Abstract
Objective
Based on a maladaptive coping explanation, the relationship between major depression (MD) and obesity could be strong among nonsmokers, who may engage in unhealthy eating and sedentary behavior to cope with depression. By contrast, the MD-obesity association could be weak among smokers, who can use tobacco (instead of food or sedentary behavior) to cope with mood symptoms. This study examined smoking status and tobacco dependence as moderators of the MD-obesity link.
Design
Correlational, cross-sectional population-based survey of 41,654 US adults.
Main Outcome Measures
Obesity (BMI ≥ 30 kg/m2) and quantitative BMI value.
Results
Current smoking status moderated the association between past-year MD and current obesity, as well as the link between MD and body mass index (BMI) value (ps ≤ .0001). MD predicted obesity and BMI among nonsmokers (ps < .0001) but did not do so in smokers (ps ≥ .10). Similar findings emerged with tobacco dependence as the moderator. Each finding persisted after accounting for demographics, psychiatric variables, and potential confounds.
Conclusion
Tobacco use characteristics appear to moderate the MD-obesity association in the US population. These findings may shed light on the mechanisms linking MD and obesity and have implications for identifying which individuals may benefit most from obesity interventions that target depressive symptoms.
Keywords: Major Depression, Tobacco Use, Smoking, Obesity, Body Mass Index
The relationship between major depression (MD) and obesity has been well studied. Evidence is suggestive of a multidirectional relationship, such that depression prospectively increases risk of obesity (Goodman & Whitaker, 2002; Pine, Goldstein, Wolk, & Weissman, 2001), obesity increases risk of depression (Herva et al., 2006; Roberts, Deleger, Strawbridge, & Kaplan, 2003), and that the two disorders are concurrently associated with one another (Carpenter, Hasin, Allison, & Faith, 2000; Simon et al., 2006), although results are not entirely consistent across investigations (Faith, Matz, & Jorge, 2002; Stunkard, Faith, & Allison, 2003). It has therefore been argued that examining depression-obesity associations in heterogeneous samples may mask important variables that moderate this relationship (Faith et al., 2002).
Extant research has consistently shown that the association between MD and obesity is conditional upon a host of moderators, including gender (Anderson, Cohen, Naumova, & Must, 2006; Barry, Pietrzak, & Petry, 2008; Carpenter et al., 2000; Herva et al., 2006; Onyike, Crum, Lee, Lyketsos, & Eaton, 2003; Richardson et al., 2003), socioeconomic status (Faith et al., 2002), education (Simon et al., 2006), ethnicity (Heo, Pietrobelli, Fontaine, Sirey, & Faith, 2006; Simon et al., 2006), age (Heo et al., 2006), genetics (Fuemmeler et al., 2009), and other factors (Friedman & Brownell, 1995). For example, the link between depression and obesity appears to be stronger in females than males. Continued identification of theory-based candidate moderators of the MD-obesity relationship is important for: (1) clarifying the mechanisms underlying this linkage; and (2) identifying individuals who may benefit most for obesity interventions that target depression.
Among the multiple mechanisms that potentially underlie the MD-obesity link, the maladaptive coping explanation is relevant to research suggesting a prospective effect of depression on subsequent obesity risk in some populations (Anderson et al., 2006; Goodman & Whitaker, 2002; Pine et al., 2001). A maladaptive coping explanation purports that MD and obesity are associated because depressed individuals engage in unhealthy eating behavior (e.g., binge eating, higher caloric intake) and sedentary behavior to cope with their depression, which could lead to increased obesity risk (Linde et al., 2004; Simon et al., 2008). Following from this hypothesis, people who engage in alternate behaviors (other than unhealthy eating and inactivity) to cope with depressive symptoms may be less susceptible to the effects of depression on obesity.
Tobacco use is one such behavior. Indeed, smoking is highly prevalent among people with MD (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Leventhal, Kahler, Ray, & Zimmerman, 2009) and is commonly cited as a means to cope with depressed mood and counteract fatigue and inactivity (Gilbert, Sharpe, Ramanaiah, Detwiler, & Anderson, 2000; Ikard, Green, & Horn, 1969; Leventhal & Avis, 1976; Piper et al., 2004; Tate, Schmitz, & Stanton, 1991). Thus, the relationship between MD and obesity may be particularly strong among nonsmokers. By contrast, the MD-obesity association may be weak among smokers, who can use tobacco to modulate their depressive states. This hypothesis is consistent with recently-supported conceptualizations that some individuals tend to substitute addictive behaviors (e.g., smoking, gambling, unhealthy eating) for one another as a result of an underlying addiction propensity, which could be due to reward dysregulation and other features associated with depression (Cowan & Devine, 2008; Sussman & Black, 2008; Warren, Frost-Pineda, & Gold, 2005).
The present study evaluated the hypothesis that tobacco use moderated the relationship between MD and obesity in a representative sample of US adults. Consistent with prior cross-sectional studies of moderators of the MD-obesity relationship (e.g., Carpenter et al., 2000), we analyzed moderational effects of past year DSM-IV diagnoses on two obesity outcomes [i.e., current body mass index (BMI) value and obese status (BMI ≥ 30)] with and without controlling for demographic, psychiatric, and substance use characteristics. Concurrent tobacco use at any level of severity could diminish the MD-obesity association. Alternatively, tobacco use only at a habitual degree could offset MD-obesity relations according to a substitute addiction framework (Sussman & Black, 2008). Accordingly, we examined two candidate tobacco use moderators: (a) smoking status (i.e., any tobacco use in the past year); and (b) tobacco dependence (TD) in the past year.
