Abstract
Purpose
Short and long sleep duration and sleep quality are associated with health including all-cause mortality, cardiovascular disease, diabetes, and obesity. Inflammation may play a role in mediating these associations.
Methods
We examined associations between inflammation and self-reported sleep characteristics in 1020 respondents of the 2000 and 2006 Social Environment and Biomarkers of Aging Study (SEBAS), a nationally representative survey of Taiwanese adults ages 53 and over. Regression models were used to estimate cross-sectional relationships between inflammation (IL-6, CRP, fibrinogen, e-selectin, sICAM-1, albumin, and WBC) and a modified Pittsburgh Sleep Quality Index (PSQI), index subcomponents, and self-reported sleep duration. Change in inflammatory markers between 2000 and 2006 was also used to predict long or short sleep duration in 2006.
Results
Inflammation was not related to the overall index of sleep quality. However, longer sleep (> 8 hours) was associated with higher levels of inflammation. These associations remained after adjustment for waist circumference, self-reported health decline, diabetes, arthritis/rheumatism, heart disease, and depressive symptoms. Increases in inflammation between 2000 and 2006 were associated with long but not short sleep duration in 2006 for several markers.
Conclusions
Long sleep duration may be a marker of underlying inflammatory illness in older populations. Future studies should explore whether inflammation explains observed relationships between long sleep and mortality.
Keywords: sleep, inflammation, aging, Taiwan, CRP, IL-6
Introduction
Experimental and observational data indicate that sleep duration and other sleep characteristics are associated with a wide array of health outcomes including all-cause mortality, cardiovascular disease, diabetes, and obesity (1-3). Recent work has begun to investigate the role of inflammation in mediating associations between sleep and health outcomes (4-7). Experimentally, sleep deprivation is associated with acute elevation of both C-reactive protein (CRP) and interleukin-6 (IL-6)(8, 9), but findings in non-experimental settings have been mixed (5-7, 10-12). Associations between overall sleep quality and inflammatory markers have been more consistent but have still produced diverse findings (13). This paper examined associations between self-reported sleep duration, sleep quality and biomarkers of inflammation in a middle-aged and elderly cohort of Taiwanese adults.
Biologically, there are plausible mechanisms linking inflammation and sleep in both causal directions (14). Activation of the autonomic nervous system and elevated catecholamines during sleep deprivation can stimulate production of inflammatory mediators (10). Sleep deprivation also leads to blood pressure elevations that may increase endothelial shear stress (the force of flowing blood over the surface of the endothelium) (15), resulting in endothelial production of inflammatory mediators such as IL-6 and adhesion molecules such as e-selectin and soluble intercellular adhesion molecule-1 (sICAM-1) (10, 16). In the reverse direction, cytokines may have direct effects on the brain, including the regions involved in sleep regulation (14, 17). Cytokines are important regulators of host defense to infection, and the sleep-inducing impact of cytokines such as IL-6, as well as other immune cells that increase expression of these cytokines, may predispose persons with elevated levels of inflammation to longer sleep durations (18).
This study seeks to fill several gaps in the current literature. First, while studies of sleep duration and outcomes such as obesity, diabetes, and mortality have focused on the U-shaped risk of both short and long sleep, studies on sleep duration and inflammation have focused either on short sleep duration or modeled sleep duration linearly, both approaches potentially missing an effect at longer durations. Second, the majority of existing studies of sleep characteristics and inflammation have examined Western populations. A biological necessity, sleep is also a complex behavior influenced by cultural and social factors, such as the encouragement or discouragement of naps, traditional care giving roles, or the amount of time typically devoted to work. Thus, cross-cultural studies may help elucidate disease etiology in settings where the determinants of sleep habits differ (19). Lastly, most studies on sleep characteristics and inflammation have looked at young adult populations, but differences in these relationships may emerge in older populations, among whom chronic disease and higher levels of inflammation are more prevalent (20, 21). It is possible that the mechanisms through which sleep affects inflammatory processes (and vice versa) vary across the life span. In particular, long sleep is most prevalent in those over age 60, and a lengthening of sleep duration may coincide with the aging process and declining health (22-24).
Methods
Sample
Data are from the 2000 and 2006 Social Environment and Biomarkers of Aging Study (SEBAS) of Taiwanese adults ages 53 and over in 2006. All participants were part of the ongoing Taiwan Longitudinal Study of Aging (TLSA) initiated in 1989, with interviews conducted every 3-4 years and with periodic inclusion of younger refresher cohorts (25). In 2000, a demographic and health update, blood and urine specimens, and a medical examination were collected on a subset of respondents (SEBAS I, see (26) for a description). Six years later, a follow-up (SEBAS II) was conducted with those who completed the 2000 exam and survived to 2006 as well as with a sample of respondents aged 53 to 60 who were first interviewed in the 2003 wave of TLSA. Written informed consent was obtained for participation in both the in-home interview and hospital visit in 2000 and 2006; all SEBAS protocols were approved by human subjects committees in Taiwan, and at Georgetown and Princeton Universities.
