Abstract
Tregs not only keep immune responses to autoantigens in check, but also restrain those directed toward pathogens and the commensal microbiota. Control of peripheral immune homeostasis by Tregs relies on their capacity to accumulate at inflamed sites and appropriately adapt to their local environment. To date, the factors involved in the control of these aspects of Treg physiology remain poorly understood. Here, we show that the canonical Th2 transcription factor GATA3 is selectively expressed in Tregs residing in barrier sites including the gastrointestinal tract and the skin. GATA3 expression in both murine and human Tregs was induced upon TCR and IL-2 stimulation. Although GATA3 was not required to sustain Treg homeostasis and function at steady state, GATA3 played a cardinal role in Treg physiology during inflammation. Indeed, the intrinsic expression of GATA3 by Tregs was required for their ability to accumulate at inflamed sites and to maintain high levels of Foxp3 expression in various polarized or inflammatory settings. Furthermore, our data indicate that GATA3 limits Treg polarization toward an effector T cell phenotype and acquisition of effector cytokines in inflamed tissues. Overall, our work reveals what we believe to be a new facet in the complex role of GATA3 in T cells and highlights what may be a fundamental role in controlling Treg physiology during inflammation.
Introduction
Impaired function or homeostasis of Tregs has been implicated in the development of several autoimmune and inflammatory diseases. In addition to controlling autoimmune responses, Tregs regulate immunity to infection of viral, bacterial, or parasitic origin and can restrain responses to tumors or grafts (1–3). Thus, Tregs control a large array of immune responses in the context of various polarized settings as well as in distinct microenvironments. This implies that maintenance of peripheral homeostasis relies on the capacity of Tregs to appropriately adapt to defined settings while maintaining their regulatory capacity. Such control is particularly critical in tissues highly exposed to microbial stimulation and environmental insults such as the gastrointestinal (GI) tract or the skin.
Sustained expression of Foxp3 in Tregs is critical for maintaining regulatory function (4). Signaling events and factors downstream of TCR signaling, such as calcium signaling and the NF-κB and Ras-ERK pathways, have been shown to be important for both the induction and maintained expression of Foxp3 (5). For instance, signaling through cooperation of NFAT/Foxp3 complexes and the NF-κB pathway through c-Rel and binding of Runx1–Cbf-β complex to the CNS2 loci in the Foxp3 gene, are critical for sustained Foxp3 expression (6–10). In addition, Tregs are highly dependent upon survival factors, in particular IL-2 (11–13). Although the molecular control of Tregs is well characterized, how the integration of these factors contributes to the maintenance of a Treg program in the context of inflammation is poorly understood.
Accumulation and survival of Tregs at the site of inflammation is also critical for their ability to regulate ongoing immune responses (3, 14–20). Moreover, recent evidence suggests that the capacity of Tregs to control defined polarized settings can be associated with the acquisition of specific transcription factors. For instance, T-bet, IRF4, and STAT3 can contribute to the capacity of Tregs to control Th1, Th2, and Th17 responses, respectively (21–23). Via expression of these factors, Tregs can partially mimic the phenotype of the effector T cells, a property that could endow them with finely tuned homing, survival, or functional properties (3). However, in some extreme settings, such adaptation can also be associated with the expression of effector cytokines (24–26). For instance, we have previously shown that in the context of a lethal oral infection with Toxoplasma gondii, T-bet expression by Tregs was associated with their production of IFN-γ (18), and recent evidence suggests that in experimental diabetes, a significant fraction of IFN-γ–producing effector T cells may have derived from cells previously expressing Foxp3 (27). Furthermore, both in humans and mice, Tregs that reside in the intestine can express RORγt, which is associated with an ability to produce IL-17 (28, 29), and fate-mapping strategies revealed that a significant proportion of IL-17 effector T cells in the gut had at one time expressed Foxp3 (30).
Tregs are mostly self reactive when emerging from the thymus and believed to be further shaped for self reactivity in the periphery (31). This suggests that if armed with effector cytokines such as IFN-γ or IL-17, Tregs could contribute to tissue damage and/or become ineffective in regulating effector responses (32–35). In support of this, a recent report identified a population of Tregs expressing IFN-γ in the context of autoimmunity in humans (36). Thus, Treg plasticity is likely to be tightly controlled. A corollary to this idea is that in most circumstances, and particularly in settings where the host survives severe inflammation, Tregs remain stable and rarely express effector cytokines (37).
Because of the cardinal role of Tregs in maintaining peripheral homeostasis, a better understanding of the factors controlling their maintenance of a program compatible with regulatory function as well as their accumulation in inflamed tissues is clearly needed. In the present study, we have shown that the canonical Th2 transcription factor GATA3 plays a critical role in Treg physiology by preventing excessive polarization toward an effector phenotype and enhancing their capacity to accumulate within inflamed tissues.
Results
Foxp3+ Tregs from barrier sites express high levels of GATA3.
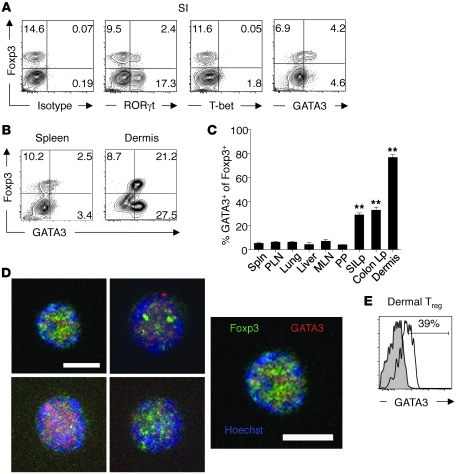
Previous work has shown that at sites undergoing constitutive inflammation such as the GI tract, a substantial fraction of Tregs expressed the transcription factor associated with Th17 lineage polarization, RORγt (ref. 30 and Figure 1A). On the other hand, Tregs from this site did not express T-bet, the transcription factor associated with Th1 lineage (Figure 1A). Intriguingly, a third of Tregs residing in the small and large intestinal lamina propria (Lp) also expressed the canonical Th2 transcription factor GATA3 (Figure 1, A and C). Evaluation of GATA3 expression at other locations revealed that 80% of dermal Tregs also expressed this transcription factor (Figure 1, B and C). The expression of GATA3 by Tregs was less pronounced in lymphoid organs as well as in other tissues, such as the lung and liver (Figure 1, B and C). In contrast, the expression of GATA3 in Foxp3– T cells was less pronounced in comparison with Tregs (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI57456DS1). Confocal imaging of purified Foxp3+ T cells confirmed the nuclear localization of GATA3 in small intestine Lp (SILp) Tregs (Figure 1D). Thus, a significant fraction of Tregs residing at barrier sites express the canonical Th2 transcription factor GATA3. Of note, coexpression of GATA3 with Foxp3 was not restricted to mice, as a high frequency of Foxp3hiCD3+ dermal cells from rhesus macaques also expressed GATA3 under steady-state conditions (Figure 1E).
Figure 1. Dermis and gut Lp Tregs express high levels of GATA3.
(A) Gut Tregs express RORγt and GATA3. Cells were harvested from SILp, stained for T-bet, RORγt, GATA3, and Foxp3, and assessed by flow cytometry. Plots are gated on live CD4+TCR-β+ cells; isotype for RORγt. (B) GATA3 is highly expressed in dermis Tregs. Cells were harvested from spleen and ear pinna, stained for GATA3 and Foxp3, and assessed by flow cytometry. Plots are gated on live CD4+TCR-β+ cells. Numbers in quadrants refer to the percentage of each subset. (C) Barrier tissue resident Tregs express higher levels of GATA3 than peripheral Tregs. Percentages of Foxp3+CD4+TCRβ+ cells expressing GATA3 in spleen (Spln), peripheral LN (PLN), lung, liver, mesenteric LN (MLN), Peyer patches (PP), SILp, colon Lp, and dermis. **P < 0.001. n = 3 mice per group. Data are presented as mean ± SEM. (D) Nuclear localization of GATA3. Foxp3eGFP+ cells were purified by sorting from the SILp of naive Foxp3eGFP mice and stained for Foxp3 (green), GATA3 (red), and Hoescht (blue), and localization of proteins was assessed by confocal microscopy. Right image is enlargement of top left image. Scale bars: 5 μm. (E) GATA3 is expressed by dermis Tregs in rhesus macaque. Cells were harvested from the dermis and stained for CD3, GATA3, and Foxp3. Number refers to frequency of GATA3+ cells. Plot is gated on live Foxp3+CD3+CD8– cells (filled histogram, isotype control; open histogram, GATA3). Data shown are representative of 3 independent experiments (A and B) and 1 experiment (E) with similar results.
GATA3 is upregulated in Tregs following TCR engagement.
