), and attachment and penetration of monocyte-like cells (arrows in F) in the interstitial region. Scale bars: 2 μm (C and F); 1 μm (D and E).
The number of microvessels in the inner stripe of the outer medulla declines after IRI, potentially facilitated by the downregulation of angiogenic factors such as VEGF and upregulation of inhibitors of angiogenesis (31, 35). This reduced number of vessels is associated with chronic hypoxia (31), which can be expected to lead to increased tubular injury and tubulointerstitial fibrosis. This can be reinforcing and progressive, since increased fibrosis will further compromise the microvasculature and further decrease the availability of oxygen and nutrients to the tubules, enhance tubular stress and epithelial cell injury, possibly interfere with normal regenerative processes, and lead to further fibrosis (31, 36). Remaining vessels may have blood flow compromised by endothelial cell swelling. There are also other functional consequences of vessel dropout, including the development of salt-sensitive hypertension and altered concentrating ability, perhaps direct reflections of local areas of hypoxia, especially in the medulla (31).
Inflammation
The immune response.
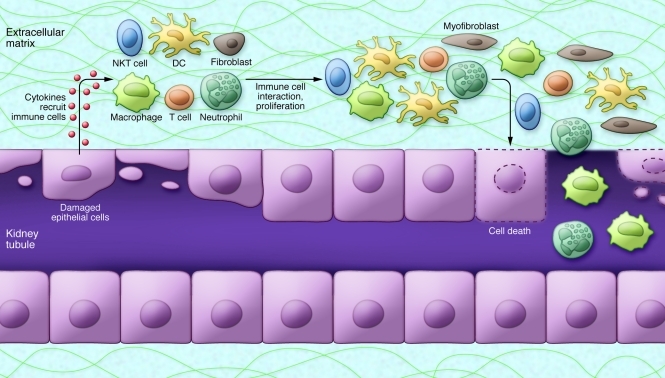
Both innate and adaptive immune responses are important contributors to the pathology of ischemic injury. The innate component is responsible for the early response to injury in a non-antigen-specific fashion and comprises neutrophils, monocytes/macrophages, DCs, NK cells, and natural killer T (NKT) cells. The adaptive component, activated by specific antigens, is initiated within hours and lasts over the course of several days after injury. The adaptive response includes DC maturation and antigen presentation, T lymphocyte proliferation and activation, and T to B lymphocyte interactions (Figure 4).
Figure 4. Immune response in ischemic AKI.
The injured tubular epithelium releases proinflammatory cytokines and chemokines, which aid in recruiting immune cells. Epithelial cells also express adhesion molecules, TLRs, and T cell costimulatory molecules, which activate the immune cells and amplify the inflammatory responses. Neutrophils, macrophages, and natural killer T (NKT) cells cause direct injury to tubular epithelial cells. DCs are involved in both the innate and adaptive immune responses through secretion of inflammatory cytokines and presentation of antigens to T lymphocytes.
Tubular cells contribute to inflammation.
The tubular epithelium is not merely a passive victim of injury but also an active participant in the inflammatory response in kidney IRI. In addition to generating proinflammatory and chemotactic cytokines such as TNF-α, MCP-1, IL-8, IL-6, IL-1β, TGF-β, RANTES, and epithelial neutrophil-activating protein 78 (ENA-78), which activate inflammatory cells (26), tubular cells also express Toll-like receptors (TLRs), complement and complement receptors, and costimulatory molecules, which regulate T lymphocyte activity (Figure 4).
TLRs are a family of evolutionarily conserved transmembrane receptors and prototypic pattern recognition receptors (PRRs), which detect exogenous microbial products (37) or endogenous ligands from host material released during injury, including high-mobility group box 1, hyaluronan, and biglycan (38). During AKI, renal tubular epithelial cells express increased amounts of both TLR2 and TLR4, which modulate the degree of injury (39). Activation of TLRs initiates a proinflammatory response marked by the release of cytokines/chemokines, which attract inflammatory cells. Although evidence implicates TLRs in the pathophysiology of ischemic AKI, the processes involved in controlling the upregulated protein expression and the cellular localization of the molecules are not clearly defined, nor has this been well studied in humans.
In addition to its role in generating mediators that contribute to the inflammatory response, the proximal tubular epithelium expresses MHC II and therefore can present antigen to T cells and express costimulatory molecules (40). Proximal tubule cells respond to T cell ligands through activation of cell surface receptors (41). CD4+ cells express CD40 ligand, which interacts with CD154 to stimulate MAPK activation, MCP-1 and IL-8 production, and TNF receptor–associated factor 6 (TRAF6) recruitment in proximal tubule cells (41). CD40 ligation also induces RANTES production by human renal tubular epithelia, an effect that is amplified by production of IL-4 and IL-13 by Th2 cells, a subpopulation of T cells (42). Ischemia/reperfusion increases expression of B7-1 and B7-2, costimulatory tubule cell molecules that interact with CD28 on T lymphocytes and facilitate cytokine production (43).
Immune/inflammatory cell subgroups.
Neutrophils, monocytes/macrophages, DCs, and T cells are important contributors to ischemic kidney injury and repair. Neutrophils attach to the activated endothelium and accumulate in the kidney both in animal models and in human AKI (33, 44–46), particularly in the peritubular capillary network of the outer medulla, as early as 30 minutes after reperfusion. They produce proteases, myeloperoxidase, reactive oxygen species, and cytokines, which leads to increased vascular permeability and reduced tubular epithelial and endothelial cell integrity (47), aggravating kidney injury (46). IL-17 produced by neutrophils regulates IFN-γ–mediated neutrophil migration into the mouse kidney after IRI (48).
Two types of blood monocytes have been identified in mice. Monocytes (49), having a CD11b+CCR2loGr-1–Ly6C–CX3CR1hi phenotype, migrate to uninjured tissues rapidly upon leaving the bone marrow and differentiate into resident macrophages and DCs. In contrast, a second monocyte subset (CD11b+CCR2hiLy6ChiGr-1intCX3CR1lo) infiltrates inflamed kidney tissue and differentiates into macrophages and DCs. Migration to the tissue and differentiation to resident, “inflamed” macrophages (M1 type) or DCs is determined by differential pathological conditions. Macrophage numbers increase in the mouse kidney at 1 hour after reperfusion, peaking at 24 hours and persisting for 7 days (48). This infiltration is facilitated by the CCR2 (48) and CX3CR1 signaling pathways (50). M1 macrophages produce large amounts of reactive oxygen and nitrogen intermediates and inflammatory cytokines (including IL-1β and TNF-α) that drive a polarized Th1 immune response. M2 macrophages are diverse, generally believed to be “pro-repair,” and can be generated when monocytes are exposed to IL-4 or IL-13, immune complexes, IL-10, and glucocorticoid hormones. M2 macrophages mainly provoke a Th2 response.