Sixty-eight percent of those interviewed in SEBAS I and 81 percent in SEBAS II agreed to participate in the physical examination. Previous analysis suggests that estimates from the SEBAS exams are unlikely to be seriously biased by non-participation in the presence of controls for age (27-29). The physical examination followed a similar protocol in both waves. Several weeks after the household interview, participants collected a 12-hour overnight urine sample (7pm to 7am), fasted overnight, and visited a nearby hospital the following morning for an examination that included collection of a blood specimen and measurements of blood pressure and anthropometry. Compliance in the collection of the blood and 12-hour urine samples was high: 96 percent in 2000 and 88 percent in 2006 fasted overnight and provided a suitable urine specimen. Blood and urine specimens were analyzed at Union Clinical Laboratories in Taipei.
This paper used data from 639 survivors of SEBAS I who participated in the SEBAS II examination plus 397 persons aged 53-60 who were first interviewed in 2003. Sixteen observations were dropped for missing data on one or more covariates, leaving a total of 1020 respondents.
Measures
Sleep
Sleep questions were asked for the first time in 2006. A shortened version of the Pittsburgh Sleep Quality Index (PSQI) captured five dimensions of sleep over the month prior to interview (30): subjective sleep quality, sleep latency (time to fall asleep), sleep duration (during the night), sleep efficiency (hrs asleep/hrs in bed), and sleep dysfunction (daytime sleepiness).1 As with the full-length PSQI, each dimension was scored on a 0-3 scale, with higher numbers reflecting worse sleep quality. The overall (shortened) PSQI was a sum of the five subcomponents, with a range of (0-15). In accordance with the traditional PSQI scale, sleep duration was coded categorically (0=≥7 hours, 1=≥6 to <7 hours, 2=≥5 to <6 hours, and 3=<5 hours). Because of potential associations between both long and short sleep duration and health outcomes, we also categorized sleep duration as <6 hours, 6-8 hours, and >8 hours for separate non-linear analyses.
Inflammatory markers
Inflammatory markers were assayed from overnight fasting serum samples. We examined all inflammation-related markers measured in SEBAS I and II: C-Reactive protein (CRP), interleukin-6 (IL-6), white blood cell count (WBC), fibrinogen (SEBAS II only), albumin, e-selectin, and sICAM-1; measures of IL-6, e-selectin, sICAM-1, and CRP for SEBAS I were derived from frozen specimens collected in 2000 and assayed between 2007 and 2009. CRP, an acute phase protein, is an important component of the non-specific innate immune system response to infection and injury and is often used as a general marker of systemic inflammation (32, 33). IL-6 is the primary inflammatory cytokine responsible for upregulation of CRP (34). Fibrinogen is an acute phase protein that plays an important role in blood clotting (34). Albumin is involved in the transport of small molecules in the blood and in the maintenance of osmotic pressure, with low levels associated with inflammation (35). E-selectin and sICAM-1 are adhesion molecules that mediate the attachment of monocytes and lymphocytes to endothelial cells (36, 37). Levels of soluble adhesion molecules reflect the process of inflammation of vessel walls. White blood cells (leukocytes) are infection fighting blood cells that are also used as a measure of systemic inflammation (38).
Serum albumin was assayed using the bromcresol green method (DL=1.0 g/dL, CV=1.5 percent). Leukocyte (WBC) count was determined by direct current (DL=0.02×103/μL, CV=1.5 percent). Fibrinogen was measured using the coagulation method. E-selectin and sICAM-1 were each assayed using ELISA (R&D Systems, Quantikine kit). CRP samples were measured with a high sensitivity immunoturbidimetry assay (Bayer ADVIA1800 for 2000 samples and Roche Cobas Integra 800-CRPLX for 2006 samples; DL=0.012mg/dL 2000 samples and 0.071 mg/dL 2006 samples). IL-6 was measured using ELISA; (R&D Systems, Inc., Minneapolis, MN, DL=0.7 pg/mL; CV=12.1 percent).
Inflammatory markers were treated as continuous variables, with natural log transformations for skewed distributions where appropriate (CRP, IL-6, sICAM-1 and e-selectin). For CRP, the only marker with established clinical cut-offs, we also constructed two dichotomous measures: > 3.0mg/L, the cut-off for increased cardiovascular disease risk; and >10.0mg/L, the cut-off for evidence of current or recent infection (39). From the 2006 markers, we created an inflammation index (0-7) that counted the number of markers for which an individual was in the highest risk quartile. From this index we also created an indicator for high inflammation based on whether the individual was in the top quartile of risk for 3 or more markers (27% of the sample). In an effort to eliminate persons experiencing acute infection rather than chronic inflammation, all models (excepting those with CRP>10mg/L as a predictor) were rerun excluding individuals with WBC count >10 ×103/μL (3.2%) and separately CRP >10mg/L (17.7%), with similar substantive results (available upon request).