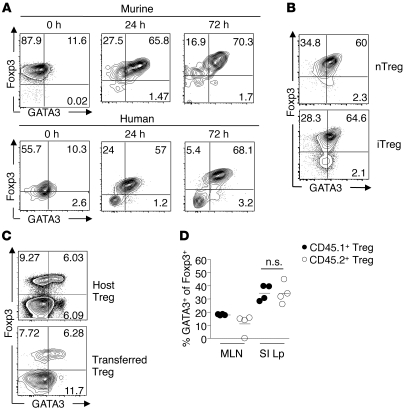
Previous work has demonstrated that GATA3 can be induced in conventional CD4+ T cells following TCR stimulation (38–40). To assess whether a similar mechanism could account for GATA3 expression by Tregs, highly purified Foxp3+CD4+ T cells were cocultured with splenic DCs (SpDCs) in the presence of anti-CD3 antibody. As early as 24 hours after stimulation, up to 65% of Tregs expressed high levels of GATA3 (Figure 2A). Addition of recombinant IL-2 (rIL-2) did not significantly increase the level of GATA3 expression by Tregs at 24 hours, but was required for sustained GATA3 expression at 72–96 hours (data not shown). Addition of IL-2 alone was sufficient to induce low levels of GATA3 expression by Tregs, but optimal expression was observed in the context of TCR stimulation (Supplemental Figure 2). GATA3 expression by Tregs was further confirmed by assessment of mRNA expression (Supplemental Figure 3).
Figure 2. TCR activation triggers GATA3 expression in mouse and human Tregs.
(A) Expression of GATA3 by nTregs. Murine: sort-purified CD4+eGFP+ cells from peripheral LNs of Foxp3eGFP mice were cultured as described in methods. Human: sort-purified CD4+CD25+CD127lo cells from peripheral blood were cultured as described in Methods. Cells were stained for Foxp3 and GATA3 and analyzed by flow cytometry. Numbers in quadrants refer to the percentage of each subset. Plots are gated on live CD4+ cells. (B) Expression of GATA3 by in vitro iTregs. Sort-purified CD4+eGFP+ cells cultured in the presence of 1 ng/ml rIL-2 and anti-CD3 antibody (nTreg, top panel) and sort-purified CD4+eGFP– cells incubated with rIL-2 and TGF-β (iTreg, bottom panel). Numbers in quadrants refer to the percentage of each subset. Plots are gated on live CD4+ T cells. (C) Expression of GATA3 by in vivo iTregs. OT-II-Foxp3eGFP– cells were transferred into CD45.1+ hosts and fed ovalbumin in their drinking water for 7 days. Cells were harvested from the MLN and SILp, stained for Foxp3 and GATA3, and analyzed by flow cytometry. Plots are gated on live CD4+TCRβ+ cells from the SILp. Numbers in quadrants refer to the percentage of each subset. (D) Graphical representation of FACS plots in C (CD45.1+ host cells, closed circles; CD45.2+OT-II transferred cells). Each circle represents 1 mouse, and crossbars depict the mean of 3 mice analyzed. Data shown are representative of at least 3 independent experiments with similar results.
We next wanted to determine whether human Tregs expressed GATA3 following TCR engagement. To this end, CD4+CD25brightCD127lo Tregs were purified by sorting from the peripheral blood of healthy donors and cultured on plate-bound anti-CD3 antibody and anti-CD28 antibody in the presence of rIL-2 for 3 days. After sorting, a small fraction of human Tregs isolated from PBMCs expressed low levels of GATA3 (Figure 2A). Notably, GATA3 expression was dramatically enhanced in human Tregs as early as 24 hours and was sustained for the duration of the 3-day culture (Figure 2A). Thus, both mouse and human Tregs can express GATA3 upon TCR engagement.
To assess whether inducible Treg (iTreg) cells could also upregulate GATA3, naive CD4+Foxp3eGFP– cells were cultured in the presence of anti-CD3 antibody, TGF-β, and rIL-2 for 4 days. After 96 hours of culture, we found that in vitro–induced murine Tregs expressed GATA3 to the same extent as in vitro–activated natural Tregs (nTregs) (Figure 2B). Because we and others had previously shown that the gut-associated lymphoid tissue (GALT) is a preferential site for the induction of Tregs (41, 42), we next assessed whether GATA3 could also be expressed by mucosal-induced Tregs. To this end, CD4+Foxp3– T cells from Foxp3eGFP OTII transgenic mice were adoptively transferred into congenic CD45.1+ C57BL/6 recipient mice that received OVA protein dissolved in their drinking water for 7 consecutive days. At the conclusion of the experiment, OTII iTregs from the SILp expressed a comparable level of GATA3 to host Tregs (Figure 2, C and D).
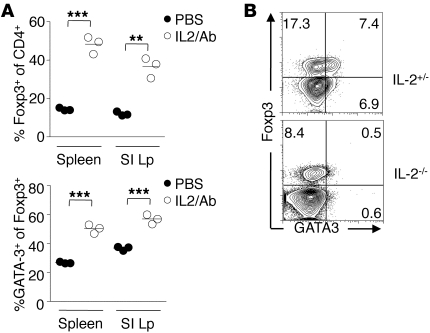
To determine whether GATA3 expression by Tregs was restricted to the GI tract or the skin environment, we utilized an approach previously shown to lead to systemic activation and expansion of Tregs (43). As previously described, following injection of mice with rIL-2 coupled with anti–IL-2 antibody, Tregs proliferated, as evidenced by increased expression of Ki-67 (Supplemental Figure 4) and their increased frequency (Figure 3A). Under these conditions, GATA3 expression by Tregs was upregulated systemically (Figure 3A). To further explore the potential role of IL-2 in controlling GATA3 expression by Tregs, we assessed the expression of this transcription factor in IL-2–deficient mice. In these mice, the frequency of Tregs is dramatically reduced in the periphery (44, 45), but to a lesser extent in the GI tract (18). We found that in contrast with those from control mice, Tregs from the small intestine Lp of IL-2–deficient mice did not express GATA3 (Figure 3B). Thus, Tregs from both mice and humans can readily express GATA3 upon TCR engagement and/or exposure to IL-2. Our data suggest that expression of this transcription factor by Tregs at barrier sites reflects their status of activation rather than an adaptation to a defined milieu.
Figure 3. GATA3 expression by Tregs is positively regulated by IL-2.
(A) Induction of GATA3 expression by IL-2/anti–IL-2 treatment. Naive C57BL/6 mice were treated with rIL-2/anti–IL-2 complexes for 6 days. Cells were harvested, and their expression of GATA3 was evaluated by flow cytometry. Each circle represents 1 mouse, and crossbars depict the mean of 3 mice analyzed. Graph represents the percentage of live Foxp3+CD4+TCRβ+ expressing GATA3 in the spleen and SILp. **P ≤ 0.0014; ***P < 0.0007. (B) IL-2–deficient mice express reduced levels of GATA3 in SILp Tregs. Cells were isolated from the SILp of 8-week-old Il2+/– control mice or IL-2–deficient mice (Il2–/–) and stained for Foxp3 and GATA3. Plots are gated on live CD4+TCRβ+. Numbers in quadrants refer to the percentage of each subset. Data shown are representative of at least 2 independent experiments with similar results.
Cytokine control of GATA3 expression by Tregs.
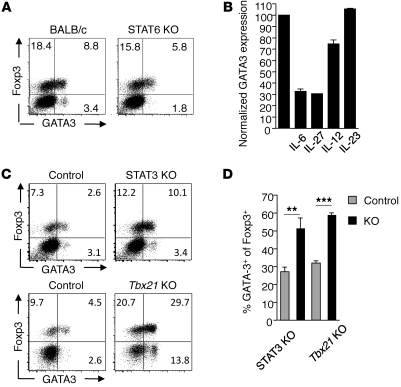
Because Th2 cytokines have been shown to contribute to the upregulation of GATA3 by conventional CD4+ T cells (46–49), we assessed whether the same factors could account for constitutive GATA3 expression by Tregs in tissue. To this end, we examined GALT Tregs in the context of loss of function of the shared IL-4 and IL-13 receptor (IL-4Rα KO), loss of Th2 cytokines (IL-4 KO and IL-13 KO), or molecules involved in their signaling (STAT6 KO). As expected, steady-state expression of GATA3 by conventional Foxp3– T cells was dependent on STAT6 signaling (Figure 4A). In contrast, the pattern of GATA3 expression by Tregs was unaltered in the aforementioned mice (Figure 4A and Supplemental Figure 5). Thus, constitutive expression of GATA3 by Tregs is independent of Th2-associated cytokines (Figure 4A and Supplemental Figure 5). Although Th2 factors were not involved in the constitutive expression of GATA3, we postulated that exposure to Th2 cytokines may positively regulate its expression. Indeed, addition of exogenous IL-4 to in vitro–stimulated Tregs enhanced GATA3 expression (Supplemental Figure 6). However, increased levels of GATA3 by Tregs in vitro or in vivo was not associated with the expression of effector Th2 cytokines.
Figure 4. Cytokine regulation of GATA3 expression by Tregs.