In mice, depletion of kidney and spleen macrophages using liposomal clodronate prior to renal IRI prevented AKI, while adoptive transfer of macrophages restored the AKI response (51), attesting to the importance of these cells in injury to the organ. Macrophages are also important for tissue repair, however. At 3–5 days after the initial injury, when the tubule cell proliferation and repair process is well established, pro-repair M2 macrophages expressing high levels of mannose receptor and arginase-1 predominate in the tissue (52).
A network of DCs and macrophages exists in the normal kidney serving to constantly sample the environment (53). During tubular injury, DCs are activated and can in turn activate naive T cells by presenting antigen, expressing costimulatory molecules, and producing cytokines, thus linking the innate immune response to adaptive immunity. A kidney DC subset has also been shown to play an important role in recovery or regeneration processes after IRI (53). Notably, there is some controversy as to the distinction between DCs and tissue macrophages (54).
Both the early and later phases of AKI are characterized by infiltration of T lymphocytes, which, like macrophages and DCs, can facilitate injury but also promote repair after IRI (55). CD4+ cells, in the presence of costimulation with CD28, have been implicated in the potentiation of IRI (56). By contrast, T cell receptor β (TCRβ)+CD4+CD25+Foxp3+ regulatory T cells (Tregs) are antiinflammatory lymphocytes that infiltrate the kidney after 3 or 10 days in the mouse model of ischemia and facilitate repair after IRI (57). Rag1–/– mice lack T and B cells and are not protected from AKI induced by ischemia (55, 58). Multiple groups have detected no difference in serum creatinine 24 hours after IRI in mice with depletion of either TCRαβ or TCRγδ (55, 59, 60), although some have found reduced structural damage (60, 61) and/or improved survival and lower serum creatinine (60) at later time points after reperfusion.
Complement
The complement system is an important contributor to inflammation after IRI, but the kidney is unique in that activation after IRI occurs predominantly, if not exclusively, by the alternative pathway (62). Complement upregulates expression of endothelial cell adhesion molecules (63). DCs covalently fix marked amounts of macrophage-derived C3, the most abundant complement protein in the circulation, on their surface (64). This C3 binding promotes maturation of DCs, which in turn activate T cell responses.
Deposition of C3 along renal tubular cells can be seen as early as 6 hours after reperfusion in a mouse isograft model. This is associated with dislocation and decrease of complement inhibitor Crry on the tubular basolateral surface (65). Heterozygosity of Crry in mice increases susceptibility to severe ischemic injury (66). Crry, together with factor H, a serum alternative pathway regulatory protein, regulates complement activation on the basolateral surface of tubule epithelial cells. Congenital deficiency of Crry or reduced basolateral expression on injured cells permits spontaneous complement activation and tubular injury (67). Selective inhibition of the alternative pathway protects the kidney from ischemic injury (68, 69). CXC chemokine production by tubular epithelial cells requires activation of the alternative complement pathway (70). In addition, C5b-9 complex (membrane attack complex [MAC]) and C5a also contribute to ischemic AKI (71).
Endogenous inhibitors of inflammation
There are several endogenous inhibitors of inflammation that limit damage to the kidney following ischemia. Ischemia results in an increase in epithelial cell heme oxygenase–1, which confers an antiinflammatory response and protects against IRI (72). Tamm-Horsfall protein (THP) also confers a protective effect, perhaps by downregulating expression of TLR4 in proximal tubular cells in the outer medulla (73).
Resolvins (Rvs) and protectin D1 (PD1) are natural antiinflammatory compounds that are derived from the omega-3 fatty acid docosahexaenoic acid. Administration of D series resolvins (RvDs) or PD1 to mice before ischemia resulted in reduced leukocyte accumulation, TLR-mediated activation of macrophages, functional and morphological kidney injury, and postischemic fibrosis (74). Lipoxin A4 (75) and hepatic growth factor (HGF) also limit renal IRI as endogenous immunoregulatory factors (76). Single Ig IL-1–related receptor (SIGIRR), also known as Toll–IL-1 receptor 8 (TIR8), is a member and inhibitor of the TLR/IL-R family that is highly expressed in the kidney tubular cells and intrarenal myeloid cells. SIGIRR deficiency aggravated postischemic AKI in rodent models of IRI in association with increased innate immune signaling in intrarenal DCs and monocytes (77).
Characteristics of tubular injury
Proximal tubule.
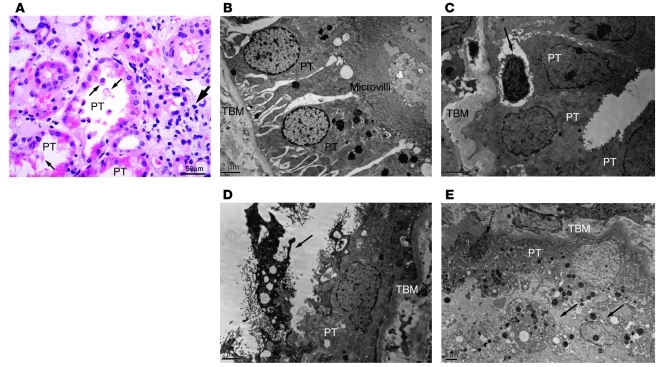
Epithelial cell injury associated with ischemia/reperfusion is most apparent in the S3 segment of the proximal tubule in most animal models of ischemia (78). The appearance of casts and tubular cells in the urine confirms that there is tubular cell damage and death by apoptosis and/or necrosis. While there is some controversy as to the relative extent of proximal versus distal tubule injury in humans (79), more recent studies using biomarkers of proximal tubule injury, such as kidney injury molecule–1 (KIM-1) ectodomain in the urine following ischemia, reveal significant proximal tubule injury in humans (80, 81). While the degree of histologic injury on biopsy is variable, this may be related to technical limitations such as infrequent sampling and the fact that biopsy needle samples primarily capture the cortex, missing injury to the outer medulla. Clearly, in many patients there are very dramatic signs of tubule epithelial injury on biopsy (Figure 5, B–E).
Figure 5. Pathology after ischemia in humans.
(A) Outer medulla in human ischemic AKI. The proximal tubules (PT) lose brush border, and cells are released into the lumen (thin arrows). Inflammatory cells are seen in the interstitial compartment (thick arrow). Light microscopy: original magnification, ×400; scale bar: 50 μm. (B) Electron microscopy sections through normal human proximal tubules. (C–E) Human AKI. (C) In ischemic AKI, lymphocytes are seen infiltrating into the tubule wall (arrow). (D and E) Loss of brush border and contraction of the cell (arrow in D and dashed arrow in E) with necrosis (D and E) of proximal tubules are shown. Cellular debris is apparent in the lumen (solid arrows in E). Scale bars: 2 μm (B–E). TBM, tubular basement membrane.