We adjusted for the following potentially inflammation-related health conditions in 2006: measured waist circumference, self-reported health decline (worse health than one year ago), diabetes, arthritis/rheumatism, heart disease, and depressive symptoms. Diabetes, arthritis/rheumatism and heart disease were based on the question “has a doctor ever told you” that you have the condition. Depressive symptoms were measured using a 10-item Center for Epidemiologic Studies Depression Scale (CES-D) questionnaire, excluding the one question pertaining to sleep quality. Shortened versions of the traditional 20 question CES-D have shown reliability in Western as well as Chinese populations (40, 41), and this subset has been found to predict mortality in the broader TLSA (42).
Statistical Analysis
First, we estimated linear regression models with each inflammatory marker as an outcome to examine cross-sectional associations between individual markers and the overall modified PSQI as well as the five PSQI sleep components, adjusted for age and sex. PSQI subcomponents were entered linearly (0-3); results were similar when scores were used as indicator variables. Linear probability regression models were run for the dichotomous CRP variables, the overall inflammation index, and the dichotomous indicator of high overall inflammation (results using logit models for binary outcomes were very similar).
Next, we ran analogous models for each inflammatory marker using a categorical measure of sleep duration (<6 hours, 6-8 hours, >8 hours) as the predictor, with and without adjustment for potential health mediators. Finally, because of the possibility that inflammation can promote sleep (18), we tested whether increases in inflammation between 2000 and 2006 predicted sleep duration in 2006. This analysis included all markers except fibrinogen (which was collected only in 2006), and was based on respondents who participated in both waves (n=639). For each biomarker, we used a multinomial logit model with sleep duration coded categorically as the dependent variable, adjusted for age, sex and baseline inflammation levels.
Because some studies have found sex differences in relationships between inflammation and sleep (13, 43), we also tested for interactions by sex; these interactions were not statistically significant and thus were not included in our final models.
Results
The average age of the sample was 66 years, with 6.4 average hours of reported sleep (Table 1). Overall, there were few associations between the inflammatory markers and the PSQI subcomponents or the overall PSQI (Table 2). There were no significant associations for CRP, IL-6, fibrinogen, WBC, or albumin. sICAM-1 showed marginally significant positive associations with the overall index (p≈0.057), sleep efficiency (p≈0.092), and a significant association with the index measure of short sleep duration (p≈.012). Self-reported sleep quality was also positively associated with e-selectin (p≈.041). In all of these cases, the direction of effect suggested that poorer sleep quality was associated with higher levels of these markers. There were no associations between any of the PSQI subcomponents and the overall inflammation index.
Table 1. Descriptive Statistics, SEBAS II.
| Variable | Mean | S.D. |
|---|---|---|
| Age | 66.107 | 10.376 |
| Male | 0.529 | 0.499 |
| Hours of Sleep | 6.409 | 1.471 |
| <6 hours | 25.0% | |
| 6-8 hours | 64.0% | |
| >8 hours | 11.0% | |
| PSQI Sleep Index (0-15) | 3.807 | 3.398 |
| Sleep Quality (0-3) | 1.190 | 0.817 |
| Sleep Latency (0-3) | 0.896 | 1.076 |
| Sleep Efficiency (0-3) | 0.544 | 0.975 |
| Sleep Dysfunction (0-3) | 0.287 | 0.761 |
| Sleep Duration (0-3) | 0.848 | 1.011 |
| C-Reactive Protein (CRP)mg/L | 2.632 | 0.717 |
| CRP> 3.0 mg/L | 0.177 | 0.380 |
| CRP> 10.0 mg/L | 0.040 | 0.195 |
| Interleukin-6 (pg/mL) | 4.015 | 8.253 |
| sICAM-1 (ng/mL) | 270.125 | 96.265 |
| E-selectin (ng/mL) | 42.334 | 32.071 |
| White Blood Cell Count (103/μL) | 6.091 | 1.729 |
| Albumin (g/dL) | 4.400 | 0.300 |
| Fibrinogen (mg/dL) | 330.580 | 69.090 |
| Inflammation Index (0-7) | 1.741 | 1.626 |
| Inflammation Index >3 | 26.9% | |
| Worse health than a year ago | 39.5% | |
| Ever had diabetes | 16.9% | |
| Ever had arthritis | 17.7% | |
| Ever had heart disease | 18.0% |
N=1020
Table 2. Association of sleep subcomponents with inflammation, OLS regressions.