(A) GATA3 expression in SILp Tregs is independent of STAT6 signaling. Cells were isolated from the SILp of naive BALB/c or STAT6-deficient mice and stained for expression of Foxp3 and GATA3. Plots are gated on live CD4+TCR-β+ cells. Numbers in quadrants refer to the percentage of each subset. (B) IL-6 and IL-27 impair expression of GATA3 by Tregs in vitro. CD4+eGFP+ T cells were cultured with SpDCs in the presence of anti-CD3 antibody and rIL-2 for 5 days in the presence of indicated cytokines. GATA3 expression was normalized to 100% of the IL-2 alone conditions. (C) STAT3 and T-bet negatively regulate GATA3 expression in SILp Tregs. Cells were isolated from the SILp of littermate control or Stat3f/f-CD4Cre (STAT3 KO) or Tbx21-deficient mice and stained for expression of Foxp3 and GATA3 intracellularly. FACS plots of control are gated on live CD4+TCRβ+ cells. n = 3 mice per group. **P < 0.008; ***P ≤ 0.0003. (D) Graphical representation of FACS plots in C. Data shown are representative of 2 (A, C, and D) and 3 (B) independent experiments with similar results. Data are presented as mean ± SEM.
Previous work has established that several inflammatory cytokines can oppose GATA3 expression in conventional CD4+ T cells (50, 51). To evaluate whether these inflammatory cytokines could also regulate the induction of GATA3 in Tregs, we stimulated FACS-sorted Tregs with anti-CD3 antibody in the presence of SpDC and various inflammatory mediators and assessed GATA3 expression in these cells by flow cytometry. Addition of IL-6 or IL-27 prevented GATA3 expression by Tregs (Figure 4B). In a less dramatic manner, exposure to IL-12 also led to a reduced GATA3 expression, while IL-23 alone had no effect on the expression of GATA3 by Tregs, possibly due to lack of IL-23 receptor expression on these cells (Figure 4B). As a corollary to this observation, mice in which IL-6/IL-27 signaling is altered (STAT3f/f-CD4Cre) (52, 53) had significantly increased frequencies of Tregs expressing GATA3 in the Lp compared with control mice (Figure 4, C and D). Similarly, mice in which Th1 responses are abrogated (T-bet–deficient mice, Tbx21 KO) also had increased frequencies of GATA3-expressing Tregs in the Lp (Figure 4, C and D). Taken together, our results show that inflammatory cytokines leading to Th1 or Th17 induction, such as IL-12, IL-27, and IL-6, can oppose GATA3 expression by Tregs.
GATA3-deficient Tregs fail to accumulate in tissues and express effector cytokines.
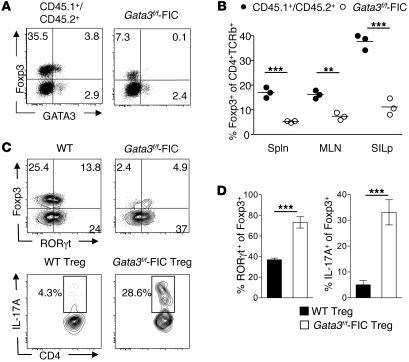
To further evaluate the role of GATA3 in the control of Tregs, Gata3flox/flox Foxp3-IRES-Cre (Gata3f/f-FIC) mice were generated. Young mice in which GATA3 has been selectively deleted from Foxp3-expressing cells did not exhibit any overt inflammatory disorder at steady state, and Tregs from lymphoid organs or residing in tissue were present at a frequency similar to that seen in control mice (data not shown). Thus, GATA3 is not essential for Treg survival under homeostatic conditions. To assess the role of GATA3 in Tregs after disruption of homeostasis, competitive mixed bone marrow chimeras were generated by lethally irradiating CD45.1+ congenic mice and transferring CD45.1+CD45.2+ WT bone marrow in combination with bone marrow from CD45.2+ Gata3f/f-FIC mice. Conventional T cell chimerism was equivalent between GATA3-deficient and control T cells (data not shown). Strikingly, we found that Gata3f/f-FIC Tregs failed to compete with WT Tregs (Figure 5, A and B). We also utilized a complementary approach to delete GATA3 from Foxp3+ Tregs without manipulating Foxp3 alleles. Gata3f/f-OX40Cre mice represent a useful tool for deleting GATA3 from Foxp3-expressing cells because Tregs constitutively express OX40 (54) and OX40 is expressed late during thymic development (54, 55). Similar data were observed in Gata3f/f-OX40Cre chimeric mice (Supplemental Figure 7, A and B). Of note, the frequency of GATA3-deficient Tregs was decreased in comparison with that of WT Tregs in all tissues analyzed (Figure 5B and Supplemental Figure 7, A and B). Consistent with the pattern of expression of GATA3 at steady state, decreased frequency of Tregs was more pronounced in the GI tract compared with other compartments (Figure 5B and Supplemental Figure 7, A and B). Under this setting, the level of tissue inflammation is heightened, as evidenced by increased IFN-γ and IL-17 production by effector T cells compared with that seen in nonirradiated mice (data not shown). Tregs from the SILp were assessed for the expression of T-bet, RORγt, and the effector cytokines IFN-γ and IL-17A. Notably, Gata3f/f-FIC Tregs significantly upregulated the expression of RORγt in comparison with the WT Tregs (Figure 5C). Additionally, Gata3f/f-FIC Tregs expressed high levels of the effector cytokine IL-17A (Figure 5, C and D) compared with WT Tregs. We did not observe the expression of T-bet or IFN-γ in either Gata3f/f-FIC or WT Tregs in this setting (data not shown). In agreement with our data using Gata3f/f-FIC donors, we found that Gata3f/f-OX40 Tregs that reside in the GALT also expressed increased levels of RORγt and IL-17A compared with WT Tregs in mixed bone marrow chimeras (Supplemental Figure 8). Thus, under homeostatic proliferation conditions, GATA3-deficient Tregs not only fail to accumulate in tissues but also acquire effector cytokine production.
Figure 5. GATA3-deficient Tregs do not accumulate in inflamed tissues.
(A–D) GATA3-deficient Tregs do not compete with WT Tregs. Ten weeks after reconstitution, cells were harvested from the SILp and stained for congenic markers CD45.1, CD45.2, CD4, CD25, Foxp3, and TCR-β. FACS plots are gated on live CD4+TCR-β+ cells and appropriate congenic markers. Numbers in quadrants refer to the percentage of each subset. (B) Graphical representation of percentage of Foxp3+CD4+TCR-β+ cells in the spleen, mesenteric lymph node, and SILp. Each circle represents 1 mouse, and crossbars depict the mean of 3 mice analyzed. *P < 0.05; **P < 0.01; ***P < 0.001 compared with CD45.1+/CD45.2+ Tregs. (C) GATA3-deficient Tregs express high levels of RORγt and IL-17A. Cells were harvested from the SILp as in A and then stained for congenic markers, CD4, TCR-β, Foxp3, GATA3, and RORγt (top panels) or stimulated for 3 hours with PMA/ionomycin and brefeldin A and then stained for congenic markers, CD4, TCR-β, Foxp3, and IL-17A (bottom panels). Plots are gated on congenic markers CD4+TCR-β+ (top panels) and Foxp3+ (bottom panels). Numbers in quadrants refer to the percentage of each subset. (D) Graphical representation of data in above FACS plots. n = 3 mice per group. ***P ≤ 0.0004. Data shown are representative of 3 independent experiments with similar results. Data are presented as mean ± SEM.
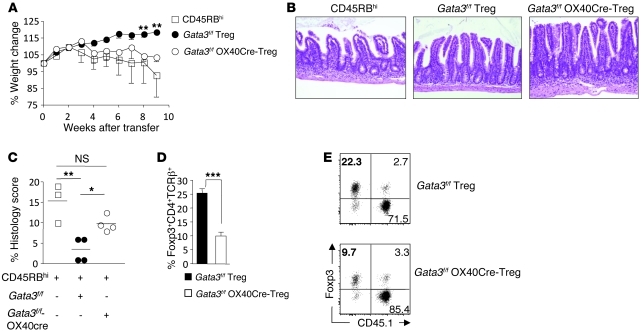
To assess whether absence of GATA3 could have functional consequences on the ability of Tregs to control tissue inflammation, we utilized a well-described model of T cell transfer colitis (56). Colitogenic CD4+CD45RBhi T cells were transferred into Rag1-deficient (Rag-KO) mice either alone or together with Gata3f/f-OX40Cre Tregs or Gata3f/f littermate Tregs (Gata3f/f). As expected, CD4+CD45RBhi T cells led to severe inflammation associated with significant weight loss that was prevented when Gata3f/f Tregs were cotransferred (Figure 6A). In contrast, Gata3f/f-OX40Cre Tregs were severely impaired in their capacity to control weight loss mediated by colitogenic effector T cells (Figure 6A). Consistently, mice cotransferred with Gata3f/f-OX40Cre Tregs had increased pathology compared with mice cotransferred with Gata3f/f Tregs (Figure 6, B and C). Although gut pathology precluded the evaluation of effector cytokine production by tissue Tregs in this setting, failure to control pathology was associated with significant reduction in the frequency of Gata3f/f-OX40Cre Tregs in the colon Lp compared with Gata3f/f Tregs (10% ± 1.4% vs. 25.4% ± 1.5%) (Figure 6, D and E). Together, these data suggest that GATA3 controls various aspects of Treg physiology in the context of inflammation including the capacity of Tregs to accumulate at inflamed sites and, in some settings, acquisition of effector cytokines.