The processes of injury and repair to the kidney epithelium are depicted schematically in Figure 6A. Ischemia results in rapid loss of cytoskeletal integrity and cell polarity (82). There is shedding of the proximal tubule brush border (44); loss of polarity with mislocalization of adhesion molecules and other membrane proteins such as the Na+K+-ATPase and β-integrins (83, 84); cytokine-induced disruption of cell-matrix adhesion dependent on β integrins (26); and disruption of cell-cell interactions at adherent and tight junctions (12, 85). There are also changes in actin localization from apical to lateral cell membrane (86, 87).
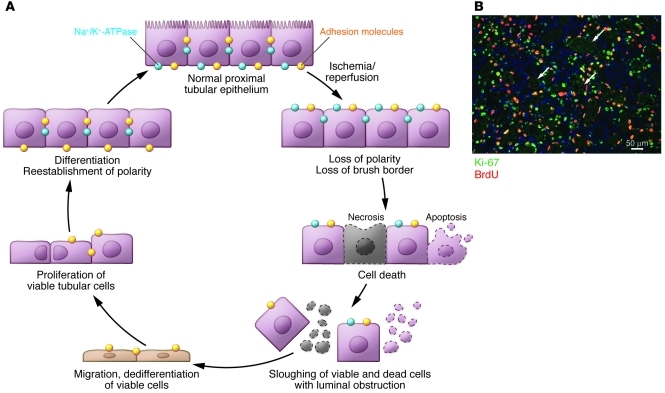
Figure 6. Normal repair in ischemic AKI.
(A) The current understanding of tubular injury and repair after ischemic AKI. With IRI, the normally highly polar epithelial cell loses its polarity and brush border with proteins mislocated on the cell membrane. With increasing time/severity of ischemia, there is cell death by either necrosis or apoptosis. Some of the necrotic debris is released into the lumen. Viable epithelial cells migrate and cover denuded areas of the basement membrane. These cells undergo division and replace lost cells. Ultimately, the cells go on to differentiate and reestablish the normal polarity of the epithelium. (B) The photomicrograph shows a vigorous repair process after ischemic injury in the mouse. Cells that have entered the cell cycle are stained with Ki-67. Cells specifically in the S phase of the cell cycle have taken up BrdU, which had been injected into the animal. Arrows point to some of the cross sections of tubules that are filled with cellular debris. Scale bar: 50 μm. Image in B reproduced with permission from Nature Medicine (128).
Under normal circumstances, epithelial cells communicate with one another via tight junctions and adhesion junctions, which are regulated by the F-actin cytoskeleton. In turn, the cytoskeleton is regulated by the Rho family of GTPases, which are activated in response to ischemia. Downstream effectors of Rho GTPases include the Rho-associated coiled-coil–forming protein kinase (ROCK). ROCK is a negative regulator of the pro-survival PI3K/AKT pathway. Activation of ROCK has been implicated in increased cell apoptosis, and inhibitors of ROCK have been reported to attenuate IRI (88).
With severe injury, viable and nonviable cells are desquamated, leaving the basement membrane as the only barrier between the filtrate and the peritubular interstitium. The increase in permeability results in backleak of glomerular filtrate from the tubular lumen to the intersitium. The cells and their debris that detach from the basement membrane combine with proteins present in the tubular lumen such as THP and fibronectin (89) to form casts that can obstruct the tubule, increasing intratubular pressure; these casts are detected in the urine as a hallmark of AKI in humans.
AKI results in the activation of a large number of genes (90, 91), among which KIM-1 (90, 92) and neutrophil gelatinase-associated lipocalin (NGAL) (93) are the most highly upregulated in the proximal and distal tubules, respectively. Both are also present in the urine of animals and patients with AKI and have been found to be useful noninvasive biomarkers of injury (94, 95). While produced in the distal nephron and many other organs, NGAL is filtered and reabsorbed by the normal proximal tubule. KIM-1 is a phosphatidylserine receptor that recognizes and directs apoptotic cells to lysosomes in proximal tubular cells. It also mediates phagocytosis of necrotic cells and oxidized lipoproteins by renal proximal tubular cells. In addition to facilitating clearance of the apoptotic debris from the tubular lumen, KIM-1 may play an important role in limiting the immune response to injury, since phagocytosis of apoptotic bodies is one mechanism for limiting the proinflammatory response (96). KIM-1 has been reported to be an endogenous ligand for leukocyte mono-Ig-like receptor 5 (LMIR5), and the KIM-1–LMIR5 interaction has been implicated in neutrophil recruitment (97). The ectodomain of KIM-1 is shed into the urine of human and rodent kidneys with renal injury and serves as a biomarker for the early diagnosis of AKI in humans and rodents (98).
NGAL is an iron-transporting protein (99), and iron has been proposed to play an important role in protection of the proximal tubule. Intravenous administration of purified recombinant NGAL results in uptake by proximal tubular cells, where it inhibits apoptosis, enhances proliferation, and provides significant functional and pathological protection in murine models of renal IRI (100, 101). NGAL forms a complex with iron-binding siderophores and iron (102). Iron scavenging by deferoxamine, or apotransferrin, an endogenous iron-binding protein, protects against ischemia/reperfusion–mediated tubular injury and organ failure by abrogating superoxide formation. The mechanism underlying this effect has been postulated to be the sequestration of iron via siderophores to stop inappropriately liganded iron from producing damaging oxygen radicals (100).
Proteins upregulated in the proximal tubule and believed to be protective against injury include heme oxygenase (72) and heat shock proteins (103). Heme oxygenase activity is the rate-limiting step in the degradation of heme to biliverdin, releasing iron and carbon monoxide (CO). Many of the products of heme oxygenase action are cytoprotective. CO exerts vasorelaxant, antiinflammatory, and antiapoptotic effects.
Autophagy also plays an important role in proximal tubule cell survival after IRI in rodents (104). When the ability of the cells to undergo autophagy is blocked, the cells accumulate malformed mitochondria and ubiquitin-positive cytoplasmic inclusions, accumulate p62, and have increased propensity to become apoptotic.
Distal tubule.