Method
Sample
Participants were respondents in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC; Wave 1, 2001– 2002). All participants were civilian, non-institutionalized, residents of the United States aged 18 or older. African-Americans and Hispanic-Americans were over-sampled and each group accounted for approximately 20 percent of the sample. Young adults between ages18 and 24 were also over-sampled by a 2.25 to 1 ratio. The data were weighted to account for over-sampling and were adjusted to be representative of the 2000 US Census results by age, ethnicity, and gender. Further details of the sampling, purpose, demographic profile, and weighting have been published elsewhere (Grant, Moore, Shepart, & Kaplan, 2003). This report utilizes the portion of the NESARC Wave 1 sample for which BMI data were available (N = 41,654, 96.7%).
Measures
BMI
Body Mass Index (BMI) was computed based on the respondents’ self-reported body weight and height (kg/m2). Although BMI does not directly measure body fat, evidence shows that BMI correlates with objective assessments of body fat, such as underwater weighing and dual energy x-ray absorptiometry (Deurenberg et al., 2001; Garrow & Webster, 1985). Furthermore, even though objectively measured height and weight are the most preferable method of calculating BMIs, self-report and objectively measured height and weight are highly concordant in adults (Avila-Funes, Gutierrez-Robledo, & Ponce De Leon Rosales, 2004; Rimm et al., 1990; Spencer, Appleby, Davey, & Key, 2002).
Individuals with BMI values equal to or above 30 were classified into the obese category. We chose to focus on the distinction between obese (BMI greater or equal to 30) and nonobese (BMI less than 30) based on the growing body of evidence suggesting that obesity (but not overweight) is associated with increased mortality from cardiovascular disease and obesity-related cancers (Flegal, Graubard, Williamson, & Gail, 2007). Because pregnancy can inflate BMI values, we re-ran all of the following analyses after excluding those who reported that they were currently pregnant (n = 453, 1%). None of the results were affected, thus the analyses reported herein utilize the entire sample.
AUDADIS-IV
The Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS-IV; Grant, Dawson et al., 2003) was used to apply DSM-IV criteria to determine substance use, mood, and anxiety disorders in the past 12 months and over lifetime (APA, 1994). The AUDADIS-IV also includes questions to assess tobacco use patterns. Following from previous moderational analyses of the MD-obesity relationship which relied on MD during the past 12 months (Carpenter et al., 2000), we analyzed: (1) MD Status (presence vs. absence of DSM-IV-defined major depressive episode in past 12 months); (2) smoking status (presence vs. absence of use of one or more types of tobacco in the past 12 months); and (3) TD status (presence vs. absence of DSM-IV-defined nicotine dependence in past 12 months). Classification of MD excluded substance-induced MD because the psychoactive effects of substance misuse influence depressive symptoms (Schuckit et al., 2007), associate with smoking (Grant et al., 2004), and may therefore affect the findings. The reliability and validity of the AUDADIS-IV for classifying smoking status, TD, and MD in the present sample and numerous other population surveys has been excellent (Canino et al., 1999; Chatterji et al., 1997; Grant, Dawson et al., 2003).
Procedure
Potential respondents were contacted in writing and informed the nature of the study, the uses of survey data from the NESARC, that participation was voluntary, and about confidentiality laws protecting their individual information. The response rate was 81% and one adult from each household was selected for the interview. After informed consent was obtained, interviewers from the US Census Bureau conducted in-person interviews and recorded responses into a laptop computer. The US Census Bureau and the US Office of Management reviewed and approved all consent procedures, research measures and protocol.
Statistical Analysis
Initial regression models tested the univariate effects of MD, smoking status, and TD on obesity status and BMI value. Regression models were used to test the hypothesized interaction between MD and tobacco variables in predicting obesity outcomes. To this end, four sets of baseline models were tested: (1) MD × smoking status predicting obesity status, controlling for MD and smoking status; (2) MD × smoking status predicting BMI value, controlling for MD and smoking status; (3) MD × TD predicting obesity status, controlling for MD and TD; and (4) MD × TD predicting BMI value, controlling for MD and TD. These four models were retested after adjusting for demographic (age, sex, ethnicity/race, marital status, education, urbanicity) and psychiatric (lifetime history of anxiety, manic, personality, alcohol use, and drug use disorder) variables. Models predicting obesity status (BMI ≥ 30 vs. BMI < 30) utilized logistic regression. Models predicting BMI used linear regression. Interactions were deconstructed using simple effect analyses which tested the influence MD on obesity/BMI in subsamples stratified on smoking/TD status. Supplemental analyses to examine confounds (e.g. medication use, smoking heaviness), appetite changes, the temporal status of moderational effects, moderation by quit attempts in past year, and three way interactions with gender were evaluated using similar methods and are described in greater detail below. All analyses were conducted in SAS using the PROC SURVEY procedures (SAS Institute Inc., 2009), which account for complex sampling methodology of the NESARC and utilizes the recommended sampling weights to approximate the US population (Grant, Moore et al., 2003). Means and percentages reported are weighted estimates (±SE).