Figure 6. GATA3-deficient Tregs do not protect from colitis.
(A) Rag-KO mice received either CD4+CD45RBhiCD45.1+ alone (white squares) or in combination with CD4+CD25brightCD45.2+ littermate Gata3f/f Tregs (black circles) or Gata3f/f-OX40Cre Tregs (black circles). **P < 0.001. Mice were weighed weekly and (B) tissue samples taken for H&E staining. Original magnification, ×20. (C) Graphical representation of histological score of mice; see Methods for scoring. Each symbol represents 1 mouse, and crossbars depict the mean of 3–4 mice analyzed. *P < 0.0114, Gata3f/f Treg compared with Gata3f/f-OX40Cre Tregs; **P < 0.0089, CD45RBhi compared with Gata3f/f Tregs. (D and E) Gata3f/f-OX40Cre Tregs were harvested from the SILp and stained for congenic markers CD45.1, CD45.2, CD4, TCR-β, and Foxp3; graph depicts percentage of Foxp3+CD4+ cells from colons of recipient mice. ***P < 0.0003. Numbers in quadrants refer to the percentage of each subset. Data shown are representative of 3 independent experiments with similar results. Data are presented as mean ± SEM.
GATA3 controls Treg polarization.
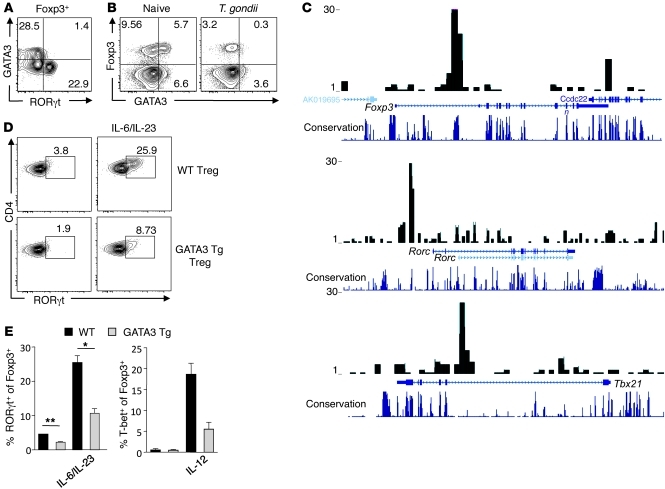
The intriguing observation that under homeostatic proliferation, GATA3-deficient Tregs could produce effector cytokines prompted us to assess the role of GATA3 in controlling Treg polarization. A canonical feature of the transcription factors involved in Th cell differentiation is their capacity to crossregulate each other’s expression. To evaluate whether such reciprocal control could also occur in Tregs, we assessed their relative expression of transcription factors in tissues at steady state and during inflammation. As previously discussed, a significant proportion of Tregs residing in the SILp expressed RORγt (Figure 1A), with a subfraction of these cells able to release IL-17 upon activation (30). Notably, we found that the expression of GATA3 and RORγt were mutually exclusive in SILp Tregs (Figure 7A). We have recently demonstrated that following oral infection with lethal strains of T. gondii, Tregs also acquired the capacity to produce IFN-γ in a T-bet–dependent fashion (18). In the Lp of infected mice, the proportion of Tregs expressing GATA3 was significantly reduced compared with Tregs from naive mice (Figure 7B and Supplemental Figure 9). Thus, high levels of RORγt or T-bet expression by Tregs is inversely correlated with that of GATA3 expression. One possibility was that GATA3 directly opposes RORγt and T-bet. To assess whether GATA3 could directly bind to Tbx21 or Rorc, genes encoding T-bet or RORγt, respectively, we utilized a ChIP-Seq approach to map GATA3-binding sites in Tregs genome wide. We found that GATA3 was able to bind to both tbx21 and Rorc gene loci in Tregs with a similar pattern to that observed in Th2 cells (Figure 7C and ref. 57). We found that 68% of the total 2,979 GATA3-binding sites in nTregs were similarly bound by GATA3 in Th2 cells (57). Notably, there are many genes that were bound by GATA3 only in Tregs but not in Th2 cells (57). Furthermore, we found that GATA3 bound to the CNS2 element in the Foxp3 gene (Figure 7C). These results further suggest that GATA3 may contribute to several aspects of Treg function or homeostasis.
Figure 7. GATA3 controls Treg polarization.
(A) GATA3 and RORγt expression are mutually exclusive in SILp Tregs. Cells were harvested from the SILp of naive C57BL/6 mice and stained for Foxp3, RORγt, and GATA3. Plots are gated on Foxp3+. Numbers in quadrants refer to the percentage of each subset. (B) GATA3 expression in SILp Tregs is downregulated during acute Th1 T. gondii infection. Expression of GATA3 was analyzed in naive C57BL/6 mice compared with age-matched female mice infected with 40 bradyzoites T. gondii clone C1 at 9 days after infection. Cells from the SILp were isolated, and expression of Foxp3, T-bet, and GATA3 was assessed by flow cytometry. Plots are gated on live CD4+TCRβ+ cells. Numbers in quadrants refer to the percentage of each subset. (C) ChIP-Seq of GATA3 in Treg binding to Foxp3, Rorc and Tbx21. CD4+CD25+ T cells were stimulated with anti-CD3/CD28 antibody for 3 days, and ChIP-Seq analysis was performed using a specific antibody against GATA3. The sequence tags mapped to foxp3, rorc, and tbx21 are shown. (D and E) Enforced expression of GATA3 inhibits RORγt and T-bet expression in Tregs. (D) CD4+Foxp3eGFP+ cells from WT or GATA3 Tg mice were cultured in the absence or presence of rIL-6/IL-23 for 4–5 days. Cells were stained for Foxp3, T-bet, RORγt, and GATA3 and assessed by flow cytometry. Plots are gated on live CD4+Foxp3+ cells. Numbers in quadrants refer to the percentage of each subset. (E) Graphical representation of data in above FACS plots. *P ≤0.024; **P ≤ 0.0017. Data shown are representative of at least 3 independent experiments with similar results. Data are presented as mean ± SEM.
In order to specifically address the potential role of GATA3 in the regulation of T-bet and RORγt expression, we utilized Tregs that ectopically express GATA3 by transgenic expression of GATA3 under the control of the human CD2 promoter (GATA3 Tg mice) (58). Enforced expression of GATA3 did not influence the level of expression of Foxp3 by Tregs or modify their capacity to suppress T cell proliferation in vitro (data not shown). Purified Tregs from GATA3 Tg mice and littermate control mice were stimulated in vitro using anti-CD3 antibody and rIL-2 in the presence of SpDC with or without addition of Th17-polarizing cytokines (IL-6 and IL-23) or Th1-polarizing cytokine (IL-12). Upon exposure to IL-6 and IL-23 or IL-6 alone (data not shown), RORγt was induced in one-third of WT Tregs (Figure 7, D and E). Enforced expression of GATA3 by Tregs significantly limited their capacity to express RORγt (Figure 7, D and E). Similarly, following exposure to IL-12, T-bet induction was suppressed in GATA3 Tg Tregs compared with WT Tregs (Figure 7E). Thus, taken together, our data show that under inflammatory settings, GATA3 expression in Tregs limits the expression of transcription factors associated with effector T cell differentiation. In particular, GATA3 controls RORγt and aberrant production of IL-17A. Overall, these data suggest a model in which increased exposure to inflammatory signals can limit GATA3 expression, which in turn leads to increased polarization of Tregs (Supplemental Figure 10).
GATA3 controls Foxp3 expression and Treg accumulation at inflamed sites.