The straight portion of the distal tubule, the medullary thick ascending limb (MTAL) (Figure 2A), has a close spatial association with the proximal straight tubule in the outer stripe of the outer medulla. Cells from the distal nephron are more resistant to hypoxia, ischemia, and oxidative injury and remain intact during IRI. The MTAL has a greater capacity to convert from oxidative to glycolytic metabolism when mitochondrial function is limited during reduced oxygen availability (30), and hence is better poised to adapt to the increased hypoxia that characterizes ischemia. In addition, the marked increase in ERK pathway activation (105), as well as the production of antiapoptotic Bcl-2 proteins and the reparative growth factors in distal tubular cells, which act synergistically to minimize cell death, might underlie the relative resistance to ischemic injury (106, 107). Other reparative or survival growth factors synthesized in the distal nephron, including EGF, IGF-1, and HGF, may exert paracrine effects to protect the sensitive proximal tubule from injury and promote proliferation and repair of surviving proximal tubules cells via distal-proximal cell-cell crosstalk mechanisms (106, 107).
Repair of the epithelium
Normal repair.
In contrast to the heart or brain, the kidney can completely recover from an ischemic or toxic insult that results in cell death. However, this may occur less frequently in humans than previously believed, since it has been increasingly recognized that AKI, especially when there is underlying CKD, can lead to acceleration of CKD with more rapid onset of ESRD (8). Under normal circumstances, human proximal tubule cells divide at a low rate (108). Cell proliferation balances the loss of tubular epithelial cells due to cell death or release from the basement membrane into the urine (109). This low rate of turnover changes dramatically after an ischemic or toxic insult, when there is a marked increase in cell death by necrosis and apoptosis and a vigorous response to replace these cells (Figure 6B).
There had been a debate about whether the cells that replenish the epithelial cell population after injury originate from endogenous surviving epithelial cells, bone marrow stromal cells (BMSCs), or intrarenal progenitor cells. Early hypotheses (110, 111) suggested that the cells came from surviving proximal tubule cells; however, studies subsequently suggested that bone marrow–derived cells, including hematopoietic stem cells (HSCs) and mesenchymal stem cells, directly replace the epithelial cells that have been lost (112, 113). Additional analyses (114, 115), however, clarified that bone marrow–derived cells do not directly replace the tubule epithelial cells that are lost with injury, but exert paracrine effects that facilitate repair potentially by reducing inflammation; recent data suggest that this effect may be mediated by microvesicles that transfer membrane receptors, proteins, mRNAs, microRNAs, and organelles (116–118). To determine whether intrarenal progenitor cells were the origin of proliferating tubular cells after injury, genetic fate-mapping techniques were employed in transgenic mice, and the results demonstrated that surviving tubular cells proliferate and this accounts for replenishment of the tubular epithelium after ischemia (119).
When the kidney recovers after epithelial cells are lost, the surviving cells dedifferentiate, migrate along the basement membrane, proliferate to restore cell number, and then differentiate, resulting in restoration of the functional integrity of the nephron (Figure 6 and ref. 12). To some degree, repair of the kidney parallels organogenesis both in the high rate of DNA synthesis and apoptosis and in patterns of gene expression. Molecules such as vimentin (110) and neural cell adhesion molecule (NCAM) (120), which are expressed in the metanephric mesenchyme during kidney development but not in the mature nephron, are abundantly expressed in proximal tubules after IRI. The factors responsible for, and the significance of, reversion to a less-differentiated cell phenotype and its relationship to the proliferative and migratory response after renal epithelial cell injury are poorly understood.
Abnormal repair and progressive CKD after AKI.
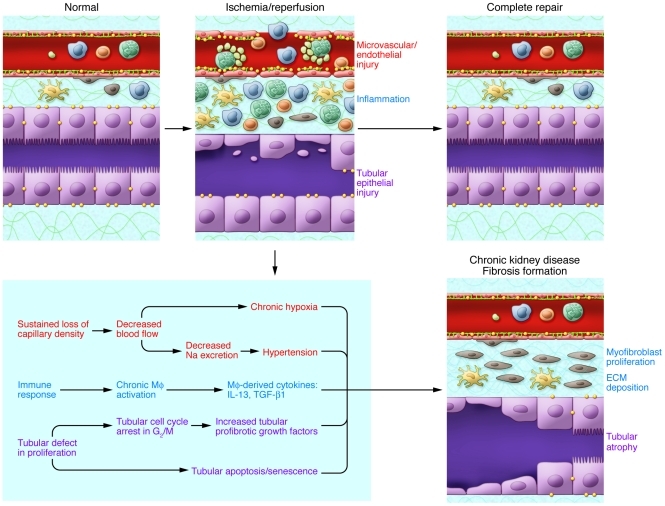
Repair after injury is frequently maladaptive (Figure 7). The development of fibrosis after acute tubular injury has important clinical consequences (9, 121). AKI can lead to incomplete tubular repair, persistent tubulointerstitial inflammation, proliferation of fibroblasts, and excessive deposition of extracellular matrix. Many injury factors (Figures 3 and 7), especially long-term hypoxia from sustained loss of peritubular microvessels (31) and disturbance of immune-respondent components such as chronic activation of macrophages (122, 123), have been suggested to contribute to postischemic fibrosis.
Figure 7. Abnormal repair in ischemic AKI.
Repair after AKI can result in incomplete repair and fibrotic lesions, which may result in progressive renal dysfunction. Factors including long-term hypoxia and hypertension result from chronic loss of peritubular microvessels. Sustained production of profibrotic cytokines such as IL-13, arginase, and TGF-β1 from the chronically activated macrophages (MΦ) contribute to postischemic fibrosis. Renal tubular epithelial cells also play a critical role in the development of fibrosis through fundamental changes in their proliferation processes, including cell cycle arrest in the G2/M phase. This results in a secretory phenotype that facilitates the production by the epithelial cells of profibrotic growth factors (including TGF-β1 and CTGF). Fibrogenesis is stimulated, and progression to chronic renal failure is accelerated.
A fundamental unanswered question in the pathogenesis of kidney fibrosis after AKI is the nature of the molecular switch that determines renal tubular reparative or atrophic/fibrotic responses to injury. Epithelial-mesenchymal transition (EMT) has been thought to be one of the major pathways toward fibrosis (124). Recent studies, however, suggest that the myofibroblasts are generated mainly from perivascular fibroblasts, or pericytes, but not due to EMT by tubular epithelial cells (119, 125, 126), although this remains quite controversial (127). The epithelial cell can generate pro-fibrogenic cytokines, including TGF-β1 and connective tissue growth factor (CTGF), whose production is enhanced by abnormalities in cell cycle progression as the surviving cells attempt to repair the epithelium. There is arrest in the G2/M phase of the cell cycle in severe or sustained kidney injury, and arrest at this phase facilitates the generation of TGF-β1 and CTGF through processes that involve activation of JNK signaling (128). These factors may then induce epigenetic changes in resident fibroblasts, including hypermethylation of RASAL1, an inhibitor of the Ras oncoprotein, which results in prolonged fibroblast activation and fibrogenesis (129).
