Abstract
Structurally diverse secondary amine-functionalized poly(propylene imine) (PPI) dendrimers capable of tunable nitric oxide (NO) release were synthesized in a straightforward, one-step manner using ring-opening or conjugate-addition reactions with propylene oxide (PO), styrene oxide (SO), acrylonitrile (ACN), poly(ethylene glycol) methyl ether acrylate (average Mn = 480) (PEG) or 1,2-epoxy-9-decene (ED). N-Diazeniumdiolate nitric oxide donors were formed on the resulting secondary amine-functionalized G2–G5 PPI dendrimers by reaction with NO gas in basic solution. The NO storage and release kinetics for the resulting dendritic scaffolds were diverse (0.9–3.8 μmol NO/mg totals and 0.3 to 4.9 h half lives), illustrating the importance of the exterior chemical modification (e.g., steric environments, hydrophobicity, etc.) on diazeniumdiolate stability/decomposition. Tunable NO release was demonstrated by combining two donor systems on the exterior of one macromolecular scaffold. Additionally, a mathematical model was developed that allows for the simulation of dual NO release kinetics using the NO release data from the two single NO donor systems. The approaches described herein extend the range and scope of NO-releasing macromolecular scaffolds by unlocking a series of materials for use as dopants in biomedical polymers or stand-alone therapeutics depending on the exterior modification.
Keywords: dendrimers, nitric oxide, tunable release, diazeniumdiolate
Introduction
Dendrimers are a family of hyperbranched macromolecules with multivalent surfaces that enable the design of targeted therapeutics agent delivery vehicles.1–4 For example, polyamidoamines,5 polyamines,6 polypeptides,7 polyesters8 and polyethers9 dendrimers have been utilized for a range of biomedical applications, including drug and gene delivery,10–22 biological imaging,23–29 and tissue engineering.30–33 Bactericidal dendrimers have been prepared by encapsulating antibacterial agents (e.g., sulfomethoxazole34) within the dendrimer or at its periphery (e.g., quaternary ammonium groups35). Anionic amphiphilic dendrimers were also reported to possess antibacterial efficacy against Gram-positive bacteria.36
Nitric oxide is an endogenously produced diatomic free radical that mediates multiple processes in mammalian physiology. Due to NO’s pharmacological potential, the synthesis of prodrugs capable of controlled exogeneous NO production is important in further understanding NO’s role in physiology and developing NO-based therapeutics. The therapeutic effectiveness of such donors depends on the target (e.g., cell type and condition), concentration and rate of NO release.37, 38 For example, Chakrapani and coworkers synthesized O2-(2,4-dinitro-5-(4-(N-methylamino) benzoyloxy)phenyl)-1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate (PABA/NO) as an anti-cancer NO prodrug with efficacy against human leukemia and A2780 human ovarian cancer xenografts.39 Both the NO payload and release kinetics were shown to influence the anticancer activity. The study of how chemical structure impacts the rate of NO release from small molecule NO donors has been an active area of research, particularly with respect to biological activity.40–45 Generally, stabilization of the N-diazeniumdiolate NO donor correlates directly with extended NO release.46
To enhance NO storage and release levels relative to small molecule NO donors, we previously reported the synthesis of N-diazeniumdiolate-functionalized poly(propylene imine) (PPI) dendrimer conjugates as macromolecular NO donors capable of storing up to ~5 μmol NO/mg with NO release kinetics dependent on the dendrimer structure.47 Although we alluded to the multivalent exterior benefit on NO storage/release and further functionalization for imaging, controlled toxicity, and/or active targeting, for example, the study of structure/nitric oxide release relationships was not systematically pursued. In subsequent work, Stasko et al. reported the synthesis of S-nitrosothiol-modified polyamidoamine (PAMAM) dendrimers with alternative decomposition mechanisms for controlled NO release.48 While capable of storing ~2 μmol NO/mg, the kinetics of NO release were found to be highly dependent on the structure of the nitrosothiol and NO release trigger (e.g., light, copper, temperature). Collectively, the dendritic effects exerted on NO donor stability and reactivity, and the benefits of multi-functionalization with imaging beacons and/or active targeting agents, highlight the tremendous potential of dendrimers as NO release therapeutics.
Herein, we describe the synthesis of N-diazeniumdiolate-functionalized PPI dendrimers using select exterior functionalities to diversify both the NO release kinetics and potential applications for which such materials may prove useful. In addition to assessing the roles of molecular weight (e.g., generation or size of dendrimer) and exterior chemical structure on NO release, we demonstrate the ability to tune NO release kinetics using multiple NO donor functionalization.