Results
Prevalence of MD, Smoking, and TD
The prevalence of MD in the past 12 months was 7.9±0.16% (n = 3416). Current smokers comprised 28±0.27% (n = 10886) of the sample. The concomitant prevalence of MD and smoking status were as follows: Nonsmoker/MD- (67.4±0.28%, n = 28730); Nonsmoker /MD+ (4.5±0.12%, n = 2038); Smoker/MD- (24.7±0.26%, n = 9552); Smoker/MD+ (33.11±0.28%, n = 1334).
The prevalence of TD in the past 12 months was 13.0±0.21% (n = 4885). The simultaneous prevalence of MD and TD status were: TD-/MD- (81.5±0.24%, n = 34327); TD-/MD+ (5.5±0.15%, n =2442); TD+/MD- (10.6±0.19%, n = 3955); TD+/MD+ (2.4±0.09%, n = 930).
Univariate Effects of MD, Smoking Status, and TD on Obesity and BMI
As illustrated in Table 1, MD was significantly associated with higher rates of obesity and higher BMI values, although the strength of effects were reduced after adjusting for demographic characteristics and lifetime psychiatric and substance use disorders. Smoking (vs. nonsmoking) status was associated with lower rates of obesity and lower BMI values. Presence of TD in the past 12 months was associated with lower BMI values. TD was not significantly associated with obesity status in unadjusted models; however, it was associated with lower obesity rates in adjusted models.
Table 1.
Univariate effects of Major Depression and Tobacco Use on Obesity
| Unadjusted | Adjustedc | |
|---|---|---|
| Outcome: Obese vs. Non-Obese (BMI ≥ 30)a | ||
| Major Depression | OR(95% CI) = 1.45(1.32–1.60), p < .0001 | OR(95% CI) = 1.30(1.17–1.45), p < .0001 |
| Smoking Status | OR(95% CI) = 0.91(0.85-0.97), p = .003 | OR(95% CI) = 0.83(0.77-0.89), p < .0001 |
| Tobacco Dependence | OR(95% CI) = 0.94(0.86-1.03), p = .20 | OR(95% CI) = 0.83(0.76-0.92), p = .0003 |
| Outcome: Continuous BMI valueb | ||
| Major Depression | t = 5.0, β = .02, p < .0001 | t = 2.0, β = .01, p = .049 |
| Smoking Status | t = -3.8, β = -.02, p = .0001 | t = -5.4, β = -.03, p < .0001 |
| Tobacco Dependence | t = -3.5, β = -.02, p = .0004 | t = -4.8, β = -.02, p < .0001 |
Note. N = 41654.
Logistic Regression Models.
Linear Regression Models.
Adjusted for age, sex, ethnicity/race, marital status, education, urbanicity, and lifetime history of anxiety, manic, personality, alcohol use, and drug use disorder
Primary Analyses
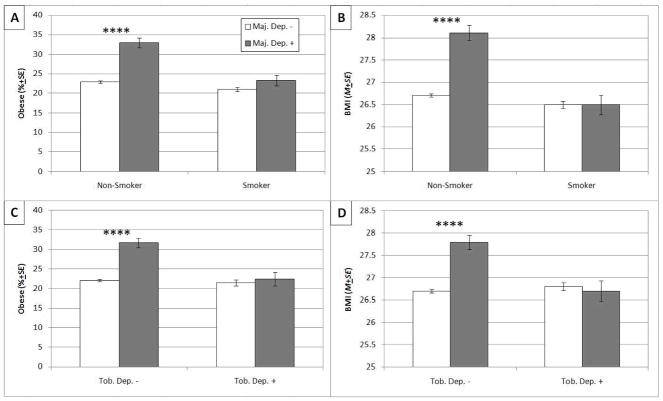
As illustrated in Table 2, there was a significant interaction between MD and smoking status in the logistic regression model predicting obesity status as well as in the linear regression model predicting BMI value. These interactions remained significant after adjusting for demographic characteristics and lifetime psychiatric and substance use disorders. Simple effect analyses in the subsample of nonsmokers showed that having a diagnosis of MD in the past 12 months was associated with increased odds of obesity and higher BMI, whereas analyses in the subsample of smokers showed no association of MD with obesity and BMI (see Table 2 and Figure 1, parts A and B).
Table 2.