Protection against injury by ischemic preconditioning
An area of increasing interest is the possibility of rendering an organ resistant to subsequent injury by a prior insult or preconditioning maneuver. Ischemic preconditioning of the kidney confers protection against a subsequent ischemic attack (130). This protective effect decreases with increasing time between the preconditioning insult and the subsequent insult, but in a mouse model, protection was measured up to 12 weeks after the initial insult (131).
A number of cellular factors have been implicated in preconditioning. Preinduction before IRI of the master regulators of genes activated by low oxygen tension, Hif1a and Hif2a, in the kidney (132) leads to protection against injury in rodents (133, 134). Mice with loss of HIF-1α or HIF-2α expression are more susceptible to renal IRI (135, 136). HIF-1α deficiency results in complete loss of ischemic preconditioning–induced cardioprotection in mice (137). Injury-induced enhancement of iNOS expression likely also contributes to kidney protection afforded by prolonged ischemic preconditioning (131). The elevated relative ratio of ERK1/2 activation to JNK or p38 activation in the preconditioned postischemic kidney is also thought to be protective against the second ischemic insult (138, 139). JNK activation is associated with cell death, and ERK activity is considered protective (105). Other candidate mediators of preconditioning include heat shock proteins (HSP27, HSP70) (131, 140), heme oxygenase (141, 142), reactive oxygen species (143), and endoplasmic reticulum stress proteins (144). Tregs have also been implicated in protection, since transfer of these cells from a preconditioned mouse to a normal mouse protected the recipient against subsequent IRI (145–147). This effect was independent of iNOS expression, and the functional benefit was dissociated from any effect on neutrophil or macrophage infiltration of the kidney after ischemia (146, 147).
Various preconditioning interventions have shown encouraging beneficial effects clinically in cardiac IRI (148); however, studies relating preconditioning to renal protection in humans are still rare. In a randomized controlled trial on patients undergoing endovascular aneurysm repair, remote ischemic preconditioning reduced the levels of urinary biomarkers (urinary retinol binding protein and albumin), reflecting kidney injury, but did not affect renal outcomes. This study was preliminary and involved only 40 patients (149).
Conclusions
The cellular contributions to the pathophysiology of ischemic renal injury are protean. AKI often occurs in the context of multiple organ failure and sepsis and involves hemodynamic alterations, inflammation, and direct injury to the tubular epithelium, followed by a repair process that restores epithelial differentiation and function. Inflammation is an important component of this disease, playing a considerable role in its pathophysiology. Significant progress has been made in defining major components of this process, yet the complex molecular and cellular interactions among endothelial cells, inflammatory cells, and the injured epithelium are poorly understood, although we are gaining ground in this quest. Recently researchers have come to realize the intrinsic capacity of the damaged proximal epithelium to repair itself by dedifferentiation and proliferation of surviving epithelial cells without a source of distinct progenitor cells. We have also identified potential pathophysiological links among injury, abnormal repair, and the profibrotic sequelae of severe injury that may help to explain why in humans AKI is such a great risk factor for progression of CKD. Better understanding of the molecular, cellular, and genetic aspects underlying kidney injury will hopefully result in the design of more targeted therapies to prevent injury and hasten repair. Progress is being made on multiple fronts, but we continue to be humbled by this disease, whose mortality rate has changed little over four decades.
Acknowledgments
The authors thank Suxia Wang for providing the electron microscopy photos. This work was supported by NIDDK-NIH grants (DK39773, DK38452, and DK72381).
Footnotes
Conflict of interest: Joseph V. Bonventre is coinventor on KIM-1 patents that have been licensed by Partners HealthCare to a number of companies. He has received royalty income from Partners Healthcare and grant funding from Johnson & Johnson and BASF. The Bonventre family has received income for consulting from Genzyme, Celgene, Sanofi-Avenits, Gilead, Merck, and PTC Therapeutics; in addition, Bonventre is on the board of directors of AMAG Pharmaceuticals, and the Bonventre family has stock ownership in AMAG Pharmaceuticals, Theravance, PatientKeeper, and Pacific Biosciences.
Citation for this article: J Clin Invest. 2011;121(11):4210–4221. doi:10.1172/JCI45161.
References
- 1.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lameire N, Van Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2(7):364–377. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 5.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72(2):208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein LB, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(2):517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 7.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishani A, et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20(1):223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50(3):811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 11.Mehta RL, et al. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66(4):1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 12.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. New Engl J Med. 1996;334(22):1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 13.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. 2009;15(6):503–508. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 14.Alejandro V, et al. Mechanisms of filtration failure during postischemic injury of the human kidney. A study of the reperfused renal allograft. J Clin Invest. 1995;95(2):820–831. doi: 10.1172/JCI117732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. . J Am Soc Nephrol. 2003;14(8):2199–2210. doi: 10.1097/01.ASN.0000079785.13922.F6. [DOI] [PubMed] [Google Scholar]
- 16.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351(2):159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 17.Karlberg L, Norlen BJ, Ojteg G, Wolgast M. Impaired medullary circulation in postischemic acute renal failure. Acta Physiol Scand. 1983;118(1):11–17. doi: 10.1111/j.1748-1716.1983.tb07234.x. [DOI] [PubMed] [Google Scholar]
- 18.Mason J, Torhorst J, Welsch J. Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int. 1984;26(3):283–293. doi: 10.1038/ki.1984.171. [DOI] [PubMed] [Google Scholar]
- 19.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conger J. Hemodynamic factors in acute renal failure. Adv Ren Replace Ther. 1997;4(2 suppl 1):25–37. [PubMed] [Google Scholar]