Experimental Section
Materials and General Considerations
Details of the synthesis of primary amine-functionalized PPI dendrimers (G1 to G5-PPI-NH2) are provided as Supporting Information. Ethylenediamine (EDA), acrylonitrile (ACN), propylene oxide (PO), styrene oxide (SO), poly(ethylene glycol) methyl ether acrylate (average Mn = 480) (PEG), and 1,2-epoxy-9-decene (ED) were purchased from Aldrich Chemical Company (Milwaukee, WI). 1,6-Hexanediamine (HDA) and sodium methoxide (5.4 M solution in methanol) was purchased from Acros Organics (Geel, Belgium). Sponge cobalt catalyst (A-8B46) was purchased from Johson Matthey Catalysts (London, UK). Common laboratory salts and solvents were purchased from Fisher Scientific (Pittsburgh, PA). All other materials were used as received without further purification unless otherwise noted. 1H nuclear magnetic resonance (NMR) spectra were recorded on Bruker (400 MHz) and Varian (600 MHz) spectrometers. Hydrogenation reactions used for the synthesis of PPI-NH2 (e.g., from G2 to G5) were carried out in a stainless steel high-pressure reactor purchased from Parr Instrument Company (Moline, IL). Agitation was provided by a Teflon-coated magnetic stirring bar. Heating was provided using a heating fabric wrapped around the reactor, and temperature was controlled using a temperature controller via a thermal coupler. Nitric oxide release was measured using Sievers 280i Chemiluminesce Nitric Oxide Analyzer (Boulder, CO) as described previously.47 The chemiluminescence analyzer was calibrated with NO gas (26.9 ppm). A parameter for converting the instrument response (ppb) to moles of NO was obtained using the conversion of nitrite standards to NO in a 0.1 M KOH/H2SO4 solution (1.31 × 10−13 moles NO/ppb).
Synthesis of Secondary Amine-Functionalized PPI Dendrimers
100 mg PPI-NH2 (e.g., from G2 to G5) was dissolved in 2 ml methanol in a 10ml vial. One equivalent of acrylonitrile (ACN), poly(ethylene glycol) methyl ether acrylate (average Mn = 480) (PEG), propylene oxide (PO), styrene oxide (SO), or 1,2-epoxy-9-decene (ED) (e.g., with respect to molar amount of primary amine functionality) was then added to the 10 mL vial. The solution was stirred at room temperature for 4 d. Solvent was removed under reduced pressure. Dendrimers were dissolved in water followed by dialysis against water and lyophilization.
Representative 1HNMR data of secondary amine-functionalized G5-PPI conjugate formed via the reactions of G5-PPI-NH2 with ACN, PEG, PO, SO, and ED (referred to hereafter as G5-PPI-ACN a-64, G5-PPI-PEG b-64, G5-PPI-PO c-64, G5-PPI-SO d-64, G5-PPI-ED e-64) are as follows: G5-PPI-ACN a-64: 1H NMR (400 MHz, CD3OD, δ): 2.87 (NHCH2CH2CN), 2.82 (NHCH2CH2CN), 2.60 (NCH2CH2CH2NH), 2.40 (NCH2CH2CH2NH), 1.60 (NCH2CH2CH2NH). 13C NMR (400 MHz, CD3OD, δ): 117, 52.4, 51.8, 44.5, 33.3, 26.1, 23.6, 16.9. G5-PPI-PEG b-64: 1H NMR (400 MHz, CD3OD, δ): 2.60 (NCH2CH2CH2NH), 2.40 (NCH2CH2CH2NH), 1.60 (NCH2CH2CH2NH), 3.40–3.70 (OCH2CH2O), 2.80 (CH2NHCH2CHCOOPEG), 2.65 (CH2NHCH2CHCOOPEG), 2.42 (CH2NHCH2CHCOOPEG). 13C NMR (400 MHz, CD3OD, δ): 172, 71.6, 70.85, 69.3, 60.1, 57.0, 51.4, 43.9, 39.1, 22.9. G5-PPI-PO c-64: 1H NMR (400 MHz, CD3OD, δ): 3.70 (CH2CH(OH)CH3), 2.60–2.62 (CH2CH(OH)CH3, NCH2CH2CH2NH), 2.40 (NCH2CH2CH2NH), 1.60 (NCH2CH2CH2NH), 1.00 (CH2CH(OH)CH3). 13C NMR (400 MHz, CD3OD, δ): 66.9, 58.2, 53.7, 52.8, 41.2, 30.8, 27.6, 24.9, 21.8. G5-PPI-SO d-64: 1H NMR (400 MHz, CD3OD, δ): 7.50–7.20 (CH2CH(OH)Ph), 3.70 (CH2CH(OH)Ph), 2.72 (CH2CH(OH)Ph), 2.60 (NCH2CH2CH2NH), 2.40 (NCH2CH2CH2NH), 1.60 (NCH2CH2CH2NH). 13C NMR (400 MHz, CD3OD, δ): 140.6, 128.2, 127.5, 127.3, 125.8, 71.9, 57.1, 52.4, 45.6, 39.8, 26.3, 23.6. G5-PPI-ED e-64: 1H NMR (400 MHz, CD3OD, δ): 5.74 (CH2CH=CH2), 4.88 (CH2CH=CH2), 3.58 (NHCH2CH(OH)CH2), 2.60 (NCH2CH2CH2NH), 2.40 (NCH2CH2CH2NH), 1.98 (CH2CH=CH2), 1.60 (NCH2CH2CH2NH), 1.2–1.4 ((CH2)5CH2CH=CH2 ). 13C NMR (400 MHz, CD3OD, δ): 140.0, 116.1, 70.5, 56.5, 52.4, 47.2, 39.2, 33.9, 30.2, 25.6, 24.9.