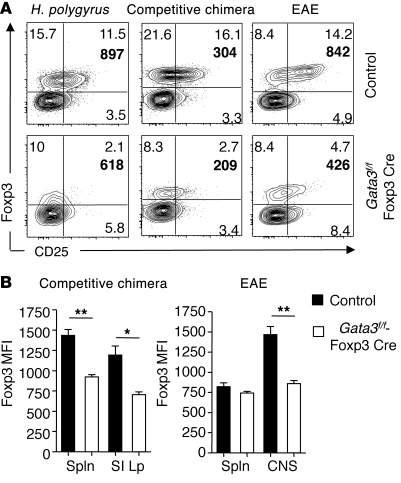
In addition to their aberrant production of cytokines in polarized settings, Tregs deficient in GATA3 failed to accumulate in tissues. Various factors may account for this defect, including impaired proliferation, survival, or stability. In vitro GATA3-deficient Tregs were comparable in their suppressive function of effector T cell proliferation as well as their capacity to survive and proliferate (Supplemental Figure 11 and data not shown). Thus, failure to control inflammation may be primarily associated with impaired maintenance at inflamed sites rather than to function or proliferation. To further explore this possibility, we evaluated the fate of Tregs under nonhomeostatic proliferation conditions. Although Tregs have been shown to control inflammation during EAE induced by suboptimal immunization, in more conventional models using high doses of peptide, Treg depletion has a minimal effect on disease progression (59, 60). Nevertheless, this later model allowed us to further probe the ability of GATA3-deficient Tregs to be maintained within a highly Th17-polarized environment (59). We designed chimeric mice with either Gata3fl/+-Foxp3Cre or Gata3fl/fl-Foxp3Cre bone marrow in a way that allowed normalizing Treg frequencies between the 2 groups. Prior to immunization for EAE, the frequency of Tregs was comparable between the 2 groups (Supplemental Figure 12). This approach allowed us to track Tregs independently of homeostatic proliferation. Following immunization with MOG35–55 in CFA, disease progression was comparable between the 2 groups. However, GATA3-deficient Tregs were significantly reduced in frequency in the inflamed central nervous environment (Figure 8A). Our data confirm that at the peak of the disease a significant proportion of Tregs express GATA3 (Supplemental Figure 13). Thus, GATA3 expression correlates with the activation status of Tregs under this setting. This observation also further supports a role for GATA3 in controlling Treg fate in highly inflamed tissues. Furthermore, GATA3-deficient Tregs expressed significantly lower levels of both CD25 and Foxp3 in the brain but not in other compartments such as the spleen (Figure 8, A and B). This decrease in Foxp3 intensity was consistently observed under various inflammatory or polarized settings including mixed bone marrow chimeras or in Gata3f/f-Foxp3Cre mice infected with Th2-inducing Heligmosomoides polygyrus (Figure 8, A and B). Consistent with previous findings that depletion of Tregs does not alter worm burden (61), fecundity of worms, as assessed by fecal egg counts, was unaffected in the absence of GATA-3 in Tregs at the time point analyzed. Although we cannot exclude the possibility that long term pathology may be increased in the absence of GATA3 expression by Tregs, at the time point examined, we did not observe gross changes in pathological outcome.
Figure 8. GATA3 maintains Foxp3 and CD25 expression in Tregs during inflammation.
(A) GATA3-deficient Tregs express lower levels of Foxp3 and CD25 during inflammation. Cells were harvested from the SILp for H. polygyrus infection, brain and spinal cord for EAE, and SILp for competitive bone marrow. Cells were stained for CD4, TCRβ, CD45.1, CD45.2, Foxp3, and CD25. FACS plots are gated on live CD4+TCRβ+ cells and congenic markers. Numbers in quadrants refer to the percentage of each subset; bold number in upper right quadrant of plot is the MFI of CD25 on Foxp3+ cells. (B) Graph depicts the MFI of Foxp3 on Foxp3+ cells. *P ≤ 0.014; **P ≤ 0.0012. Data are presented as mean ± SEM.
Further supporting the idea that GATA3 function is revealed under extreme settings, reduced Foxp3 expression was most notable at sites of inflammation or in highly polarized settings (Figure 8, A and B). Recent findings demonstrated that a conserved noncoding DNA sequence element (CNS2) at the Foxp3 locus encodes information associated with the stability of the Treg population. Intriguingly, as previously mentioned, our ChIP-seq data showed that GATA3 specifically binds to the CNS2 element of foxp3, suggesting a link between this regulatory element and GATA3. These results suggest that GATA3 is necessary for promoting the accumulation of Tregs at inflamed sites, a phenomenon that can be at least in part associated with the capacity to sustain Foxp3 expression.
Discussion
Our data revealed an unexpected role for the canonical Th2 transcription factor GATA3 in controlling certain key features of Treg physiology during inflammation. In particular, GATA3 restrains excessive polarization and inflammatory cytokine production by Tregs. Furthermore, our data demonstrate that Treg intrinsic expression of GATA3 is required for the maintenance of high-level Foxp3 expression and promotes Treg accumulation at inflamed sites. Altogether, our work reveals a new facet in the complex role of GATA3 in T cell fate and highlights its fundamental role in controlling Treg physiology during inflammation.
While we found that GATA3 represents a default response for Tregs upon TCR engagement, increased exposure to inflammatory signals can limit its expression. Our work supports the following model in which, under physiological or mild inflammation, intrinsic expression of GATA3 by Tregs can partially restrain expression of transcription factors associated with effector T cell lineage and sustain high levels of Foxp3 expression. In extreme settings, sustained inflammatory responses and loss of GATA3 could lead to an unleashed polarization by Tregs and their potentially harmful production of effector cytokines as well as loss of their regulatory phenotype.
In the present study, we found that GATA3 is highly expressed in Tregs at barrier sites such as the skin and the gut and is readily expressed in Tregs following TCR activation. Gene expression analysis previously reported the coexpression of Foxp3 and GATA3 on Tregs (62). Furthermore, iTregs derived from naive human cells can express high levels of GATA3 (63), and in mice, a small fraction of Tregs residing in the spleen expressed GATA3 (64). Physical interaction between Foxp3 and GATA3 has been previously reported; however, the consequence of this interaction remains unclear (65, 66). Our present data obtained both in vivo and in vitro further confirm that committed and induced Tregs from both mice and humans coexpress Foxp3 and GATA3.
Because overexpression of GATA3 or exposure to IL-4 can inhibit the induction of Foxp3 during iTreg differentiation, previous reports have suggested that GATA3 and Foxp3 coexpression were incompatible in Tregs (63, 67, 68). We could argue that these data are associated with the high level of GATA3 expression reached under these overexpression conditions (63, 67, 68). It also remains unclear in this setting whether IL-4–dependent GATA3 or GATA3-dependent IL-4 production is responsible for suppressing Foxp3 induction (63). Another possible explanation for these apparently conflicting results may be associated with the mechanism by which GATA3 is induced. Indeed, in the above-mentioned settings, GATA3 was induced in an IL-4/STAT6–dependent manner (63, 66–68). However, we found that constitutive expression of GATA3 by Tregs in vivo was independent of the IL-4/STAT6 signaling pathway, suggesting that its expression is induced via other means in Tregs.
GATA3 expression by conventional CD4+ T cells can be induced by other factors such as TCR, IL-2, Wnt, or Notch (38–40, 69, 70). Our in vitro data revealed that in the absence of inflammatory mediators, GATA3 was ubiquitously induced in Tregs following TCR engagement in both mice and human Tregs, suggesting that under neutral conditions, GATA3 is a default response to TCR stimulation. This finding is consistent with our observation that at steady state, GATA3 is primarily expressed in Tregs residing in tissues that exhibit constitutive low-level inflammation, such as the GI tract and the skin. Our data also support the idea that IL-2 contributes to the expression of GATA3 by Tregs. Indeed, treatment of mice with IL-2/anti–IL-2 complex led to a systemic and significant increase of GATA3 expression by Tregs, while expression of GATA3 by Tregs was abolished in IL-2–deficient mice. Because IL-2–deficient mice develop pathology over time, ongoing inflammation could also account for the effect observed. However, in our facility, we found that these mice do not develop inflammation before 15 weeks of age, suggesting that lack of GATA3 expression by Tregs in 8-week-old mice is more likely a consequence of IL-2 deficiency than increased inflammation. A role for IL-2 in promoting GATA3 expression is further supported by the observation that during acute T. gondii infection in which IL-2 is limiting, Tregs fail to express GATA3. However in both settings, we cannot fully exclude the possibility that upregulation of IL-27 or IL-6, cytokines we have shown to inhibit GATA3 expression by Tregs, could also contribute to this effect. Intriguingly, IL-2 alone induces low levels of GATA3 by Tregs. Previous work demonstrated that IL-2 could directly trigger a low level activation by Tregs (71, 72), and our present results support the idea that GATA3 expression may be part of this response. Although IL-2 is not absolutely required for early GATA3 expression by Tregs in the context of TCR engagement, we found that IL-2 is required to sustain its expression. As previously shown for conventional T cells, IL-2 may promote or maintain GATA3 expression in a STAT5-dependent manner (69). Although dissociating the role of IL-2 in Treg survival versus GATA3 induction remains difficult at this point, we could speculate that both roles may account for optimal and sustained GATA3 expression upon activation. In addition, steady-state expression of GATA3 by tissue-resident Tregs is likely to reflect their status of activation and in particular recent TCR engagement in the context of IL-2 rather than an adaptation to a defined environment.
One canonical feature of the transcription factors involved in Th cell differentiation is their capacity to crossregulate their expression. Our data support the idea that such a mechanism of regulation also occurs in Tregs. Indeed, in vivo expression of GATA3 at steady state was mutually exclusive with that of RORγt expression in GALT Tregs. Similarly, T-bet–expressing Tregs that arose during acute T. gondii infection did not coexpress GATA3. While GATA3 expression appears to be a default response of Tregs under neutral conditions, cytokines associated with either Th1 or Th17 differentiation can oppose its upregulation. Notably, we found that cytokines signaling through STAT3 (such as IL-6, IL-27) or STAT4 (such as IL-12) limited GATA3 expression by Tregs. In conventional T cells, signaling of IL-12 through STAT4 inhibits the expression of GATA3 in Th1 cells, and downstream of STAT4, T-bet can also downregulate the expression of GATA3 (69, 73–75). Similar mechanisms may account for the negative regulation of GATA3 expression in Tregs. Of note, the degree of inhibition was not as profound in the presence of IL-12 compared with STAT3-signaling cytokines. This latter finding is consistent with the fact that unless exposed to a highly Th1-polarizing milieu, Tregs poorly expressed IL-12Rβ2 and are consequently ill equipped to optimally respond to this cytokine (18, 21). Our data indicate that inflammatory cytokines directing Tregs toward a Th1 or Th17 phenotype are associated with the reduction of GATA3 expression, and conversely, enhanced GATA3 expression occurs in situations in which signaling via these cytokines is limited.