Interactive effects of Major Depression and Tobacco Use on Obesity, and Simple Effect analyses
| Unadjusted | Adjustede | |
|---|---|---|
| Outcome: Obese vs. Non-Obese (BMI ≥ 30)c | ||
| Smoking Status × MD interactiona | Wald χ2= 14.4, p = .0001 | Wald χ2= 14.3, p < .0001 |
| Effect of MD among Nonsmokers | OR(95% CI) = 1.72(1.53–1.94), p < .0001 | OR(95% CI) = 1.43(1.25–1.63), p < .0001 |
| Effect of MD among Smokers | OR(95% CI) = 1.15(0.97–1.37), p = .10 | OR(95% CI) = 1.12(0.93–1.36), p = .23 |
| TD × MD interactionb | Wald χ2= 11.5, p = .0007 | Wald χ2= 12.5, p = .0004 |
| Effect of MD among TD- respondents | OR(95% CI) = 1.65(1.47–1.84), p < .0001 | OR(95% CI) = 1.40(1.24–1.58), p < .0001 |
| Effect of MD among TD+ respondents | OR(95% CI) = 1.08(0.81–1.34), p = .49 | OR(95% CI) = 1.06(0.83–1.35), p = .67 |
| Outcome: Continuous BMI valued | ||
| Smoking Status × MD interactiona | F = 24.6, p < .0001 | F = 21.1, p < .0001 |
| Effect of MD among Nonsmokers | F = 56.4, p < .0001 | F = 34.0, p < .0001 |
| Effect of MD among Smokers | F = 0.2, p = .68 | F = 0.4, p = .55 |
| TD × MD interactionb | F = 13.6, p = .0002 | F = 13.5, p = .0002 |
| Effect of MD among TD- respondents | F = 49.1, p < .0001 | F = 26.7, p < .0001 |
| Effect of MD among TD+ respondents | F = 0.1, p = .72 | F < 0.01, p = .98 |
Note. N = 41654. Smoking Status = Smoked Tobacco in past 12 months (Nonsmoker, n = 30768; Smoker = 10886); MD = Major Depression in past 12 months excluding substance-induced (MD-, n = 38238; MD+, n = 3416); TD = Tobacco Dependence in past 12 months (TD-, n = 36769; TD+, n = 4885).
Effects of interaction term in models including MD, smoking status, and their interaction as predictors.
Effects of interaction term in models including MD, TD, and their interaction as predictors
Logistic Regression Models.
Linear Regression Models.
Adjusted for age, sex, ethnicity/race, marital status, education, urbanicity, and lifetime history of anxiety, manic, personality, alcohol use, and drug use disorder
Figure 1.
(Part A) Weighted percentage (±SE) of obesity (BMI ≥ 30 kg/m2) by Smoking and Major Depression (MD) Status in the past 12 months. (Part B) Weighted mean (±SE) BMI value by Smoking and MD Status in the past 12 months. Nonsmoker/MD- (n = 28730); Nonsmoker/MD+ (n = 2038); Smoker/MD- (n = 9552); Smoker/MD+ (n = 1334). (Part C) Weighted percentage (±SE) of obesity by Tobacco Dependence (TD) and MD Status in the past 12 months. (Part D) Weighted mean (±SE) BMI value by TD and MD Status in the past 12 months. TD-/MD- (n = 34327); TD-/MD+ (n =2442); TD+/MD- (n = 3955); TD+/MD+ (n = 930). **** MD+ vs. MD- contrast significant (p < .0001)
A similar pattern of findings emerged when TD was incorporated as the moderator variable. Significant interactions between MD and TD were found in models predicting obesity status and BMI value. These interactions remained significant after adjusting for demographic characteristics and lifetime psychiatric disorders (see Table 2). Simple effect analyses in the subsample of respondents without TD showed a positive MD diagnosis was associated with increased prevalence of obesity and higher BMI, whereas analyses in the subsample of respondents with TD showed no association of MD with obesity and BMI (see Table 2 and Figure 1, parts C and D).
Supplemental Analyses
Confound checks
Because depressed smokers may smoke more heavily than non-depressed smokers, differences in smoking severity could potentially explain the interaction. Therefore, we retested the effects of the hypothesized interactions in models that controlled for cigarettes smoked per day, the interaction between cigarettes smoked per day and MD status, as well as the other demographic and psychiatric variables. As in the primary adjusted analyses, four models were tested: (1) MD × smoking status predicting obesity status; (2) MD × smoking status predicting BMI value; (3) MD × TD predicting obesity status; (4) MD × TD predicting BMI value. The interactions remained significant in each model (Wald χ2s ≥ 9.4, ps ≤ .002; Fs ≥ 11.1, ps ≤ .0009), indicating that smoking heaviness did not account for the moderational effect of TD and smoking status.
Depressed smokers may be more likely to receive antidepressant medications that assist in smoking cessation, such as bupropion, that cause less weight gain than depressed nonsmokers, which could potentially explain the hypothesized interaction. Therefore, we retested each of the four adjusted models in the subsample of respondents who reported that they were not prescribed medication for depression (N = 40,441). The interactions remained significant in each model (Wald χ2s ≥ 9.2, ps ≤ .003; Fs ≥ 8.4, ps ≤ .004).
Being underweight is linked with psychopathology known to increase risk for MD (Petry, Barry, Pietrzak, & Wagner, 2008), which could have influenced the present findings. Therefore we retested each of the four models after excluding individuals classified as underweight (BMI < 18.5; n = 864). The interactions remained significant in each model (Wald χ2s ≥ 11.0, ps ≤ .009; Fs ≥ 10.6, ps ≤ .001).
Appetite changes associated with MD
Increased appetite is a symptom of MD (APA, 1994). Because tobacco is considered an appetite suppressant (Piper et al., 2004), the depressive symptom of increased appetite could be less common among depressed tobacco users, which could explain the moderational effect. We therefore recalculated each of the models after excluding individuals who endorsed this symptom on the AUDADIS-IV MD module (“wanted to eat more than usual for no special reason”; n = 3108). In each case, the interaction remained significant, although the strength of the effect was reduced [MD × smoking status predicting obesity (Wald χ2 = 10.4, p = .001) and BMI (F = 11.1, p = .0008); MD × TD predicting obesity (Wald χ2 = 4.9, p = .03) and BMI (F = 4.4, p = .04)].