Synthesis of Diazeniumdiolate-Functionalized PPI Dendrimers
One equivalent of 5.4 M sodium methoxide solution in methanol (e.g., with respect to the molar amount of primary amine functionalities in PPI-NH2 used to synthesize these secondary amine-functionalized PPI) was added to a vial containing G1 to G5 secondary amine-functionalized PPI dendrimers in methanol (2 mL), The resulting reaction solution was charged with 10 atm of NO while stirring in a stainless steel reactor. Prior to charging with NO, the reactor was flushed three times with argon followed by a series of three longer charge/discharge cycles with argon (3 × 10 min) to remove oxygen from the stirring solutions. The reactor was then filled with 10 atm of NO (purified over KOH pellets for 30 min to remove trace NO degradation products) at ambient temperature. After 3 days, the NO was expunged using the same charge/discharge procedures described above with argon to remove unreacted NO from the reaction solution.
Characterization of NO Storage and Release
Aliquots (~10–25 μL) of diazeniumdiolate-functionalized PPI as a solution in methanol (e.g., ~7–200 mM) were added to 30 mL deoxygenated phosphate buffered saline (PBS) (10 mM, pH = 7.4) at 37 °C to initiate NO release. Nitrogen was flowed through the solution at a flow rate of 70 mL/min to carry the liberated NO to the analyzer. Additional nitrogen flow was supplied to the flask to match the collection rate of the instrument at 200 mL/min. Nitric oxide analysis was terminated when levels decreased to 10 ppb NO/mg dendrimer. Chemiluminescence data for the NO-releasing dendrimers were represented as: i) total amount of NO release (t[NO], μmol NO/mg and μmol NO/μmol of secondary amine-functionalized dendrimers); ii) maximum flux of NO release ([NO]max, ppb/mg of secondary amine-functionalized dendrimers); iii) half-life (t1/2) of NO release; and, iv) conversion efficiency defined as percentages of amine functionalities in PPI (e.g., from G1 to G5) converted to diazeniumdiolate functionality (e.g., total moles of NO release divided by twice the molar amount of primary amine functionalities in PPI-NH2 used initially to synthesize secondary amine-functionalized dendrimer conjugates).
Results and Discussion
Synthesis and Characterization of Secondary Amine-Functionalized PPI Dendrimers
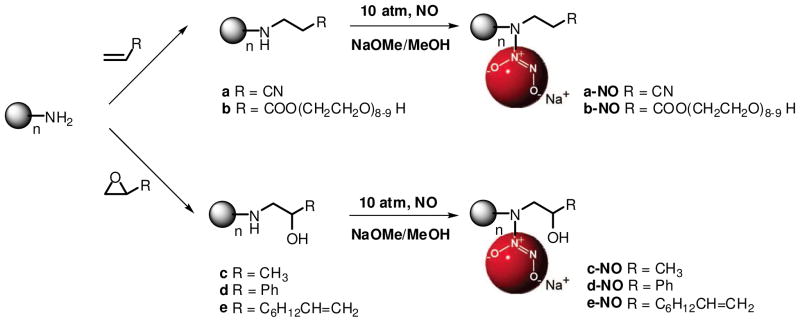
Stasko et al. previously reported an approach whereby secondary amine-functionalized PPI conjugates were reacted with NO at high pressure to form NO-releasing dendritic scaffolds with large NO storage capacity47. Analogously, primary amine-functionalized PPI dendrimers were characterized by significantly less NO storage and shorter NO release durations. These results suggest that the structural features of the dendrimer are critical in the design of diazeniumdiolate-functionalized scaffolds. Since chemical reaction of primary amine functionalities with organic compounds represents a simple approach for designing secondary amine-functionalized PPI conjugates, we targeted the synthesis of secondary amine-functionalized dendrimers using ring-opening and conjugate-addition reactions of PPI-NH2 with PO, SO, ED, ACN, and PEG (Scheme 1) to fully unlock a series of dendrimer scaffolds with diverse exterior properties (e.g., aromatic, hydrophilic, hydrophobic). Indeed, the epoxides, acrylated and acrylonitrile selected for conjugation were based on sterics, hydrophobicity, and biocompatibility. Of note, it is also possible to yield tertiary amine adducts. In addition, we carried out a series of model kinetic studies using NMR spectroscopy for conjugate-addition reactions of first generation G1-PPI-NH2 with ACN and ring-opening reactions of HDA with PO to determine the suitability of conjugate-addition and ring-opening reactions for the synthesis of secondary amine-functionalized PPI dendrimers. The results of these studies revealed large differences in the rates of reactions of G1-PPI-NH2 with ACN, and HDA with PO. The rate constants of the first conjugate-addition or ring-opening reaction (k1) were substantially larger than those of the second reactions (k2) (data not shown), providing strong support for the use of such reactions (e.g., over a period of four days, in a dilute solution, and at one equivalent of ACN, epoxides, or acrylates with respect to molar amount of PPI-NH2) to yield secondary amine-functionalized products suitable for subsequent NO release studies.