- 21.Brooks DP. Role of endothelin in renal function and dysfunction. Clin Exp Pharmacol Physiol. 1996;23(4):345–348. doi: 10.1111/j.1440-1681.1996.tb02835.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurata H, et al. Protective effect of nitric oxide on ischemia/reperfusion-induced renal injury and endothelin-1 overproduction. Eur J Pharmacol. 2005;517(3):232–239. doi: 10.1016/j.ejphar.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 23.da Silveira KD, et al. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond). 2010;119(9):385–394. doi: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 24.Conger JD. Vascular abnormalities in the maintenance of acute renal failure. Circ Shock. 1983;11(3):235–244. [PubMed] [Google Scholar]
- 25.Kwon O, Hong SM, Ramesh G. Diminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusion. Am J Physiol Renal Physiol. 2009;296(1):F25–F33. doi: 10.1152/ajprenal.90531.2008. [DOI] [PubMed] [Google Scholar]
- 26.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66(2):480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 27.Blantz RC, Deng A, Miracle CM, Thomson SC. Regulation of kidney function and metabolism: a question of supply and demand. Trans Am Clin Climatol Assoc. 2007;118:23–43. [PMC free article] [PubMed] [Google Scholar]
- 28.Brezis M, Rosen S. Hypoxia of the renal medulla — its implications for disease. N Engl J Med. 1995;332(10):647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 29.Beeuwkes R, III, Bonventre JV. Tubular organization and vascular-tubular relations in the dog kidney. Am J Physiol. 1975;229(3):695–713. doi: 10.1152/ajplegacy.1975.229.3.695. [DOI] [PubMed] [Google Scholar]
- 30.Bagnasco S, Good D, Balaban R, Burg M. Lactate production in isolated segments of the rat nephron. Am J Physiol. 1985;248(4 pt 2):F522–F526. doi: 10.1152/ajprenal.1985.248.4.F522. [DOI] [PubMed] [Google Scholar]
- 31.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72(2):151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 32.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6(7):404–414. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KJ, et al. Intercellular adhesion molecule-1 deficient mice are protected against renal ischemia. J Clin Invest. 1996;97(4):1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA. Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol. 2003;285(2):F191–F198. doi: 10.1152/ajprenal.00042.2003. [DOI] [PubMed] [Google Scholar]
- 35.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294(4):F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 36.Kwon O, Hong SM, Sutton TA, Temm CJ. Preservation of peritubular capillary endothelial integrity and increasing pericytes may be critical to recovery from postischemic acute kidney injury. Am J Physiol Renal Physiol. 2008;295(2):F351–F359. doi: 10.1152/ajprenal.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 38.Johnson GB, Brunn GJ, Platt JL. Activation of mammalian Toll-like receptors by endogenous agonists. Crit Rev Immunol. 2003;23(1–2):15–44. doi: 10.1615/CritRevImmunol.v23.i12.20. [DOI] [PubMed] [Google Scholar]
- 39.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87(9):859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 40.Wahl P, et al. Renal tubular epithelial expression of the costimulatory molecule B7RP-1 (inducible costimulator ligand). J Am Soc Nephrol. 2002;13(6):1517–1526. doi: 10.1097/01.ASN.0000017901.77985F. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Nord EP. CD40 ligation stimulates MCP-1 and IL-8 production, TRAF6 recruitment, and MAPK activation in proximal tubule cells. Am J Physiol Renal Physiol. 2002;282(6):F1020–F1033. doi: 10.1152/ajprenal.00291.2001. [DOI] [PubMed] [Google Scholar]
- 42.Deckers JG, De Haij S, van der Woude FJ, van der Kooij SW, Daha MR, van Kooten C. IL-4 and IL-13 augment cytokine- and CD40-induced RANTES production by human renal tubular epithelial cells in vitro. J Am Soc Nephrol. 1998;9(7):1187–1193. doi: 10.1681/ASN.V971187. [DOI] [PubMed] [Google Scholar]
- 43.Niemann-Masanek U, Mueller A, Yard BA, Waldherr R, van der Woude FJ. B7-1 (CD80) and B7-2 (CD 86) expression in human tubular epithelial cells in vivo and in vitro. Nephron. 2002;92(3):542–556. doi: 10.1159/000064084. [DOI] [PubMed] [Google Scholar]
- 44.Solez K, Morel-Maroger L, Sraer JD. The morphology of “acute tubular necrosis” in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine (Baltimore). 1979;58(5):362–376. [PubMed] [Google Scholar]
- 45.Awad AS, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290(6):F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 46.Awad AS, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75(7):689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130(1):41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia-reperfusion injury. Kidney Int. 2008;74(12):1526–1537. doi: 10.1038/ki.2008.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 50.Oh DJ, et al. Fractalkine receptor (CX3CR1) inhibition is protective against ischemic acute renal failure in mice. Am J Physiol Renal Physiol. 2008;294(1):F264–F271. doi: 10.1152/ajprenal.00204.2007. [DOI] [PubMed] [Google Scholar]
- 51.Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288(4):F722–F731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.John R, Nelson PJ. Dendritic cells in the kidney. . J Am Soc Nephrol. 2007;18(10):2628–2635. doi: 10.1681/ASN.2007030273. [DOI] [PubMed] [Google Scholar]
- 54.Hume DA. Macrophages as APC and the dendritic cell myth. J Immunol. 2008;181(9):5829–5835. doi: 10.4049/jimmunol.181.9.5829. [DOI] [PubMed] [Google Scholar]
- 55.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando). 2009;23(1):1–10. doi: 10.1016/j.trre.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burne MJ, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest. 2001;108(9):1283–1290. doi: 10.1172/JCI12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gandolfo MT, et al. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 2009;76(7):717–729. doi: 10.1038/ki.2009.259. [DOI] [PubMed] [Google Scholar]
- 58.Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Renal Physiol. 2002;282(2):F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 59.Faubel S, et al. Peripheral CD4 T-cell depletion is not sufficient to prevent ischemic acute renal failure. Transplantation. 2005;80(5):643–649. doi: 10.1097/01.tp.0000173396.07368.55. [DOI] [PubMed] [Google Scholar]
- 60.Hochegger K, et al. Role of alpha/beta and gamma/delta T cells in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2007;293(3):F741–F747. doi: 10.1152/ajprenal.00486.2006. [DOI] [PubMed] [Google Scholar]
- 61.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, Rabb H. Role of the T-cell receptor in kidney ischemia-reperfusion injury. Kidney Int. 2006;69(2):233–238. doi: 10.1038/sj.ki.5000038. [DOI] [PubMed] [Google Scholar]
- 62.Thurman JM, Lucia MS, Ljubanovic D, Holers VM. Acute tubular necrosis is characterized by activation of the alternative pathway of complement. Kidney Int. 2005;67(2):524–530. doi: 10.1111/j.1523-1755.2005.67109.x. [DOI] [PubMed] [Google Scholar]
- 63.Homeister JW, Lucchesi BR. Complement activation and inhibition in myocardial ischemia and reperfusion injury. Annu Rev Pharmacol Toxicol. 1994;34:17–40. doi: 10.1146/annurev.pa.34.040194.000313. [DOI] [PubMed] [Google Scholar]