Reduced appetite is also a symptom of MD that could have been overrepresented among smokers with MD. Therefore, we ran additional models after omitting participants who reported this symptom (n = 5854). Results showed the interaction terms remained significant in models predicting BMI [MD × smoking status (F = 10.3, p = .001); MD × TD (F = 7.1, p = .008)], but fell below significance in models predicting obesity status [MD × smoking status (Wald χ2 = 2.4, p = .12); MD × TD (Wald χ2 = 3.4, p = .06)].
Exploration of lifetime vs. past-year associations
We ran additional models that used lifetime indices of smoking status, tobacco dependence, and MD. In models adjusting for demographic and psychiatric variables, the interaction between lifetime depression and lifetime smoking status significantly predicted obesity status (Wald χ2 = 7.7, p = .006) and BMI (F = 13.9, p = .0002). Parallel adjusted models showed that the interaction between lifetime depression and lifetime TD significantly predicted obesity status (Wald χ2 = 4.3, p = .04) and BMI (F = 8.4, p = .004). Therefore, we tested a final set of four models adjusted for demographic and psychiatric variables, and that included the interaction between the 12-month tobacco and MD variables as well as the interaction between lifetime tobacco and MD variables. In each of these models the interaction between the 12-month variables remained significant and the interaction between the lifetime variables fell below significance, with the exception of model predicting BMI from the interactions between MD and smoking status in which both interactions were significant (12-month MD × smoking status: F = 10.2, p = .001; Lifetime MD × smoking status: F = 4.1, p = .04).
Quit attempts
To explore whether quit attempts had any bearing on these associations, we analyzed the univariate and moderational effect of the item asking respondents if they more than once wanted to stop or cut down on their smoking in the past 12 months (yes vs. no) in the subsample of current smokers. Results showed that desire to quit did not have a univariate effect on obesity or BMI, nor did it moderate the effects of MD on obesity or BMI (all ps > .24).
Moderation by gender
There have been previous reports that gender moderates the relationship between MD and obesity (Carpenter et al., 2000) as well as the relationship between smoking and MD, with stronger associations among women (Husky, Mazure, Paliwal, & McKee, 2008). Therefore we conducted an additional set of models, which added the three way interaction between gender, smoking status/TD, and depression. The three-way interaction was not significant for any of the unadjusted or adjusted models (all ps ≥ .72).
Discussion
Previous investigations have found that the relationship between MD and obesity are moderated by a variety of individual difference characteristics (Anderson et al., 2006; Barry et al., 2008; Carpenter et al., 2000; Faith et al., 2002; Fuemmeler et al., 2009; Heo et al., 2006; Herva et al., 2006; Onyike et al., 2003; Richardson et al., 2003; Simon et al., 2006). The present study extended this literature by examining tobacco use as a novel, theory-based candidate moderator of the MD-obesity relationship in a sample of nationally representative adults participating in the NESARC.
Univariate analyses showed that MD was associated with higher rates of obesity and higher BMI values. Based on maladaptive coping explanations of the MD-obesity relation, we hypothesized that tobacco use would moderate this association, such that tobacco use would diminish the strength of the relationship between MD and obesity. In support of this hypothesis, results indicated that both tobacco use indicators (i.e., past year smoking status and TD) significantly moderated the predictive influence of past-year MD on current obesity and BMI value. MD was significantly and robustly associated with obesity among nonsmokers and TD-respondents (ORs ≥ 1.65, ps < .0001), whereas the corresponding relationships among smokers and TD+ participants were substantially weaker and nonsignificant (ORs ≤ 1.10, ps ≥ .10). The same pattern was found when BMI value was the outcome. MD+ participants had higher BMIs then MD- participants only among nonsmokers and TD- respondents. Each of these findings remained consistent when adjusting for relevant sociodemographic variables and psychiatric and substance use disorders. Furthermore, supplemental analyses indicated that these associations were not entirely accounted for by extraneous confounding or explanatory factors (e.g., psychiatric medication use, smoking heaviness, underweight status). Additional analyses found that recent desire to quit did not moderate MD-obesity associations among current smokers. Given these analyses and results showing that smoking heaviness did not account for the findings, it appears that these results are specific to tobacco use indicators that differentiate smokers versus nonsmokers or people with versus without TD, and do not generalize to variables that explain individual differences among current smokers.
The moderational effect of past year MD and tobacco use indicators on obesity was unique from corresponding lifetime MD and tobacco use characteristics. There was additional evidence of a less robust, but statistically significant, lifetime MD × smoking status interaction that was unique from corresponding past-year characteristics. These findings are generally consistent with previous cross-sectional reports that other moderators impact the relationship between past-year MD and current obesity (Carpenter et al., 2000; Onyike et al., 2003).