Scheme 1.
Synthesis of secondary amine- and diazeniumdiolate-functionalized PPI conjugates for which n represents the number of primary amines on the periphery of PPI dendrimers (n = 8, 16, 32, 64).
Influence of Exterior Functionality on Nitric Oxide Release
The approach described above is based on dendrimer functionalization at their exterior to yield secondary amine-functionalized PPI dendrimers. Two practical advantages of this approach include that the synthesis is simple and the resulting structurally diverse exteriors greatly expand the potential applications for which these materials may find utility. Reactions of secondary amine-functionalized PPI (e.g., from G2 to G5) with NO under basic conditions (e.g., sodium methoxide) yielded N-diazeniumdiolate NO donor-functionalized dendrimers with diverse NO release characteristics.
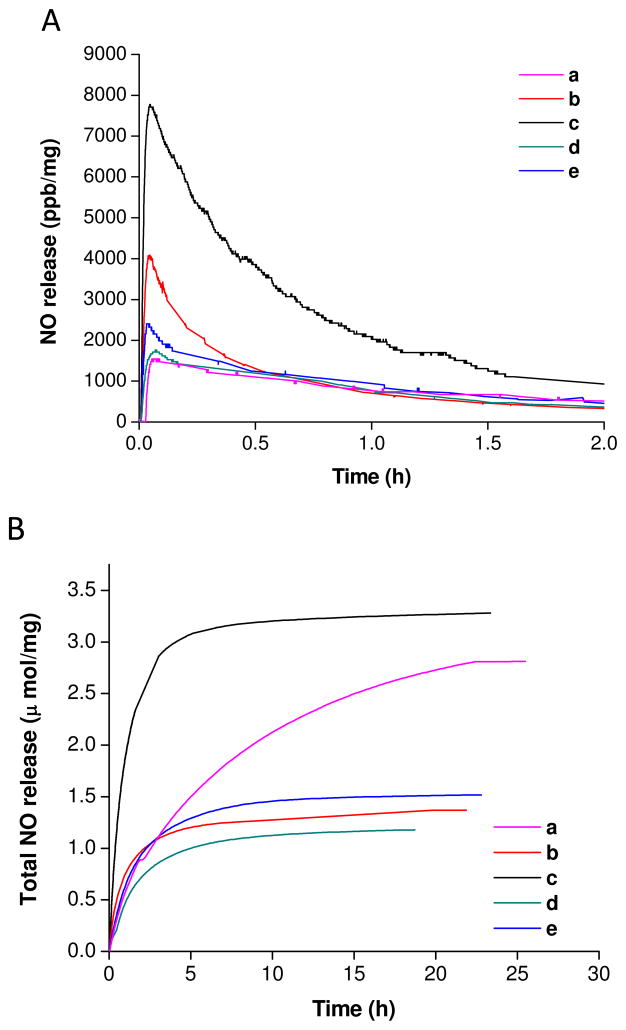
Chemiluminescence was used to characterize the NO storage and release properties (e.g., in PBS, pH = 7.4, 37 °C) for the N-diazenuimdiolate-modified PPI dendrimers. Representative NO release profiles for these dendrimers are shown in Figure 1. The workup of specific NO release parameters (e.g., total NO release, maximum flux, half-life, and conversion efficiency) are provided in Table 1. In general, the NO release results reveal high NO storage capabilities (e.g., 0.9–3.8 μmol NO/mg) and a broad range of release kinetics (e.g., NO release half-life from 0.3–4.9 h). Further inspection of these data reveals that the conversion efficiencies (e.g., 10–40%) of the dendrimers varied substantially based on the chemical modification. As shown in Table 1, G2 to G5-PPI-SO (d-NO) were characterized by lower NO donor formation (e.g., ~10–15%) versus the other PPI dendrimers (e.g., ~14–40%). The lower conversion efficiencies for PPI-SO (d-NO) may be attributed to a more sterically-hindered environment around the NO donor precursors (i.e., secondary amines), resulting in lower NO and base accessibility to the amines during the NO charging process.