- 64.Sandor N, Pap D, Prechl J, Erdei A, Bajtay Z. A novel, complement-mediated way to enhance the interplay between macrophages, dendritic cells and T lymphocytes. Mol Immunol. 2009;47(2–3):438–448. doi: 10.1016/j.molimm.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 65.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. Faseb J. 2006;20(2):217–226. doi: 10.1096/fj.05-4747com. [DOI] [PubMed] [Google Scholar]
- 66.Thurman JM, et al. Altered renal tubular expression of the complement inhibitor Crry permits complement activation after ischemia/reperfusion. J Clin Invest. 2006;116(2):357–368. doi: 10.1172/JCI24521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Renner B, et al. The complement inhibitors Crry and factor H are critical for preventing autologous complement activation on renal tubular epithelial cells. J. Immunol. 2010;185(5):3086–3094. doi: 10.4049/jimmunol.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thurman JM, et al. Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol. 2006;17(3):707–715. doi: 10.1681/ASN.2005070698. [DOI] [PubMed] [Google Scholar]
- 69.Zheng X, et al. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. 2006;82(12):1781–1786. doi: 10.1097/01.tp.0000250769.86623.a3. [DOI] [PubMed] [Google Scholar]
- 70.Thurman JM, et al. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ischemia/reperfusion. J Immunol. 2007;178(3):1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 71.Zhou W, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. 2000;105(10):1363–1371. doi: 10.1172/JCI8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70(3):432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 73.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295(2):F534–F544. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Duffield JS, et al. Resolvin d series and protectin d1 mitigate acute kidney injury. J Immunol. 2006;177(9):5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 75.Leonard MO, et al. 15-Epi-16-(para-fluorophenoxy)-lipoxin A(4)-methyl ester, a synthetic analogue of 15-epi-lipoxin A(4), is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13(6):1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- 76.Franquesa M, et al. Tubular epithelial cells transfected with hHGF counteracts monocyte chemotactic protein-1 up-regulation after hypoxia/reoxygenation insult. Transplant Proc. 2009;41(6):2069–2072. doi: 10.1016/j.transproceed.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Lech M, et al. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J Immunol. 2009;183(6):4109–4118. doi: 10.4049/jimmunol.0900118. [DOI] [PubMed] [Google Scholar]
- 78.Bonventre JV, Brezis M, Siegel N, Rosen S, Portilla D, Venkatachalam M. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998;275(5 pt 2):F623–F631. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 79.Heyman SN, Rosenberger C, Rosen S. Experimental ischemia-reperfusion: biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int. 2010;77(1):9–16. doi: 10.1038/ki.2009.347. [DOI] [PubMed] [Google Scholar]
- 80.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 81.Vaidya VS, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1(3):200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sutton TA, Molitoris BA. Mechanisms of cellular injury in ischemic acute renal failure. Semin Nephrol. 1998;18(5):490–497. [PubMed] [Google Scholar]
- 83.Gailit J, Colflesh Rabiner, I, Simone J, Goligorsky MS. Redistribution and dysfunction of integrins in cultured renal epithelial cells exposed to oxidative stress. Am J Physiol. 1993;264(1 pt 2):F149–F157. doi: 10.1152/ajprenal.1993.264.1.F149. [DOI] [PubMed] [Google Scholar]
- 84.Zuk A, Bonventre JV, Brown D, Matlin KS. Polarity, integrin, and extracellular matrix dynamics in the postischemic rat kidney. Am J Physiol. 1998;275(3 pt 1):C711–C731. doi: 10.1152/ajpcell.1998.275.3.C711. [DOI] [PubMed] [Google Scholar]
- 85.Bush KT, Keller SH, Nigam SK. Genesis and reversal of the ischemic phenotype in epithelial cells. . J Clin Invest. 2000;106(5):621–626. doi: 10.1172/JCI10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molitoris BA, Dahl R, Geerdes A. Cytoskeleton disruption and apical redistribution of proximal tubule Na+-K+-ATPase during ischemia. . Am J Physiol. 1992;263(3 pt 2):F488–F495. doi: 10.1152/ajprenal.1992.263.3.F488. [DOI] [PubMed] [Google Scholar]
- 87.Brown D, Lee R, Bonventre JV. Redistribution of villin to proximal tubule basolateral membranes after ischemia and reperfusion. Am J Physiol. 1997;273(6 pt 2):F1003–F1012. doi: 10.1152/ajprenal.1997.273.6.F1003. [DOI] [PubMed] [Google Scholar]
- 88.Prakash J, et al. Inhibition of renal rho kinase attenuates ischemia/reperfusion-induced injury. . J Am Soc Nephrol. 2008;19(11):2086–2097. doi: 10.1681/ASN.2007070794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zuk A, Bonventre JV, Matlin KS. Expression of fibronectin splice variants in the postischemic rat kidney. Am J Physiol Renal Physiol. 2001;280(6):F1037–F1053. doi: 10.1152/ajprenal.2001.280.6.F1037. [DOI] [PubMed] [Google Scholar]
- 90.Amin RP, et al. Identification of putative gene based markers of renal toxicity. Environ Health Perspect. 2004;112(4):465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Villanueva S, Cespedes C, Vio CP. Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R861–R870. doi: 10.1152/ajpregu.00384.2005. [DOI] [PubMed] [Google Scholar]
- 92.Ichimura T, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 93.Mishra J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 94.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaidya VS, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamanishi Y, et al. TIM1 is an endogenous ligand for LMIR5/CD300b: LMIR5 deficiency ameliorates mouse kidney ischemia/reperfusion injury. J Exp Med. 2010;207(7):1501–1511. doi: 10.1084/jem.20090581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonventre JV, Yang L. Kidney injury molecule-1. Curr Opin Crit Care. 2010;16(6):556–561. doi: 10.1097/MCC.0b013e32834008d3. [DOI] [PubMed] [Google Scholar]
- 99.Bao G, et al. Iron traffics in circulation bound to a siderocalin (Ngal)-catechol complex. Nat Chem Biol. 2010;6(8):602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mishra J, et al. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2004;15(12):3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 101.Mori K, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chien CT, Lee PH, Chen CF, Ma MC, Lai MK, Hsu SM. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J Am Soc Nephrol. 2001;12(5):973–982. doi: 10.1681/ASN.V125973. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z, et al. Induction of heat shock protein 70 inhibits ischemic renal injury. Kidney Int. 2011;79(8):861–870. doi: 10.1038/ki.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kimura T, et al. Autophagy Protects the Proximal Tubule from Degeneration and Acute Ischemic Injury. J Am Soc Nephrol. 2011;22(5):902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.di Mari JF, Davis R, Safirstein RL. MAPK activation determines renal epithelial cell survival during oxidative injury. Am J Physiol. 1999;277(2 pt 2):F195–F203. doi: 10.1152/ajprenal.1999.277.2.F195. [DOI] [PubMed] [Google Scholar]