Recent reports provide evidence that a degree of plasticity through the acquisition of specific transcription factors in Tregs was required for them to exhibit control in defined polarized settings (21–23). Such plasticity, however, is not without consequences, as we and others have shown that in extreme inflammatory settings or in defined compartments, Tregs can express effector cytokines (18, 24–27). For instance, previous reports demonstrated that a subfraction of RORγt+ Tregs can produce IL-17A (33). We found that in the gut, expression of RORγt was exclusive with that of GATA3 in Tregs and that following reconstitution in mice, GATA3-deficient Tregs produced increased levels of IL-17A compared with WT Tregs. We have also previously shown that in the context of lethal T. gondii infection, T-bet–expressing Tregs produce high levels of IFN-γ (18). In both instances, effector cytokine production by Tregs occurred in situations in which GATA3 was reduced or absent. Previous work supported the idea that in some settings in which Foxp3 expression is reduced by genetic manipulation, Foxp3-low cells expressing GATA3 aberrantly produced IL-4 (66). However, in our Th2 setting, loss of GATA3 was not associated with production of IL-4 or IL-13, suggesting that loss of Foxp3, rather than induction of GATA3, confers a Th2 phenotype to Tregs.
Our data obtained both in vivo and in vitro suggest that one function of GATA3 is to control excessive Treg polarization and prevent acquisition of effector cytokines. Indeed, ChIP-seq data revealed multiple binding sites of GATA3 on both the Tbx21 and Rorc loci. Together with the distinct patterns of expression of T-bet and RORγt, this suggests that GATA3 may directly control the level of expression of these factors. In further support of this hypothesis, ectopic expression of GATA3 in Tregs reduced the induction of both transcription factors by inflammatory cytokines in vitro. Finally, in the absence of GATA3, Tregs that accumulate in the GI tract following reconstitution expressed increased levels of RORγt compared with WT Tregs and acquired the capacity to produce high levels of the effector cytokine IL-17A. While both T-bet and RORγt were controlled by GATA3 in vitro, our results in vivo support the idea that GATA3 may have a more pronounced role on the latter. Such an endogenous brake may endow Tregs with the appropriate survival/homing properties in polarized settings while restraining their potentially detrimental expression of effector functions. Further studies are required to understand the complex interplay of these transcription factors in the control of Treg adaptation versus pathogenicity during inflammation.
Accumulation of Tregs is critical for their ability to maintain tissue homeostasis (3, 14–20). The lack of spontaneous inflammatory disorders in Gata3f/f-FIC mice suggests that GATA3 is not essential for Treg steady-state homeostasis in young mice. On the other hand, a role for GATA3 in promoting Treg fitness/survival/adaptation was revealed under inflammatory or homeostatic proliferative settings. Indeed, expression of GATA3 by Tregs was required for their accumulation under various experimental settings including inflammation under nonhomeostatic proliferation conditions such as GI infection or EAE. We also found that this impaired accumulation had functional consequences for the capacity of Tregs to control colitis. Various nonexclusive factors can account for impaired accumulation in tissues including defects in proliferation, migration, or local stability/survival. We found no obvious defects in the capacity of GATA3-deficient Tregs to proliferate in vitro. Complementing these data, GATA3 was not required for expansion of Tregs following IL-2/anti–IL-2 treatment. Furthermore, GATA3 was not required for Treg suppression of effector responses in vitro, which is consistent with the lack of inflammatory disorders observed in Gata3f/f-FIC mice. These observations would argue that failure of GATA3-deficient Tregs to control colitis might be primarily associated with impaired accumulation rather than a defect in proliferation or suppression.
One striking observation was the consistent decrease in Foxp3 expression by Tregs in all inflammatory settings evaluated including Th2-polarized nematode infection or Th17-polarized EAE. Such a feature has been previously associated with poor stability of Tregs. Recent findings demonstrated that a conserved noncoding DNA sequence element (CNS2) at the Foxp3 locus encodes information associated with the stability of the Treg population (10). In particular, CNS2 is required for sustained Foxp3 expression in the progeny of dividing cells. Of note, our ChIP-seq analysis revealed that GATA3 binds to the CNS2 region in the Foxp3 gene. We also found that in the absence of GATA3, Tregs phenocopy CNS2-deficient Tregs in terms of reduced frequencies and decreased Foxp3 expression on a per cell basis during inflammation (10). Foxp3 binds to CNS2 in a Cbf-β–Runx1 and CpG DNA demethylation–dependent manner (10), and it would remain important to assess how GATA3 integrates in this module to promote Treg lineage stability. We could also speculate that reduced Foxp3 expression in the absence of GATA3 can contribute to the aberrant production of cytokines observed by these cells during inflammation. Several studies have demonstrated the importance of maintained, sufficiently high levels of Foxp3 protein expression to preserve full suppressor function (10, 76, 77). Thus, other factors, such as defects in function or migration, can also account for the phenotype observed. Nevertheless, impaired Treg stability via reduction of Foxp3 expression in the absence of GATA3 can be sufficient to explain the poor accumulation of Tregs in inflamed tissues or during homeostatic proliferation and the subsequent impaired control of inflammation. Importantly, the capacity of Tregs to sustain Foxp3 expression and favor their tissue accumulation was observed regardless of the polarized setting or tissue evaluated, suggesting a ubiquitous role for GATA3 in controlling Treg fate during inflammation.
Overall, our results support the idea that in a manner comparable to that of the role of GATA3 in conventional T cells, the role of this transcription factor in Tregs is likely to be complex and affect many aspects of their physiology. In this study, we uncover 2 of its potential roles. In particular, GATA3 controls excessive effector polarization by Tregs and their capacity to accumulate in tissues during inflammation. Although observed in various inflammatory settings and more recently during autoimmunity in humans (36), the role of IFN-γ or IL-17 production by Tregs remains, to date, unknown. In the context of our present study, it would remain important to further explore the contribution of aberrant polarization of Tregs versus impaired accumulation observed in the absence of GATA3 to tissue damage and/or impaired control of effector responses. We could speculate that in highly polarized settings, these events are likely to contribute in a synergistic manner to the failure of Tregs to be maintained and appropriately control tissue damage. While the identification of the factors involved in the adaptation of Tregs is likely to offer important therapeutic avenues, further characterization of elements, such as GATA3, involved in the control of Treg fate during inflammation will be equally critical to our understanding of peripheral tolerance and the development of safe therapeutic approaches.
Methods
Mice.
C57BL/6 (WT) and B6.SJL mice were purchased from Taconic Farms or bred in house. Foxp3eGFP reporter mice (Foxp3eGFP) were obtained from M. Oukka (Seattle Children’s Research Institute) (78). Gata3flox/flox-Foxp3-IRES-Cre (Gata3f/f-FIC) mice were generated by crossing C57BL/6 Gata3flox/flox (79) with Balb/c Foxp3-IRES-Cre mice (80) and subsequently backcrossed to the C57BL/6 background 4 more generations. Gata3f/f-OX40Cre mice were described in ref. 47 and bred in house. Gata3f/f-Foxp3-Cre mice were generated by crossing the Gata3flox/flox mouse (47) to the Foxp3-Cre mouse (gift from Alexander Rudensky, Sloan-Kettering Institute, New York, New York, USA) (81). Gata3 transgenic mice expressing the human CD2 promoter were bred in house (58). Stat3f/f-CD4Cre were obtained from J. O’Shea (National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIH, Bethesda, MD) (67). We crossed OT-II mice with Foxp3eGFP (OT-IIxFoxp3eGFP) mice. IL-2–deficient mice (line 159) were purchased from the Taconic National Institute of Allergy and Infectious Diseases (NIAID) exchange and crossed to the Foxp3eGFP reporter mouse to yield IL-2xFoxp3eGFP (Il2–/–) and bred in house. Mice deficient in Rag (line 146), IL-4 (line 46), IL-4Rα (line 177), IL-13 (line 178), and Stat6 (line 237) were purchased from the Taconic NIAID exchange. Mice between 6 and 14 weeks of age were used. Mice were sex- and age-matched for each experiment.
ChIP-seq analysis.
FACS-sorted CD4+CD25+ T cells were expanded with plate-bound anti-CD3/anti-CD28 in the presence of rIL-2 (100 U/ml) for 3 days. 5–10 × 106 cells were then cross-linked with formaldehyde, and the chromatin was sonicated into small fragments. Then the fragmented chromatin was immunoprecipitated with anti-GATA3 (clone L50-823; BD Biosciences). The ChIP DNA fragments of approximately 200 bp were treated and sequenced using the Illumina/Solexa 1G Genome Analyzer as previously described (82). Sequence reads were summed and displayed as a custom track on the UCSC Genome Browser.