| Variable | Log (CRP) | Log (Il-6) | Fibrinogen | Log (sICAM-1) | WBC | Albumin | E-selectin | CRP>3.0 | CRP> 10.0 | Infl. Index | High Infl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Index | 0.009 | 0.002 | 0.224 | 0.007 | 0.000 | 0.003 | 0.007 | 0.001 | -0.001 | 0.001 | -0.004 |
| (0-15) | 0.386 | 0.752 | 0.730 | 0.057* | 0.977 | 0.298 | 0.176 | 0.824 | 0.506 | 0.949 | 0.323 |
| Sleep Quality | 0.006 | 0.023 | -0.971 | 0.016 | -0.067 | 0.003 | 0.048 | -0.008 | -0.011 | -0.017 | -0.010 |
| (0-3) | 0.885 | 0.471 | 0.721 | 0.302 | 0.331 | 0.787 | 0.041** | 0.608 | 0.166 | 0.792 | 0.550 |
| Sleep Latency | 0.036 | 0.015 | 0.172 | 0.013 | -0.040 | 0.013 | 0.017 | 0.005 | 0.000 | 0.031 | -0.003 |
| (0-3) | 0.264 | 0.535 | 0.933 | 0.249 | 0.434 | 0.143 | 0.326 | 0.636 | 0.943 | 0.516 | 0.839 |
| Sleep Efficiency | 0.018 | -0.017 | -0.200 | 0.021 | 0.043 | 0.008 | 0.025 | -0.003 | -0.006 | -0.025 | -0.020 |
| (0-3) | 0.615 | 0.526 | 0.928 | 0.092* | 0.442 | 0.378 | 0.178 | 0.818 | 0.308 | 0.623 | 0.156 |
| Sleep Dysfunction | 0.064 | 0.010 | 3.147 | 0.007 | -0.015 | -0.003 | -0.035 | 0.023 | 0.008 | -0.050 | -0.023 |
| (0-3) | 0.164 | 0.767 | 0.279 | 0.673 | 0.844 | 0.811 | 0.162 | 0.161 | 0.357 | 0.461 | 0.223 |
| Sleep Duration | 0.011 | -0.004 | 1.781 | 0.029 | 0.053 | 0.011 | 0.025 | 0.003 | -0.005 | 0.030 | -0.008 |
| (0-3) | 0.749 | 0.865 | 0.401 | 0.012** | 0.326 | 0.203 | 0.174 | 0.812 | 0.365 | 0.541 | 0.558 |
Separate regression models of each biomarker on each sleep subcomponent, adjusted for age and sex.
Higher numbers indicate worse sleep quality for the modified PSQI and subcomponents
P-values in italics,
p<.10,
p<.05
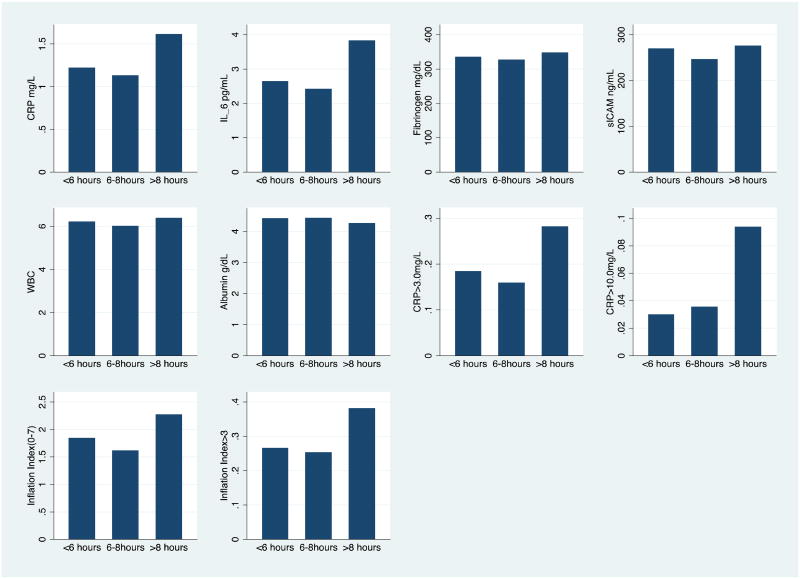
Compared with respondents who reported 6-8 hours of sleep, respondents reporting long sleep had significantly (p<.05) higher levels of CRP, IL-6, fibrinogen, WBC, and lower (worse) levels of albumin in age and sex adjusted models (crude models in Table 3). They were also more likely to have CRP levels above the clinical cut-offs for both cardiovascular disease and acute infection, and to score higher on the overall inflammation index. For respondents reporting short sleep (<6 hours), a significant relationship was found only with higher levels of sICAM-1. Adjustment for potential mediators had a modest effect on these associations. Figure 1 illustrates the significant associations between inflammatory markers and sleep duration in 2006 based on the crude models from Table 3 (for ease of interpretation, logged outcomes have been exponentiated to show the magnitude of absolute effects).