- 106.Gobe G, Zhang XJ, Willgoss DA, Schoch E, Hogg NA, Endre ZH. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol. 2000;11(3):454–467. doi: 10.1681/ASN.V113454. [DOI] [PubMed] [Google Scholar]
- 107.Gobe GC, Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol. 2007;39(9):1551–1561. doi: 10.1016/j.biocel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 108.Nadasdy T, Laszik Z, Blick KE, Johnson LD, Silva FG. Proliferative activity of intrinsic cell populations in the normal human kidney. J Am Soc Nephrol. 1994;4(12):2032–2039. doi: 10.1681/ASN.V4122032. [DOI] [PubMed] [Google Scholar]
- 109.Prescott LF. The normal urinary excretion rates of renal tubular cells, leucocytes and red blood cells. Clin Sci. 1966;31(3):425–435. [PubMed] [Google Scholar]
- 110.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93(5):2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. . J Am Soc Nephrol. 2003;14 suppl 1:S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 112.Kale S, Karihaloo A, Clark PR, Kashgarian M, Krause DS, Cantley LG. Bone marrow stem cells contribute to repair of the ischemically injured renal tubule. J Clin Invest. 2003;112(1):42–49. doi: 10.1172/JCI17856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lin F, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14(5):1188–1199. doi: 10.1097/01.ASN.0000061595.28546.A0. [DOI] [PubMed] [Google Scholar]
- 114.Duffield JS, et al. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005;115(7):1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 116.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 117.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68(5):1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78(9):838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 119.Humphreys BD, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2(3):284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 120.Abbate M, Brown D, Bonventre JV. Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Physiol. 1999;277(3 pt 2):F454–F463. doi: 10.1152/ajprenal.1999.277.3.F454. [DOI] [PubMed] [Google Scholar]
- 121.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298(5):F1078–F1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duffield JS. Macrophages and immunologic inflammation of the kidney. Semin Nephrol. 2010;30(3):234–254. doi: 10.1016/j.semnephrol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ko GJ, Boo CS, Jo SK, Cho WY, Kim HK. Macrophages contribute to the development of renal fibrosis following ischaemia/reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2008;23(3):842–852. doi: 10.1093/ndt/gfm694. [DOI] [PubMed] [Google Scholar]
- 124.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Humphreys BD, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kriz W, Kaissling B, Le Hir M. Epithelial-mesenchymal transition (EMT) in kidney fibrosis: fact or fantasy? J Clin Invest. 2011;121(2):468–474. doi: 10.1172/JCI44595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J Am Soc Nephrol. 2010;21(8):1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 128.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bechtel W, et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med. 2010;16(5):544–550. doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonventre JV. Kidney ischemic preconditioning. Curr Opin Nephrol Hypertens. 2002;11(1):43–48. doi: 10.1097/00041552-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 131.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem. 2003;278(29):27256–27266. doi: 10.1074/jbc.M301778200. [DOI] [PubMed] [Google Scholar]
- 132.Rosenberger C, et al. Expression of hypoxia-inducible factor-1alpha and -2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol. 2002;13(7):1721–1732. doi: 10.1097/01.ASN.0000017223.49823.2A. [DOI] [PubMed] [Google Scholar]
- 133.Bernhardt WM, et al. Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol. 2006;17(7):1970–1978. doi: 10.1681/ASN.2005121302. [DOI] [PubMed] [Google Scholar]
- 134.Weidemann A, et al. HIF activation protects from acute kidney injury. J Am Soc Nephrol. 2008;19(3):486–494. doi: 10.1681/ASN.2007040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kojima I, et al. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol. 2007;18(4):1218–1226. doi: 10.1681/ASN.2006060639. [DOI] [PubMed] [Google Scholar]
- 136.Hill P, et al. Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol. 2008;19(1):39–46. doi: 10.1681/ASN.2006090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cai Z, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77(3):463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 138.Park KM, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury and JNK, p38, and MAPK kinase activation by remote ischemic pretreatment. J Biol Chem. 2001;276(15):11870–11876. doi: 10.1074/jbc.M007518200. [DOI] [PubMed] [Google Scholar]
- 139.Park KM, Kramers C, Vayssier-Taussat M, Chen A, Bonventre JV. Prevention of kidney ischemia/reperfusion-induced functional injury, MAPK and MAPK kinase activation, and inflammation by remote transient ureteral obstruction. J Biol Chem. 2002;277(3):2040–2049. doi: 10.1074/jbc.M107525200. [DOI] [PubMed] [Google Scholar]
- 140.Yeh CH, Hsu SP, Yang CC, Chien CT, Wang NP. Hypoxic preconditioning reinforces HIF-alpha-dependent HSP70 signaling to reduce ischemic renal failure-induced renal tubular apoptosis and autophagy. Life Sci. 2010;86(3–4):115–123. doi: 10.1016/j.lfs.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 141.Holzen JP, et al. Influence of heme oxygenase-1 on microcirculation after kidney transplantation. . J Surg Res. 2008;148(2):126–135. doi: 10.1016/j.jss.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 142.Chok MK, et al. Renoprotective potency of heme oxygenase-1 induction in rat renal ischemia-reperfusion. Inflamm Allergy Drug Targets. 2009;8(4):252–259. doi: 10.2174/187152809789352186. [DOI] [PubMed] [Google Scholar]
- 143.Kim J, Jang HS, Park KM. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol. 2010;298(1):F158–F166. doi: 10.1152/ajprenal.00474.2009. [DOI] [PubMed] [Google Scholar]
- 144.Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal Epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. J Biol Chem. 2003;278(31):29317–29326. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- 145.Burne-Taney MJ, Liu M, Baldwin WM, Racusen L, Rabb H. Decreased capacity of immune cells to cause tissue injury mediates kidney ischemic preconditioning. J Immunol. 2006;176(11):7015–7020. doi: 10.4049/jimmunol.176.11.7015. [DOI] [PubMed] [Google Scholar]
- 146.Kinsey GR, Huang L, Vergis AL, Li L, Okusa MD. Regulatory T cells contribute to the protective effect of ischemic preconditioning in the kidney. Kidney Int. 2010;77(9):771–780. doi: 10.1038/ki.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cho WY, Choi HM, Lee SY, Kim MG, Kim HK, Jo SK. The role of Tregs and CD11c(+) macrophages/dendritic cells in ischemic preconditioning of the kidney. Kidney Int. 2010;78(10):981–992. doi: 10.1038/ki.2010.266. [DOI] [PubMed] [Google Scholar]
- 148.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204(2):334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 149.Walsh SR, et al. Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther. 2009;16(6):680–689. doi: 10.1583/09-2817.1. [DOI] [PubMed] [Google Scholar]