Given extant data that gender moderates the relationship between MD and obesity (Carpenter et al., 2000) as well as the link between smoking and MD (Husky et al., 2008) with stronger associations among women, we explored whether the interaction between MD and tobacco use was moderated by gender. Supplemental analyses indicated that the moderating influence of MD on obesity did not significantly differ by gender (i.e., a three way MD× Tobacco Use × Gender interaction; ps > .72). Thus, the extent to which tobacco use offset MD-obesity relationships was similar for both genders. There are several potential explanations for this finding. It is possible that while women may be more likely to engage in unhealthy behaviors to cope with MD, they may not be more prone than men to substitute one maladaptive coping mechanism for another. Relatedly, a prior study found little evidence of any gender differences in addiction substitution patterns, such that both men and women receiving treatment for opiate addiction exhibited similar patterns of substituting alcohol for heroin use (Almog, Anglin, & Fisher, 1993). An alternative explanation of the lack of gender differences is that both men and women may both use tobacco to counteract the depressogenic effects of obesity (i.e., using tobacco to manage the mood-dysregulating effects of stigma, social isolation, and immobility associated with obesity).
There are several plausible accounts of the primary finding that tobacco use moderated MD-obesity associations in the overall sample. Previous investigations suggest that depression is associated with higher caloric intake (Rohde et al., 2008; Simon et al., 2008), more frequent binge eating (Linde et al., 2004), and less physical activity (Rohde et al., 2008) in an effort to cope with depressive disturbance, and that some of these relationships mediate the association between depression and obesity (Rohde et al., 2008). Thus, it is possible that individuals who do not use tobacco may be especially vulnerable to the effects of MD on these intermediate processes that increase obesity risk. Alternatively, tobacco use, which has been reported as a means to manage depressed mood and counter fatigue and inactivity (Gilbert et al., 2000; Ikard et al., 1969; Leventhal & Avis, 1976; Piper et al., 2004; Tate et al., 1991), may potentially offset MD-related obesity risk.
Other research indicates that individuals with underlying dysregulation of brain reward systems may substitute drug use and unhealthy eating for one another because both food and drugs share mood-altering properties, and therefore help individuals overcome reward deficits (Sussman & Black, 2008). Accordingly, tobacco may compete with food for brain reward sites, especially among individuals with MD, who have been demonstrated to exhibit brain reward system dysregulation (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002). Therefore, substitute addiction processes (Sussman & Black, 2008) may potentially explain why tobacco may reduce the association between MD and obesity. However, both tobacco dependence and any past-year tobacco use both had moderational effects. Thus, any substitution that may have been operating was not limited to addictive patterns of tobacco use.
Although the impetus for this analysis was based on the notion that tobacco use may offset the effect of MD on obesity, the cross-sectional nature of the study allows for alternative causal explanations of the present findings. Previous evidence also suggests a prospective effect of obesity on depression, which may be due to difficulty coping with stigma, negative self-image, social isolation, and other factors (Roberts et al., 2003). Perhaps tobacco use can buffer against the negative psychological effects of being obese. Alternatively, tobacco use may inversely associate with shared factors that underlie the covariation of depression and obesity, such as neural catecholamine abnormalities, genetic factors, and personality traits associated with a propensity to experience negative emotions (Fuemmeler et al., 2009; Hainer, Kabrnova, Aldhoon, Kunesova, & Wagenknecht, 2006).
One can also interpret the present findings as indicative that MD acts as a moderator of the association between tobacco use and obesity, such that MD amplifies the inverse relation between tobacco use and obesity (see Figure 1). Indeed univariate analyses showed that tobacco use tended to be associated with lower rates of obesity and lower BMIs. Consistent with this finding, previous reports have found that tobacco use is associated with lower rates of obesity (Bakhshi et al., 2008; Molarius, Seidell, Kuulasmaa, Dobson, & Sans, 1997). It is possible that the extent to which nicotine has appetite suppressant and metabolic-enhancing effects that reduces obesity risk is greater in people with MD. However, while research suggests that depressed smokers exhibit greater sensitivity to the effects of nicotine on mood (Faith, Flint, Fairburn, Goodwin, & Allison, 2001; Farmer et al., 2002; Pomerleau et al., 2005; Spring et al., 2008), they do not appear to differentially sensitive to nicotine’s appetite-suppressant effects (Spring et al., 2008). Another related explanation is that the type of depression that leads people to smoke involves depressive episodes that include reduced appetite, which could impact BMI. Supplemental analyses eliminating respondents with reduced appetite demonstrated that endorsing this symptom may have accounted for some of the moderational effects on categorical obesity outcomes, but did not entirely account for moderational effects on BMIs. This pattern suggests that loss of appetite may explain some of the moderating effect of tobacco use on of MD’s association with extreme BMI levels, but not gradual BMI differences.
Finally, it should be noted that although our results indicate that tobacco use reduces the MD-smoking association, smoking elevates the risk of cardiovascular disease and various cancers and smoking cessation reduces these risks (Ockene, Kuller, Svendsen, & Meilahn, 1990). Therefore, we are not advocating for increased tobacco use as a strategy to prevent or treat obesity in depressed individuals. Instead, it is important to consider the health impacts of these behavioral mechanisms in terms of the complex and interacting systems in which they function.
This study had several limitations, including a cross-sectional correlational design, self-report of height and weight for BMI calculation, no biochemical verification of tobacco use, lack of data on underlying mechanisms (e.g., physical activity patterns, diet, and coping responses to depressed mood), and absence of additional measures of adiposity other than BMI. Despite limitations, this study had several offsetting strengths, such as the use of a nationally representative sample, exploration across multiple moderators (i.e., smoking status and TD) and multiple outcomes (i.e., obesity status and BMI), use of valid clinical interview assessments to generate DSM-IV MD and TD diagnoses, and rigorous examination of extraneous confounding and explanatory variables.