Table 3. Regression coefficients for association of short and long sleep duration with inflammation.
| Variable | Ln (CRP) | Ln (IL-6) | Fibrinogen | Ln(sICAM-1) | WBC | Albumin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |
| < 6 hours | 0.036 | 0.049 | 0.031 | 0.016 | 6.488 | 7.932 | 0.075 | 0.068 | 0.204 | 0.225 | -0.006 | 0.018 |
| 0.647 | 0.533 | 0.602 | 0.788 | 0.195 | 0.116 | 0.007** | 0.0145** | 0.107 | 0.076* | 0.766 | 0.387 | |
| 6-8 hours | ref | |||||||||||
| >8 hours | 0.261 | 0.213 | 0.317 | 0.280 | 16.306 | 21.810 | 0.078 | 0.092 | 0.392 | 0.423 | -0.117 | -0.104 |
| 0.031** | 0.100* | 0.000** | 0.004** | 0.034** | 0.009** | 0.066* | 0.047** | 0.044** | 0.045** | 0.001** | 0.003** | |
| E-Selectin | CRP>3.0 | CRP>10.0 | Infl. Index | High Inflammation | ||||||||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||
| < 6 hours | 0.063 | 0.051 | 0.018 | 0.019 | -0.009 | -0.007 | 0.129 | 0.088 | -0.008 | -0.210 | ||
| 0.138 | 0.220 | 0.525 | 0.491 | 0.516 | 0.621 | 0.269 | 0.445 | 0.806 | 0.501 | |||
| 6-8 hours | ||||||||||||
| >8 hours | 0.060 | -0.006 | 0.105 | 0.091 | 0.049 | 0.050 | 0.423 | 0.356 | 0.081 | 0.064 | ||
| 0.364 | 0.933 | 0.014** | 0.051* | 0.025** | 0.030** | 0.018** | 0.064* | 0.102 | 0.225 | |||
Crude models control for age and sex; Adjusted models control for age, sex, waist circumference, reported health decline, diabetes, arthritis, heart disease, and depressive symptom score.
P-values in italics,
p<.10
p<.05
Figure 1.
Mean predicted values of inflammatory outcomes for each level of sleep duration, adjusted for age and sex (predicted at actual levels of observed covariates, based on crude models from Table 3). All differences between >8 hours and 6-8 hours shown here were significant at p<.10. Differences between <6 and 6-8 hours only significant for sICAM.
In the presence of controls for baseline levels, increases in IL-6 and s-ICAM-1 and decreases (worsening) in albumin between 2000 and 2006 were associated with a higher risk of long but not short sleep duration in 2006 (Table 4). Higher baseline levels of IL-6 and e-selectin also predicted long sleep duration six years later independent of the change in levels, while higher baseline levels of sICAM-1 predicted short sleep duration. Given the six-year period between measurement of inflammation and sleep duration, these results suggest that the contemporaneous inflammation-long sleep duration association may not be driven solely by acute illness but likely reflects longer-term physiological processes.
Table 4. Sleep duration in 2006 and change in inflammation in 2000-2006: Multinomial logits.
| RRR | p-value | RRR | p-value | |
|---|---|---|---|---|
| <6 hours | >8 hours | |||
| 2000 Ln(IL-6) | 1.034 | 0.789 | 1.731 | 0.001** |
| Change in Ln(IL-6) | 1.102 | 0.421 | 1.685 | 0.001** |
| 2000 albumin | 1.11 | 0.79 | 0.439 | 0.128 |
| Change in albumin | 1.266 | 0.513 | 0.335 | 0.018** |
| 2000 WBC | 1.095 | 0.163 | 1.093 | 0.34 |
| Change in WBC | 1.085 | 0.207 | 1.129 | 0.176 |
| 2000 Ln(CRP) | 1.1014 | 0.873 | 1.157 | 0.215 |
| Change in Ln(CRP) | 0.965 | 0.705 | 1.078 | 0.562 |
| 2000 Ln(e-selectin) | 1.386 | 0.112 | 1.924 | 0.028** |
| Change in Ln(e-selectin) | 0.965 | 0.868 | 0.747 | 0.364 |
| 2000 (Ln(sICAM-1)) | 2.055 | 0.013** | 1.622 | 0.208 |
| Change in Ln(sICAM-1) | 1.591 | 0.251 | 4.000 | 0.01** |
N=624: 6-8 hours of sleep as the reference category for outcome
Relative Risk Ratios (exp(b)) reported, P-values in italics,
p<.10,
p<.05
Models adjust for age and sex.