Phenotypic analysis.
Cells from spleen, mesenteric LN, intestinal epithelial lymphocytes, Lp, and Peyer patches were prepared as previously described (42). Cells from the dermis were prepared as previously described (83). Single-cell suspensions were incubated with anti-FcεIII/II receptor. Cells were stained with fluorochrome-conjugated antibodies against surface markers CD4 (RM4-5), CD8a (53-6.7), CD25 (PC61.5), TCR-β chain (H57-597), CD45.1 (A20), and CD45.2 (clone 104), in HBSS for 20 minutes on ice and then washed. Live/Dead Fixable Blue Cell Stain Kit (Invitrogen) was used to exclude dead cells. For Foxp3 (FJK-16s) staining, cells were subsequently stained using the Foxp3 staining set (eBioscience) according to the manufacturer’s protocol. Nuclear Ki-67 staining was performed using an antibody against human Ki-67 (B56) from BD Biosciences — Pharmingen. For GATA3, RORt, and T-bet staining, anti-GATA3 (L50-823; BD Biosciences — Pharmingen), anti-GATA3 (TWAJ), anti-RORt (AFKJS-9), and anti-T-bet (eBio4B10) from eBioscience were used. Cell acquisition was performed on an LSRII machine using FACSDiVa software (BD Biosciences). For each sample, at least 300,000 events were collected. Data were analyzed using FlowJo software (TreeStar).
Murine T cell purification.
Single-cell suspensions of spleen and peripheral LNs extracted from naive Foxp3eGFP, GATA3 Tg, and C57BL/6 WT mice were enriched for CD4+ T cells by a negative selection using an autoMACs (Miltenyi Biotec). The enriched fraction was further labeled with fluorescent dye–conjugated mAbs, including CD4 (RM4-5), CD25 (7D4), CD44 (IM7), and CD45RB (16A) (all from eBioscience), and sorted by flow cytometry on a FACSAria (BD Biosciences). Purified CD4+CD25–CD44loeGFP– T cells (<0.5% Foxp3+) were used for in vitro conversion assays. Purified CD4+eGFP+ or CD4+CD25bright cells were used for Treg-DC incubation.
In vitro restimulation and intracellular cytokine detection.
RPMI 1640 supplemented with 10% FBS, penicillin, streptomycin, gentamicin, HEPES, glutamine, nonessential amino acids, and 50 mM of β-mercaptoethanol was used for in vitro restimulation. For basal cytokine detection, spleen, mesenteric lymph node (MLN), and lamina propria (LP) single-cell suspensions were cultured in duplicate at 1 × 106 cells/ml in a 96-well U-bottom plate and stimulated with 50 ng/ml PMA (Sigma-Aldrich) and 5 μg/ml ionomycin (Sigma-Aldrich) in the presence of brefeldin A (GolgiPlug, BD Biosciences). After 4 hours, dead cells were stained as described previously, washed twice with PBS, and fixed using paraformaldehyde 2% (Electron Microscopy Sciences) solution. Cells were then stained with fluorochrome-conjugated antibodies against TCR-β chain (H57-597), CD4 (RM4-5), IFN-γ (XMG1.2), IL-17A (17B7), IL-10 (JES5-16E3), IL-2 (JES6-1A12), Foxp3 (FJK-16s), or isotype controls: rat IgG1 (eBRG1), rat IgG2a (eBR2a), rat IgG2b (eB149/10H5), and mouse IgG1 (clone P3) in the presence of anti-FcεIII/II receptor for 60 minutes in FACS buffer containing 0.5% saponin. All antibodies were purchased from eBioscience or BD Biosciences.
Murine Treg stimulation.
3–5 × 104 CD4+Foxp3eGFP+ T cells were cocultured in each well with SpDCs purified from naive mice at a 1:1 ratio in complete medium with soluble anti-CD3 mAb (0.25 μg/ml) (BD Bioscience), and 1–5 ng/ml of rIL-2 (PeproTech) was supplemented in cocultures. After incubation for 24 hours with SpDC alone or in the presence of anti-CD3 for 72 hours, rIL-2 was added at the beginning of the culture. Various combinations of cytokines including 10 ng/ml of rIL-6, IL-12, IFN-γ, or mAb anti–IL-12/23p40, or 10 μg/ml of anti–IFN-γ and anti–IL-4 antibody were added at the start of coculture as described above. After 4- to 5-day culture at 37°C, 5% CO2, cells were harvested and stained for Foxp3, GATA3, T-bet, RORγt, and cytokines as previously described.
Human Treg purification and activation.
PBMCs were prepared over Ficoll-Paque Plus gradients (GE Healthcare). CD4+ T cells were purified by positive selection using Miltenyi beads over the autoMACS cell separator (both from Miltenyi Biotec). The cells were labeled with CD4 FITC, CD45RA PECY5, and CD25 PE, purchased from Invitrogen, and CD127 Alexa Fluor 647 from BD Biosciences — Pharmingen. Labeled CD4+ cells were sorted with FACSAria Flow Cytometer (BD Biosciences) for 2 populations: Tregs, CD4+CD127–CD25hi, and naive T cells, CD4+CD127+CD25–CD45RA+. The post-sort purity was higher than 99%. Other than fresh time points, all FACS-sorted cells (1 × 106 cells per well) were activated in vitro with plate-bound anti-CD3 and anti-CD28 (2 μg/ml each) in 24-well culture plates coated overnight at 4°C. The culture medium consisted of RPMI (Lonza) with 10% heat-inactivated serum and 50 IU/ml IL-2 (Hoffman-LaRoche). Human Tregs were cultured under activating conditions in the presence of anti–IL-4 and anti–IFN-γ (R&D Systems). Cells were stimulated for 1–3 days, then washed and stained for intracellular expression of FoxP3 and GATA3.
Oral antigen administration.
CD4+Foxp3–CD44lo T lymphocytes from the secondary LNs and spleen of OT-IIxFoxp3eGFP mice (CD45.2) were purified by FACS sorting and adoptively transferred into naive B6.SJL recipient mice (CD45.1). Each mouse received 1.5–2 × 106 cells and then 1.5% OVA solution dissolved in drinking water (grade V; Sigma-Aldrich) for 6 consecutive days. On day 7, spleen, MLN, and LP were collected from B6.SJL hosts, and Foxp3 and GATA3 expression were assessed in transferred cells. Spleen, MLN, and LP single-cell suspensions were prepared and stained as described above.
IL-2 in vivo treatment.
rIL-2 and anti–IL-2 antibody complexes were administered or not administered intraperitoneally for 5 consecutive days. The complex was formed by incubating 1.5 μg recombinant mouse IL-2 and 15 μg of functional grade purified anti-mouse IL-2 (clone: JES6-1A12; eBioscience) for 5 minutes at room temperature.
Parasite and infection protocol.
ME-49 type II strain (ATCC # 50840) of T. gondii was used for production of tissue cysts in C57BL/6 mice. Tissue cysts used in experiments were obtained from mice that were inoculated 1–2 months previously with 5 cysts by gavage. Animals were sacrificed, brains were removed and homogenized in 1 ml of PBS, pH 7.2, and tissue cysts were counted on the basis of 3 or more aliquots of 20 μl. For challenge studies, 8- to 12-week-old mice were used for all studies. Mice were infected by oral route. Parental ME-49 was electroporated with RFP and selected for red fluorescence. STAg was prepared as previously described (84). Mice were perorally infected with the 150 infective stage larvae (L3) H. polygyrus.
Confocal analysis.
Foxp3eGFP+ T cells from the spleen and SILp were sorted from naive Foxp3eGFP reporter mice. Cells were subsequently stained for Foxp3 (FITC), GATA3 (AF647), and Hoechst 33342 (Invitrogen) as previously described. Images were acquired on a Yokogawa CSU-22 spinning disc confocal unit installed on an IX-71 inverted Olympus microscope. The confocal microscope is equipped with 5 lasers delivering the wavelengths 405, 440, 488, 514, 561, and 640 nms via a single mode optic fiber. The confocal contains a quad dichroic (for 405, 488, 561, and 640 nm excitations), and corresponding emission filters are installed in a filter wheel before the camera. Images were acquired using an EMCCD camera (Quant-EM; Photometrics) and either a ×60, 1.45 NA TIRF or a ×100 1.4 NA oil immersion objective. All hardware was controlled using Metamorph software. Image analysis was also performed using Metamorph software.
T cell transfer model of colitis.
CD4+ T cells were enriched by negative selection with the CD4+ T cell Isolation Kit (Miltenyi Biotec). Naive CD4+CD45RBhi T cells were purified (>98%) from spleens and peripheral LNs of congenic C57BL/6 mice via FACS sorting as previously described (85) with a cell sorter BD Aria II (BD). CD45.2+CD4+CD25+ Tregs were isolated from Gata3f/f or Gata3f/f-OX40Cre mice from the spleens and peripheral LNs and cotransferred at a 1:3 ratio with naive CD4+CD45RBhi T cells. T cell suspensions were washed in sterile HBBS, and female Rag1–/– recipient mice received 3 × 105 CD45.1+CD4+CD45RBhi T cells by intraperitoneal injection. For cotransfer experiments, CD4+CD25+ Tregs (6 × 104 cells) were transferred per mouse.