To our knowledge, this is the first study to examine whether tobacco use moderates the association between MD and obesity. The unique moderational relationship demonstrated herein warrants future investigation as it points to putative etiological mechanisms that could account for the relationship between MD and obesity. Additional clarification of the temporal and causal nature of this moderational relationship using longitudinal and experimental research designs would be informative. In addition, future investigations directly measuring unhealthy eating and physical inactivity in addition to affective coping patterns as potential mechanisms explaining our findings would be useful for understanding the etiological processes linking MD and obesity. Such research could also have implications for matching obesity interventions to patients with individual difference factors that predict favorable treatment response. For example, the present findings may suggest that obesity interventions which target depressive symptoms may be more effective among nonsmokers. However, further research is required to evaluate the clinical validity of this prediction.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/HEA
References
- Almog YJ, Anglin MD, Fisher DG. Alcohol and heroin use patterns of narcotics addicts: Gender and ethnic differences. The American Journal of Drug and Alcohol Abuse. 1993;19(2):219–238. doi: 10.3109/00952999309002682. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Cohen P, Naumova EN, Must A. Association of depression and anxiety disorders with weight change in a prospective community-based study of children followed up into adulthood. Arch Pediatr Adolesc Med. 2006;160(3):285–291. doi: 10.1001/archpedi.160.3.285. [DOI] [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; 1994. [Google Scholar]
- Avila-Funes JA, Gutierrez-Robledo LM, Ponce De Leon Rosales S. Validity of height and weight self-report in Mexican adults: results from the national health and aging study. J Nutr Health Aging. 2004;8(5):355–361. [PubMed] [Google Scholar]
- Bakhshi E, Eshraghian MR, Mohammad K, Foroushani AR, Zeraati H, Fotouhi A, et al. Sociodemographic and smoking associated with obesity in adult women in Iran: results from the National Health Survey. J Public Health (Oxf) 2008;30(4):429–435. doi: 10.1093/pubmed/fdn024. [DOI] [PubMed] [Google Scholar]
- Barry D, Pietrzak RH, Petry NM. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Ann Epidemiol. 2008;18(6):458–466. doi: 10.1016/j.annepidem.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canino G, Bravo M, Ramirez R, Febo VE, Rubio-Stipec M, Fernandez RL, et al. The Spanish Alcohol Use Disorder and Associated Disabilities Interview Schedule (AUDADIS): reliability and concordance with clinical diagnoses in a Hispanic population. J Stud Alcohol. 1999;60(6):790–799. doi: 10.15288/jsa.1999.60.790. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000;90(2):251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterji S, Saunders JB, Vrasti R, Grant BF, Hasin D, Mager D. Reliability of the alcohol and drug modules of the Alcohol Use Disorder and Associated Disabilities Interview Schedule--Alcohol/Drug-Revised (AUDADIS-ADR): an international comparison. Drug Alcohol Depend. 1997;47(3):171–185. doi: 10.1016/s0376-8716(97)00088-4. [DOI] [PubMed] [Google Scholar]
- Cowan J, Devine C. Food, eating, and weight concerns of men in recovery from substance addiction. Appetite. 2008;50(1):33–42. doi: 10.1016/j.appet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Deurenberg P, Andreoli A, Borg P, Kukkonen-Harjula K, de Lorenzo A, van Marken Lichtenbelt WD, et al. The validity of predicted body fat percentage from body mass index and from impedance in samples of five European populations. Eur J Clin Nutr. 2001;55(11):973–979. doi: 10.1038/sj.ejcn.1601254. [DOI] [PubMed] [Google Scholar]
- Dietz WH. Overweight in childhood and adolescents. The New England Journal of Medicine. 2004;350(9):822–857. doi: 10.1056/NEJMp048008. [DOI] [PubMed] [Google Scholar]
- Faith MS, Flint J, Fairburn CG, Goodwin GM, Allison DB. Gender differences in the relationship between personality dimensions and relative body weight. Obes Res. 2001;9(10):647–650. doi: 10.1038/oby.2001.86. [DOI] [PubMed] [Google Scholar]
- Faith MS, Matz PE, Jorge MA. Obesity-depression associations in the population. J Psychosom Res. 2002;53(4):935–942. doi: 10.1016/s0022-3999(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Farmer A, Redman K, Harris T, Mahmood A, Sadler S, Pickering A, et al. Neuroticism, extraversion, life events and depression. The Cardiff Depression Study. Br J Psychiatry. 2002;181:118–122. doi: 10.1017/s0007125000161823. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Brownell KD. Psychological correlates of obesity: moving to the next research generation. Psychol Bull. 1995;117(1):3–20. doi: 10.1037/0033-2909.117.1.3. [DOI] [PubMed] [Google Scholar]
- Fuemmeler BF, Agurs-Collins T, McClernon FJ, Kollins SH, Garrett ME, Ashley-Koch AE. Interactions between genotype and depressive symptoms on obesity. Behav Genet. 2009;39(3):296–305. doi: 10.1007/s10519-009-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrow JS, Webster J. Quetelet's index (W/H2) as a measure of fatness. Int J Obes. 1985;9(2):147–153. [PubMed] [Google Scholar]
- Gilbert D, Sharpe P, Ramanaiah N, Detwiler F, Anderson A. Development of a Situation x Trait Adaptive Response (STAR) model-based smoking motivation questionnaire. Personality and Individual Differences. 2000;29(1):65–84. [Google Scholar]
- Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110(3):497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Grant B, Moore T, Shepart J, Kaplan K. Source and Accuracy Statement for Wave 1 of the 2001–2202 National Epedimeologic Survey on Alcohol and Related Conditions (NESARC) Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou PS, Kay W, Pickering R. The Alcohol Use Disorder and Associated Disabilities Interview Schedule-IV (AUDADIS-IV): reliability of alcohol consumption, tobacco use, family history of depression and psychiatric diagnostic modules in a general population sample. Drug Alcohol Depend. 2003;71(1):7–16. doi: 10.1016/s0376-8716(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Hainer V, Kabrnova K, Aldhoon B, Kunesova M, Wagenknecht M. Serotonin and norepinephrine reuptake inhibition and eating behavior. Ann N Y Acad Sci. 2006;1083:252–269. doi: 10.1196/annals.1367.017. [DOI] [PubMed] [Google Scholar]
- Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes (Lond) 2006;30(3):513–519. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- Herva A, Laitinen J, Miettunen J, Veijola J, Karvonen JT, Laksy K, et al. Obesity and depression: results from the longitudinal Northern Finland 1966 Birth Cohort Study. Int J Obes (Lond) 2006;30(3):520–527. doi: 10.1038/sj.ijo.0803174. [DOI] [PubMed] [Google Scholar]
- Husky MM, Mazure CM, Paliwal P, McKee SA. Gender differences in the comorbidity of smoking behavior and major depression. Drug Alcohol Depend. 2008;93(1–2):176–179. doi: 10.1016/j.drugalcdep.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikard F, Green D, Horn D. A scale to differentiate between types of smoking as related to the management of affect. The International Journal on Addictions. 1969;4(4):649–659. [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Zimmerman M. Refining the depression-nicotine dependence link: patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addict Behav. 2009;34(3):297–303. doi: 10.1016/j.addbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal H, Avis N. Pleasure, addiction, and habit: factors in verbal report of factors in smoking behavior? J Abnorm Psychol. 1976;85(5):478–488. doi: 10.1037//0021-843x.85.5.478. [DOI] [PubMed] [Google Scholar]
- Linde JA, Jeffery RW, Levy RL, Sherwood NE, Utter J, Pronk NP, et al. Binge eating disorder, weight control self-efficacy, and depression in overweight men and women. Int J Obes Relat Metab Disord. 2004;28(3):418–425. doi: 10.1038/sj.ijo.0802570. [DOI] [PubMed] [Google Scholar]
- Molarius A, Seidell JC, Kuulasmaa K, Dobson AJ, Sans S. Smoking and relative body weight: an international perspective from the WHO MONICA Project. J Epidemiol Community Health. 1997;51(3):252–260. doi: 10.1136/jech.51.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockene JK, Kuller LH, Svendsen KH, Meilahn E. The relationship of smoking cessation to coronary heart disease and lung cancer in the Multiple Risk Factor Intervention Trial (MRFIT) Am J Public Health. 1990;80(8):954–958. doi: 10.2105/ajph.80.8.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003;158(12):1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70(3):288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001;107(5):1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, et al. A multiple motives approach to tobacco dependence: the Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) J Consult Clin Psychol. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine Tob Res. 2005;7(1):91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, et al. A longitudinal evaluation of adolescent depression and adult obesity. Arch Pediatr Adolesc Med. 2003;157(8):739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1(6):466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003;27(4):514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- Rohde P, Ichikawa L, Simon GE, Ludman EJ, Linde JA, Jeffery RW, et al. Associations of child sexual and physical abuse with obesity and depression in middle-aged women. Child Abuse Negl. 2008;32(9):878–887. doi: 10.1016/j.chiabu.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows (Version 9.2) Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Pierson J, Trim R, Nurnberger JI, et al. A comparison of factors associated with substance-induced versus independent depressions. J Stud Alcohol Drugs. 2007;68(6):805–812. doi: 10.15288/jsad.2007.68.805. [DOI] [PubMed] [Google Scholar]
- Simon GE, Ludman EJ, Linde JA, Operskalski BH, Ichikawa L, Rohde P, et al. Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry. 2008;30(1):32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5(4):561–565. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- Spring B, Cook JW, Appelhans B, Maloney A, Richmond M, Vaughn J, et al. Nicotine effects on affective response in depression-prone smokers. Psychopharmacology (Berl) 2008;196(3):461–471. doi: 10.1007/s00213-007-0977-7. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003;54(3):330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- Sussman S, Black DS. Substitute addiction: a concern for researchers and practitioners. J Drug Educ. 2008;38(2):167–180. doi: 10.2190/DE.38.2.e. [DOI] [PubMed] [Google Scholar]
- Tate JC, Schmitz JM, Stanton AL. A critical review of the Reasons for Smoking Scale. J Subst Abuse. 1991;3(4):441–455. doi: 10.1016/s0899-3289(10)80025-2. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: altered response to dextroamphetamine. Arch Gen Psychiatry. 2002;59(5):409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24(3):95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]