Discussion
From our cross-sectional data, we found virtually no relationship between biomarkers of inflammation and the overall sleep quality index or its subcomponents. In contrast, we found a strong relationship between long sleep duration and most markers of inflammation, with little evidence of associations between short sleep duration and inflammation. Increases in inflammation between 2000 and 2006, determined from longitudinal data, were associated with an increased relative risk of long sleep duration in 2006, with no association found for short sleep duration. This result may support the hypothesis that high inflammation is related to long sleep duration via the worsening of co-morbidities associated with inflammation.
This work is consistent with some, but not all, previous research on inflammation and sleep. For example, Okun et al. (2009) found that poor sleep quality as measured by the PSQI was associated with higher CRP but not with IL-6 or tumor necrosis factor-alpha (TNF-α) in a sample of 43 young U.S. women, with no associations between inflammatory markers and sleep duration as measured continuously, consistent with our primarily negative findings for sleep quality and continuous sleep duration(5). Friedman et al. (2005) identified relationships between IL-6 and the PSQI global score at the bivariate level but not after adjustment for sociodemographic factors in a sample of 74 U.S. women aged 61-90 (7). Better sleep efficiency, as measured by the in-home NightCap monitoring system, was related to significantly lower levels of IL-6 in this sample. McDade et al. (2006) found higher CRP in those reporting taking longer than 30 minutes to fall asleep, but noassociation with sleep duration or other PSQI measures of sleep quality among 188 Chicago adults aged 52-70, similar to our negative findings for the PSQI(12).
Two studies have found associations between self-reported sleep and inflammation for women but not men, in contrast to our findings of no interactions by sex. Miller et al. (2009) recently found lower IL-6 in women sleeping 8 compared to 7 hours and higher CRP for women sleeping 5 hours or less compared to 7 hours, but no associations for men in the Whitehall II sample of 4600 individuals (43). Using the PSQI, Suarez (2008) found that overall sleep quality and difficulty falling asleep were associated with higher fibrinogen, IL-6, and CRP only for women in a sample of 210 young adults in North Carolina (13). These differences may reflect different associations between sex, psychosocial factors, and sleep disturbance in American and British compared with Taiwanese women, or differences in these relationships at younger compared with older ages.
Only one previous study to our knowledge has found associations between longer sleep duration and increased levels of inflammatory markers. Patel et al (2009) found that additional hours of self-reported sleep were associated with higher levels of CRP and IL-6, while short duration during observed polysomnography was associated with higher levels of TNF-α in a sample of 614 adults from the Cleveland Family Study (6). In the only study of sleep quality and inflammation in a non-Western population, no associations between self-reported sleep quality and IL-6 and CRP were found for Chinese adults aged 50-70 (44), consistent with our findings.
Many studies have found associations between long sleep and mortality, even adjusting for multiple chronic conditions (45). Recent work from the U.S. found that this relationship held for the elderly but not middle-aged adults; the authors concluded that the association between long-sleep and mortality is a consequence of underlying medical conditions and age-related sleep changes (46). The current study suggests that inflammation may be a mediator of the relationship between underlying health, long sleep, and mortality. In individuals with sleep disorders, circulating levels of IL-6 predict severity of daytime sleepiness, and experiments have shown that administration of IL-6 leads to somnolence (47). Sleep is also known to dramatically increase during the last few weeks or months of life, and sleep-inducing effects of cytokines may contribute to this phenomenon (23). While less supported, it is also possible that long sleep directly causes inflammation. Long sleep has been associated with increased sleep fragmentation and more frequent awakenings, which can influence cytokine expression (22).
Our study is limited by a one-time measure of sleep characteristics. Because our analyses are primarily cross-sectional, no causal relationship between increased levels of inflammatory markers and longer sleep duration can be established. While the association between long sleep and inflammation was not mediated by measures of chronic conditions in our sample, it is possible that unmeasured disease processes with an inflammatory component are a source of both inflammation and long sleep duration. This explanation would be consistent with our findings that increases in inflammation between waves predicted long sleep duration in the second wave, and broader findings in the literature of an association between long sleep and mortality. An additional limitation is that self-reported sleep quality and duration are likely measured with error, which could contribute to our null findings for the PSQI sleep components and inflammation. Objective monitoring methods such as wrist-watch actigraphy have recently beenemployed in population surveys to measure more precisely various aspects of sleep quality and sleep duration (48).