Figure 1.
(A) Real time NO release profile for NO-releasing G4-PPI dendrimer conjugates; and (B) plot of t[NO] vs time for NO-releasing PPI dendrimer conjugates.
Table 1.
Nitric oxide release characteristics for PPI dendrimers in PBS (pH = 7.4) at 37 °C.
| Dendrimer | t[NO] (μmol NO/mg)a | t[NO] (μmol NO/μmol)b | [NO]max (ppb/mg)c | [NO]max (ppb/μmol)d | t½ (h) | Conversion (%) | |
|---|---|---|---|---|---|---|---|
| a-8-NO | G2-PPI-ACN-NO | 3.57 | 4.18 | 1529 | 1788 | 4.81 | 26.1 |
| a-16-NO | G3-PPI-ACN-NO | 3.33 | 8.35 | 1100 | 2758 | 4.84 | 26.1 |
| a-32-NO | G4-PPI-ACN-NO | 2.45 | 12.7 | 1547 | 7977 | 4.82 | 19.9 |
| a-64-NO | G5-PPI-ACN-NO | 1.68 | 17.7 | 881 | 9282 | 4.88 | 13.8 |
|
| |||||||
| b-8-NO | G2-PPI-PEG-NO | 1.11 | 5.10 | 3771 | 17291 | 0.67 | 31.9 |
| b-16-NO | G3-PPI-PEG-NO | 1.08 | 10.1 | 2309 | 21564 | 1.11 | 31.6 |
| b-32-NO | G4-PPI-PEG-NO | 1.37 | 25.8 | 4088 | 77035 | 0.84 | 40.3 |
| b-64-NO | G5-PPI-PEG-NO | 1.17 | 44.3 | 1886 | 71403 | 1.22 | 34.6 |
|
| |||||||
| c-8-NO | G2-PPI-PO-NO | 2.99 | 3.63 | 17130 | 20725 | 0.30 | 22.7 |
| c-16-NO | G3-PPI-PO-NO | 3.22 | 8.38 | 9617 | 24888 | 0.62 | 26.2 |
| c-32-NO | G4-PPI-PO-NO | 3.27 | 17.5 | 7762 | 41481 | 0.78 | 27.3 |
| c-64-NO | G5-PPI-PO-NO | 3.78 | 41.1 | 6839 | 74250 | 1.06 | 32.1 |
|
| |||||||
| d-8-NO | G2-PPI-SO-NO | 1.14 | 1.95 | 8495 | 14496 | 1.47 | 12.2 |
| d-16-NO | G3-PPI-SO-NO | 0.91 | 3.30 | 2363 | 8462 | 0.97 | 10.3 |
| d-32-NO | G4-PPI-SO-NO | 1.18 | 8.70 | 1720 | 12609 | 1.43 | 13.6 |
| d-64-NO | G5-PPI-SO-NO | 1.25 | 18.5 | 2215 | 32843 | 1.62 | 14.5 |
|
| |||||||
| e-8-NO | G2-PPI-ED-NO | 1.95 | 3.87 | 5648 | 11178 | 0.81 | 24.2 |
| e-16-NO | G3-PPI-ED-NO | 1.64 | 6.75 | 2733 | 11279 | 1.71 | 21.1 |
| e-32-NO | G4-PPI-ED-NO | 1.51 | 12.8 | 2401 | 20217 | 1.34 | 19.9 |
| e-64-NO | G5-PPI-ED-NO | 1.86 | 31.6 | 3190 | 54262 | 1.88 | 24.7 |
total amount of NO release (μmol) per milligram of secondary amine-functionalized PPI.
total amount of NO release (μmol) per micromole of secondary amine-functionalized PPI.
maximum flux of NO release (ppb) per milligram of secondary amine-functionalized PPI.
maximum flux of NO release (ppb) per micromole of secondary amine-functionalized PPI.