Pathology assessment.
Mice were euthanized 8 weeks after induction of colitis. Small and large intestines were removed and immediately fixed in a solution containing 10% formalin. Paraffin-embedded sections were cut at 0.5 μm and stained with H&E. The sections of the small and large intestine were examined histologically. Inflammation was scored on the following modified scale (86) of 0–5: 0, within normal limits; 1, minimal inflammatory leukocyte infiltrates multifocally or locally; 2, mild inflammatory leukocytic and granulocytic infiltrates within the Lp; 3, mild to moderate inflammatory infiltrates in the Lp and submucosa, alterations in intestinal crypts, local to diffuse thickening of the mucosal epithelium; 4, moderate to marked inflammatory infiltrates of primarily viable and degenerate neutrophils, eosinophils, lymphocytes, plasma cells admixed with cellular debris within the Lp and submucosa with separation or loss of crypts; and 5, marked to severe inflammation diffusely with altered or complete loss of normal histologic structures and abundant inflammatory infiltrates that may extend transmurally (with peritonitis).
RNA purification and quantitative PCR.
Total RNAs were isolated using a combination of TRIzol (Invitrogen) and an RNeasy Kit (QIAGEN). cDNAs were prepared using SuperScript III Reverse Transcriptase (Invitrogen). Quantitative PCR was performed on a 7900HT Sequence Detection System (Applied Biosystems) using the following predesigned primer-probe sets: β-actin (Applied Biosystems) and GATA3, as previously described (47).
Experimental autoimmune encephalomyelitis.
CD45.1 congenic mice were lethally irradiated and reconstituted with either Gata3fl/+-Foxp3Cre bone marrow or Gata3fl/fl-Foxp3Cre bone marrow. Mice were allowed to reconstitute for 9 weeks before immunization for EAE. Mice were immunized with 200 μg of MOG35–55 peptide (Invitrogen) emulsified in CFA, containing 250 μg of heat-killed Mycobacterium tuberculosis (Invitrogen) subcutaneously in 100 μl total volume. On day 0 and day 2, mice were given 200 ng of pertussis toxin (List Biological Laboratories) intraperitoneally. After day 7, mice were observed daily for disease onset. Disease score was as follows: 0, no disease; 1, tail paralysis; 2, impaired gait and/or impaired righting reflex; 3, partial hind limb paralysis; 4, full hind limb paralysis; 5, front limb paralysis and complete hind limb paralysis; 6, moribund.
Statistics.
Groups were compared with Prism software (GraphPad) using a 2-tailed unpaired Student’s t test. Data are presented as mean ± SEM. P < 0.05 was considered significant.
Study approval.
All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care–accredited animal facility at the NIAID and housed in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals under an animal study proposal approved by the NIAID Animal Care and Use Committee. Peripheral blood was obtained from healthy adult donors by the Department of Transfusion Medicine at the NIH. All subjects provided written informed consent. The acquisition of blood products was approved according to the NIAID Institutional Review Board and in accordance with the Declaration of Helsinki.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (to Y. Belkaid and W.E. Paul), and NCI, Center for Cancer Research (to R. Bhairavabhotla), NIH, National Institute of Dental and Craniofacial Research, and The Crohn’s and Colitis Foundation of America (ref #2387 to E.A. Wohlfert). We thank Rajat Varma for his assistance with the confocal microscopy. We thank Kevin Holmes and the NIAID sorting facility and Kim Beacht for technical assistance. We thank WanJun Chen, Annie Kilby, Sean Spencer, and Mike Molloy for their critical reading of the manuscript.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2011;121(11):4503–4515. doi:10.1172/JCI57456.
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*). Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DJ, Koch MA. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat Rev Immunol. 2011;11(2):119–130. doi: 10.1038/nri2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat Immunol. 2007;8(3):277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 5.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Ruan Q, et al. Development of Foxp3(+) regulatory T cells is driven by the c-Rel enhanceosome. Immunity. 2009;31(6):932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isomura I, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206(13):3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol. 2003;74(6):961–965. doi: 10.1189/jlb.0603272. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 13.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178(1):280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 14.Yuan Q, et al. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204(6):1327–1334. doi: 10.1084/jem.20062076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang SG, et al. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132(3):966–981. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLachlan JB, Catron DM, Moon JJ, Jenkins MK. Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity. 2009;30(2):277–288. doi: 10.1016/j.immuni.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31(5):772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207(4):699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grinberg-Bleyer Y, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med. 2010;207(9):1871–1878. doi: 10.1084/jem.20100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10(6):595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458(7236):351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326(5955):986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5(11):1149–1156. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 25.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201(12):1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106(12):4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beriou G, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453(7192):236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7(4):401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 32.Almeida AR, Zaragoza B, Freitas AA. Competition controls the rate of transition between the peripheral pools of CD4+CD25- and CD4+CD25+ T cells. Int Immunol. 2006;18(11):1607–1613. doi: 10.1093/intimm/dxl093. [DOI] [PubMed] [Google Scholar]
- 33.Osorio F, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008;38(12):3274–3281. doi: 10.1002/eji.200838950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39(4):948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3(+) regulatory T cells in human autoimmune disease. Nat Med. 2010;17(6):673–675. doi: 10.1038/nm.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329(5999):1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19(1):83–94. doi: 10.1016/S1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- 39.Scheinman EJ, Avni O. Transcriptional regulation of GATA3 in T helper cells by the integrated activities of transcription factors downstream of the interleukin-4 receptor and T cell receptor. J Biol Chem. 2009;284(5):3037–3048. doi: 10.1074/jbc.M807302200. [DOI] [PubMed] [Google Scholar]
- 40.Yu Q, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10(9):992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204(8):1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311(5769):1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 44.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10(4):371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 45.Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169(9):4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 46.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89(4):587–596. doi: 10.1016/S0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5(11):1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 48.Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. J Exp Med. 2005;202(6):793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16(1):3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 50.Kusam S, Toney LM, Sato H, Dent AL. Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J Immunol. 2003;170(5):2435–2441. doi: 10.4049/jimmunol.170.5.2435. [DOI] [PubMed] [Google Scholar]
- 51.Lucas S, Ghilardi N, Li J, de Sauvage FJ. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100(25):15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 53.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23(9):513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 54.Takeda I, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172(6):3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 55.Klinger M, Kim JK, Chmura SA, Barczak A, Erle DJ, Killeen N. Thymic OX40 expression discriminates cells undergoing strong responses to selection ligands. J Immunol. 2009;182(8):4581–4589. doi: 10.4049/jimmunol.0900010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 57.Wei G, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity. 2011;35:299–311. doi: 10.1016/j.immuni.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lesourne R, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10(8):840–847. doi: 10.1038/ni.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78(3):399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 60.Kohm AP, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176(6):3301–3305. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 61.Rausch S, et al. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39(11):3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 62.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445(7130):936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 63.Mantel PY, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5(12):e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Souabni A, Flavell RA, Wan YY. An intrinsic mechanism predisposes Foxp3-expressing regulatory T cells to Th2 conversion in vivo. J Immunol. 2010;185(10):5983–5992. doi: 10.4049/jimmunol.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(–) effector T cells. Nat Immunol. 2008;9(12):1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maruyama T, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol. 2010;12(1):86–95. doi: 10.1038/ni.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104(46):18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hadjur S, et al. IL4 blockade of inducible regulatory T cell differentiation: the role of Th2 cells, Gata3 and PU.1. Immunol Lett. 2009;122(1):37–43. doi: 10.1016/j.imlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 69.Guo L, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci U S A. 2009;106(32):13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amsen D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27(1):89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bensinger SJ, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172(9):5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walsh PT, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116(9):2521–2531. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaplan MH, Sun YL, Hoey T, Grusby MJ. Impaired IL-12 responses and enhanced development of Th2 cells in Stat4-deficient mice. Nature. 1996;382(6587):174–177. doi: 10.1038/382174a0. [DOI] [PubMed] [Google Scholar]
- 74.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 75.Usui T, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203(3):755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 77.Kitoh A, et al. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31(4):609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 79.Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity. 2003;19(6):863–875. doi: 10.1016/S1074-7613(03)00328-5. [DOI] [PubMed] [Google Scholar]
- 80.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 81.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28(4):546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 82.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Suffia I, Reckling SK, Salay G, Belkaid Y. A role for CD103 in the retention of CD4+CD25+ Treg and control of Leishmania major infection. J Immunol. 2005;174(9):5444–5455. doi: 10.4049/jimmunol.174.9.5444. [DOI] [PubMed] [Google Scholar]
- 84.Grunvald E, et al. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect Immun. 1996;64(6):2010–2018. doi: 10.1128/iai.64.6.2010-2018.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190(7):995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.