Our study contributes to the sparse literature on long sleep duration and inflammation, which may be particularly salient for older populations. The current study's strengths include a large sample size compared with previous studies, an extensive array of inflammatory markers—including measures of change for most markers-- and one of the first investigations of these associations in a non-Western sample. Afternoon naps are customary among the elderly in Taiwan, with some evidence suggesting that nappers have better nocturnal sleep quality(49). Since the PSQI asks only about nighttime sleep duration, it is possible that total sleep duration is longer than reported, making short nighttime sleep duration less detrimental and longer nighttime sleep duration a stronger indicator of poor health.
Future work on sleep duration and inflammation should move beyond an exclusive focus on short sleep and examine the potential importance of long sleep as a marker of underlying inflammatory illness. Prospective studies of sleep and inflammatory markers can help shed light on whether these relationships are causal or are driven by other factors. Such studies could also help elucidate the role of inflammation in the observed association between long sleep and mortality, contributing to our understanding of sleep as both a cause and consequence of ill health.
Acknowledgments
This work was supported by the National Institutes of Health grants R01AG016790 and R01AG16661(from the National Institute on Aging) and R24HD047879 (from The Eunice Kennedy Shriver National Institute of Child Health and Human Development).
Abbreviations
- CRP
C-reactive protein
- IL-6
interleukin-6
- sICAM-1
soluble intercellular adhesion molecule-1
- SEBAS
Social Environment and Biomarkers of Aging Study
- TLSA
Taiwan Longitudinal Study of Aging
- WBC
white blood cell count
- ELISA
Enzyme linked immunoassay
- TNF-α
tumor necrosis factor-alpha
- PSQI
Pittsburgh Sleep Quality Index
Footnotes
The original PSQI contains two additional subcomponents: sleep disturbances and use of sleep medication (30). Subsequent research has shown that the sleep medication component is not significantly associated with the global PSQI score, and sleep disturbances had a low correlation with the global score (31).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hu F, Stampfer M, Speizer F, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–4. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between Reduced Sleep and Weight Gain in Women. Am J Epidemiol. 2006 November 15;164(10):947–54. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Medicine Reviews. 2010;14(3):191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liukkonen T, Rasanen P, Ruokonen A, et al. C-reactive protein Levels and Sleep Disturbances: Observations Based on The Northern Finland 1966 Birth Cohort Study. Psychosom Med. 2007 October 1;69(8):756–61. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 5.Okun ML, Coussons-Read M, Hall M. Disturbed sleep is associated with increased C-reactive protein in young women. Brain, Behavior, and Immunity. 2009;23(3):351–4. doi: 10.1016/j.bbi.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009;32(2):200–4. doi: 10.1093/sleep/32.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman EM, Hayney MS, Love GD, et al. Social relationships, sleep quality, and interleukin-6 in aging women. Proceedings of the National Academy of Sciences of the United States of America. 2005 December 20;102(51):18757–62. doi: 10.1073/pnas.0509281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-Reactive protein, an inflammatory marker of cardiovascular risk. Journal of the American College of Cardiology. 2004;43(4):678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Motivala S. Sleep and Inflammation: Psychoneuroimmunology in the Context of Cardiovascular Disease. Annals of Behavioral Medicine. 2011:1–12. doi: 10.1007/s12160-011-9280-2. [DOI] [PubMed] [Google Scholar]
- 10.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, Inflammatory, and Metabolic Consequences of Sleep Deprivation. Progress in Cardiovascular Diseases. 2009;51(4):294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum C-reactive protein (CRP)--no association with sleep duration or sleep disordered breathing. Sleep. 2007;30(8):991–6. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and Behavioral Predictors of Inflammation in Middle-Aged and Older Adults: The Chicago Health, Aging, and Social Relations Study. Psychosom Med. 2006 May 1;68(3):376–81. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 13.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: Evidence for gender disparity. Brain, Behavior, and Immunity. 2008;22(6):960–8. doi: 10.1016/j.bbi.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Opp M. Sleeping to fuel the immune system: mammalian sleep and resistance to parasites. BMC Evolutionary Biology. 2009;9(1):8. doi: 10.1186/1471-2148-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu JJ, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Annals of medicine. 2009;41(1):19–28. doi: 10.1080/07853890802186921. [DOI] [PubMed] [Google Scholar]