The exterior modification also influenced the NO release kinetics. For example, both PPI-PO (c-NO) and PPI-PEG (b-NO) released NO rapidly (Table 1 and Figure 1). Such rapid NO release kinetics might be expected since the isopropyl and PEG groups are hydrophilic and facilitate water solvation favorable to diazeniumdiolate NO donor degradation. The data also indicate that the NO release half-lives for G2-G5 PPI-SO (d-NO) and PPI-ED (e-NO) are slightly longer than PPI-PO (c-NO) and PPI-PEG (b-NO). The longer NO release for PPI-SO (d-NO) and PPI-ED (e-NO) correlates well with the increased hydrophobic structure at the exteriors of these dendrimers.
The ACN modification for PPI dendrimer (a-NO) exhibited large NO storage (e.g., ~1.7–3.6 μmol NO/mg) and conversion efficiency (e.g., ~14–26%) (Table 1). Of note, past studies have indicated that the reaction of cyano-containing compounds with NO at high-pressures under basic conditions may yield C-diazeniumdiolate-functionalized products. Both NO and nitrous oxide (N2O) may be release from C-diazeniumdiolates in aqueous environments at low pH.49–51 In this context, it may be possible that the high conversion efficiencies for PPI-ACN (a-NO) arise from the contribution of NO released from C-diazeniumdiolate-functionalized products. A series of experiments were thus carried out to probe the nature of the NO release from PPI-ACN (a-NO) using G0.5-PPI, a cyano-containing compound without the capacity to form N-diazeniumdiolate due to the absence of secondary amines. The NO release from G0.5-PPI was ~4.5×10−3 μmol NO/mg, providing strong support that the high NO storage and conversion efficiency for the ACN-modified dendrimers are indeed the result of N-diazeniumdiolate functionalization.
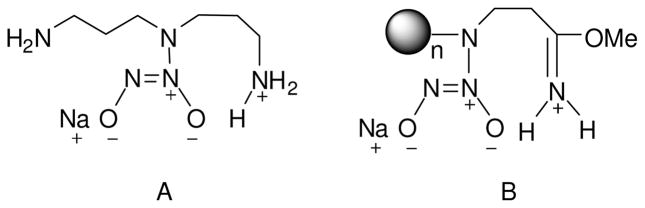
The PPI-ACN (a-NO) analogues were also characterized as having the longest NO release half-lives (e.g., ~5 h). Given the hydrophilic nature of the cyano functionality, the extended NO release is not attributable to water uptake. For example, the long half-lives of N-diazeniumdiolate- functionalized small molecule derivatives (e.g., dipropylenetriamine or DPTA-NO) have previously been attributed to diazeniumdiolate stabilization by neighboring cationic ammonium functionalities as depicted in Figure 2A.45,52,53 In this manner, the presence of neighboring cationic functionalities (e.g., protonated imidates) for PPI-ACN (a-NO) dendrimers may provide additional stabilization to the diazeniumdiolate functionality (Figure 2B).
Figure 2.
Proposed structures for stabilization of (A) diazeniumdiolate-functionalized DPTA by neighboring cationic ammonium functionality, and (B) PPI-ACN (a-NO) by neighboring cationic protonated-imidate functionality.
Since sodium methoxide was used as the base for the reaction of PPI-ACN (a) with NO (to yield diazeniumdiloate-functionalized products), the methoxide anion may also serve as a nucleophile to react with the cyano group in PPI-ACN (a) and yield imidate adducts.54 The transfer of proton from solvent (e.g., methanol) to the relatively basic nitrogen atom in the resulting imidates might thus lead to protonated-imidate functionality (Figure 2B) that is similar to cationic ammonium functionality in DPTA-NO (Figure 2A).
As shown in Table 1, both the NO storage and maximum NO flux per dendrimer molecule increased as a function of dendrimer size (i.e., generation). For example, the NO payload from G5-PPI-PO-NO (c-64-NO) was 41.1 μmol/μmol dendrimer, much greater than G2-PPI-PO-NO (c-8-NO) (e.g., 3.63 μmol/μmol dendrimer). A similar trend is observed for maximum NO flux. These results reveal the capability of larger NO-releasing PPI dendrimers to deliver significant concentrations of NO, a potentially beneficial attribute for therapeutic anti-microbial and anti-cancer applications.
Effect of Solvent for NO Conjugation
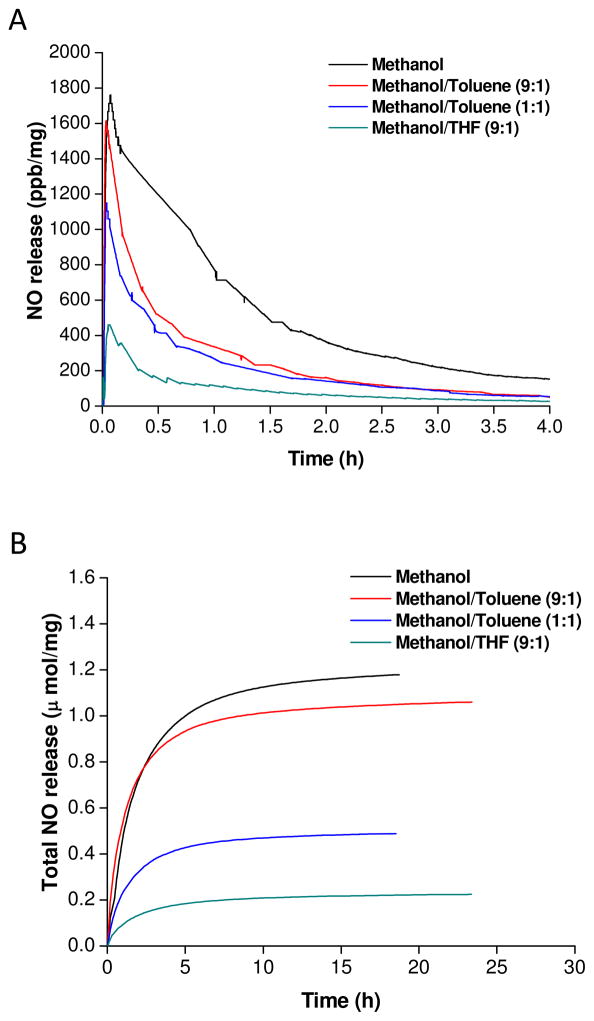
As synthesized, the amphiphilic secondary amine-functionalized dendrimers (e.g., PPI-SO, PPI-ED) possess a hydrophilic PPI core and hydrophobic periphery of aromatic rings or long alkyl chains. Different from PPI-ACN, PPI-PO and PPI-PEG, these amphiphilic dendrimers may have more packed exterior in the charging solvent due to the poor compatibility of the aromatic rings or long alkyl chains with polar solvent (e.g., methanol). We thus sought to investigate the role of charging solvent on NO donor formation efficiency using mixtures of methanol with less polar solvent (e.g., methanol/THF (9:1, v/v), methanol/toluene (9:1, v/v) and methanol/toluene (1:1, v/v)). As shown in Figure 3, the NO release data reveals an adverse effect of toluene and THF on NO conjugation to PPI-SO dendrimers. Compared to toluene, use of THF compromised the NO storage to a larger extent.
Figure 3.
(A) Real time NO release profile and (B) plot of t[NO] vs time for G4-PPI-SO-NO synthesized in different NO conjugation solvents.
The lower NO conjugation efficiency may be the result of the PPI dendrimer core collapsing in the mixture of methanol and less polar solvents (e.g., toluene and THF). Indeed, this hypothesis is supported by dynamic light scattering data (data not shown). In turn, the dendrimer backbone collapse likely contributes to a greater steric hindrance around the secondary amines and concomitant lower NO conjugation efficiency.
Characterization of PPI Conjugates Synthesized from Defined Mixtures of PO and ACN
To evaluate the ability to define NO release, we designed multi-functionalized N-diazeniumdiolate- functionalized dendrimer conjugates using G5-PPI-NH2 and defined ratios of PO and/or ACN during the functionalization step. Specifically, G5-PPI-NH2 was reacted with either PO exclusively (e.g., c-64), ACN exclusively (e.g., a-64), or three different mixtures comprised of PO and ACN at molar ratios of 3:7, 5:5, and 7:3, respectively; the PO, ACN, or defined mixtures of PO and ACN were one equivalent with respect to molar amount of primary amine functionalities in G5-PPI-NH2. A series of 1H NMR experiments were carried out on the products of these reactions to determine the actual compositions of PO and ACN conjugated to the G5-PPI-NH2 (Figure 4). A distinct resonance at 1.10 ppm was apparent corresponding to the methyl protons in the isopropyl group of the products. The chemical shift of this peak was noted in the products for reactions with PO exclusively (e.g., c-64) and those with different mixtures of PO and ACN. Further inspection of these data indicates the presence of a second distinct peak at 2.80 ppm, formed upon G5-PPI-NH2 dendrimer reaction with either ACN exclusively (e.g., a-64) or defined mixtures of PO and ACN; this peak corresponds to the methylene protons one carbon away from the cyano group of the products. The compositions of PO and ACN incorporated in the products were 27/73 (c/a (27/73)), 40/60 (c/a (40/60)), and 60/40 (c/a (60/40)), and indicate that the ACN was incorporated at ratios greater than that in the reaction mixtures likely due to more rapid reaction of G5-PPI-NH2 with ACN versus PO.
Figure 4.