- 16.Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain, Behavior, and Immunity. 2007;21(8):1050–7. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Opp MR. Cytokines and sleep. Sleep Medicine Reviews. 2005;9(5):355–64. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J Clin Endocrinol Metab. 1997 May 1;82(5):1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL. Invited Commentary: Cross-Cultural Influences on Sleep--Broadening the Environmental Landscape. Am J Epidemiol. 2008 December 15;168(12):1365–6. doi: 10.1093/aje/kwn336. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004 Jun;16(3):240–3. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- 21.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Experimental Gerontology. 2004;39(5):687–99. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Grandner MA, Drummond SPA. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Medicine Reviews. 2007;11(5):341–60. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Medicine Reviews. 2004;8(3):159–74. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Krueger PM, Friedman EM. Sleep Duration in the United States: A Cross-sectional Population-based Study. Am J Epidemiol. 2009 May 1;169(9):1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermalin A, Liang J, Chang M. Survey of Health and Living Status of the Elderly in Taiwan: Questionnaire and Survey Design. Ann Arbor, MI: University of Michigan; 1989. [Google Scholar]
- 26.Goldman N, Weinstein M, Cornman J, Singer B, Seeman T, Chang MC. Sex Differentials in Biological Risk Factors for Chronic Disease: Estimates from Population-Based Surveys. Journal of women's health. 2004;13(4):393. doi: 10.1089/154099904323087088. [DOI] [PubMed] [Google Scholar]
- 27.Goldman N, Weinstein M. Social Environment and Biomarkers of Aging Study in Taiwan (SEBAS 2000 and SEBAS 2006): Main Documentation for SEBAS Longitudinal Public Use Data. Inter-university Consortium for Political and Social Research (ICPSR) [Google Scholar]
- 28.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology. 2003;56(2):148–54. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 29.Smith KV, Goldman N. Measuring Health Status: Self-, Interviewer- and Physician Reports of Overall Health. Journal of Aging and Health. 2010 doi: 10.1177/0898264310383421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep and Biological Rhythms. 2006;4(2):129–36. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabay C, Kushner I. Acute-Phase Proteins and Other Systemic Responses to Inflammation. N Engl J Med. 1999 February 11;340(6):448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 33.Volanakis JE. Human C-reactive protein: expression, structure, and function. Molecular Immunology. 2001;38(2-3):189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 34.Crimmins EM, Vasunilashorn S, Kim JK, Alley DE. Biomarkers related to aging in human populations. Adv Clin Chemistry. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. J Epidemiol Community Health. 2008 June 1;62(6):484–91. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- 36.Ponthieux A, Herbeth B, Droesch S, Haddy N, Lambert D, Visvikis S. Biological determinants of serum ICAM-1, E-selectin, P-selectin and -selectin levels in healthy subjects: the Stanislas study. Atherosclerosis. 2004;172(2):299–308. doi: 10.1016/j.atherosclerosis.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Glowinska B, Urban M, Peczynska J, Florys B. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension, and diabetes. Metabolism. 2005;54(8):1020–6. doi: 10.1016/j.metabol.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Danesh J, Collins R, Appleby P, Peto R. Association of Fibrinogen, C-reactive Protein, Albumin, or Leukocyte Count With Coronary Heart Disease: Meta-analyses of Prospective Studies. JAMA. 1998 May 13;279(18):1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 39.Pearson TA, Mensah GA, Alexander RW, et al. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003 January 28;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 40.Boey KW. Cross-validation of a short form of the CES-D in Chinese elderly. International Journal of Geriatric Psychiatry. 1999;14(8):608–17. doi: 10.1002/(sici)1099-1166(199908)14:8<608::aid-gps991>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two Shorter Forms of the CES-D Depression Symptoms Index. J Aging Health. 1993 May 1;5(2):179–93. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 42.Collins A, Glei D, Goldman N. The role of life satisfaction and depressive symptoms in all-cause mortality. Psychology and Aging. 2009;24(3):696–702. doi: 10.1037/a0016777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller M, Kandala NB, Kivimaki M, et al. Gender differences in the cross-sectional relationships between sleep duration and markers of inflammation: Whitehall II study. Sleep. 2009;32(7):857–64. [PMC free article] [PubMed] [Google Scholar]
- 44.Haseli-Mashhadi N, Dadd T, Pan A, Yu Z, Lin X, Franco O. Sleep quality in middle-aged and elderly Chinese: distribution, associated factors and associations with cardio-metabolic risk factors. BMC Public Health. 2009;9(1):130. doi: 10.1186/1471-2458-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. Journal of Sleep Research. 2009;18(2):148–58. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 46.Gangwisch J, Heymsfield S, Boden-Albala B, et al. Sleep duration associated with mortality in the elderly, but not middle-aged, adults in a large US sample. Sleep. 2008;31(8):1087–96. [PMC free article] [PubMed] [Google Scholar]
- 47.Papanicolaou DA. The pathophysiologic roles of interleukin-6 in human disease. Annals of Internal Medicine. 1998;128(2):127–37. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 48.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively Measured Sleep Characteristics among Early-Middle-Aged Adults: The CARDIA Study. Am J Epidemiol. 2006 July 1;164(1):5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 49.Lai HL. Self-Reported Napping and Nocturnal Sleep in Taiwanese Elderly Insomniacs. Public Health Nursing. 2005;22(3):240–7. doi: 10.1111/j.0737-1209.2005.220307.x. [DOI] [PubMed] [Google Scholar]