1H NMR spectra of G5-PPI-PO (c-64) (A), G5-PPI-ACN (a-64) (E), G5-PPI-PO/ACN at molar ratios of 7:3 (B), 5:5 (C), and 3:7 (D). The actual compositions of PO and ACN incorporated into these three PPI conjugates are at molar ratios of 27/73 (B), 40/60 (C), and 60/40 (D), respectively, as determined by integrating two chemical shifts at 1.10 and 2.80 ppm.
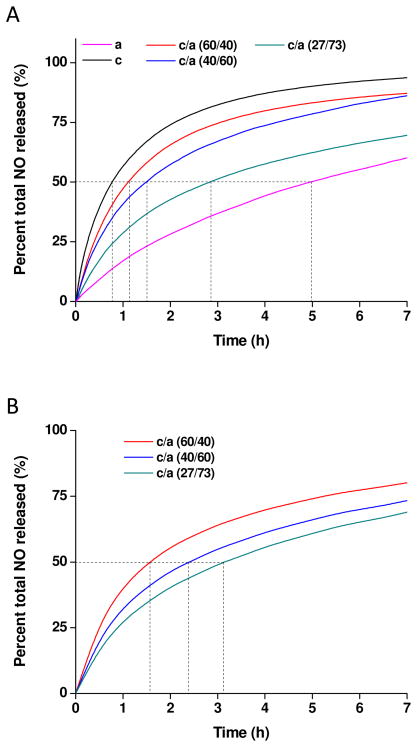
The NO release from the G5-PPI-PO/ACN conjugates at PO/ACN molar ratios of 27/73, 40/60, and 60/40, respectively, were intermediate to those synthesized based upon G5-PPI-NH2 reactions with either PO (e.g., c-64) or ACN (e.g., a-64) alone. Furthermore, the NO release profiles were influenced by the molar ratio of PO/ACN composition. For instance, the NO release was prolonged for the PPI conjugates modified with lower molar ratio of PO/ACN (half-lives of 2.90, 1.57, and 1.10 h for 27/73, 40/60, and 60/40, respectively) (Figure 5).
Figure 5.
(A) Experimental plot of percent total NO released in PBS (pH = 7.4) at 37 °C as a function of time for G5-PPI-PO (c-NO), G5-PPI-ACN (a-NO), and G5-PPI-PO/ACN conjugates; (B) Simulated plot of percent total NO released for G5-PPI-PO/ACN conjugates.
A mathematical model was developed to predict the NO release from hybrid G5-PPI-PO/ACN conjugates using the NO release data from the single G5-PPI-PO (c-64) and G5-PPI-ACN (a-64) systems. In Equation 1, a:b is the molar ratio of the PO and ACN modification in the hybrid G5-PPI-PO/ACN conjugate as determined by NMR spectroscopy, while yPO and yACN represent the normalized NO release profiles of the G5-PPI-PO (c-64) and G5-PPI-ACN (a-64) systems, respectively. Simulated NO release from a hybrid G5-PPI-PO/ACN conjugate (ya:b) was thus derived by averaging the normalized NO release from G5-PPI-PO (c-64) (yPO) and G5-PPI-ACN (a-64) (yACN) weighted with respect to the mole percentages of PO (i.e., [a/(a+b)] × 100%) and ACN (i.e., [b/(a+b)] × 100%) modification in the hybrid G5-PPI-PO/ACN conjugate.
| Eq.1 |
As shown in Figure 5, the simulated NO release from G5-PPI-PO/ACN conjugates were comparable with the experimental data, even though the experimental hybrid PPI conjugates were characterized by slightly faster NO release (e.g., NO release half-lives of 3.13, 2.42, and 1.65 h for c/a (27/73), c/a (40/60), and c/a (60/40), respectively). Nevertheless, the developed mathematical model appears to be well suited for simulating dual NO release kinetics using NO release data from single NO donor systems. For dendrimers, these results demonstrate that functionalization of PPI-NH2 with a defined mixture of PO and ACN allows for precise NO release selection with access to NO release kinetics that are intermediate to those formed upon reactions with one NO donor type exclusively.
Conclusions
To expand the utility of macromolecular NO-release scaffolds as stand-alone therapeutics, dopants for biomedical polymers, or general NO-release sources, we synthesized a series of diverse NO-releasing PPI dendrimers by straightforward chemical modification of the core dendrimer using conjugation addition (ACN and PEG) or ring opening (PO, ED, and SO) reactions. The NO release from these resulting dendrimers demonstrated that size (i.e., generation number or molecular weight) and exterior structures (e.g., steric environment, hydrophobicity, etc.) play important roles in NO release kinetics. Furthermore, the use of select NO donors with unique NO release kinetics and overall payloads may be exploited by multi-NO donor/dendrimer functionalization. The combination of structural diversity and achievable NO release (i.e., up to 3.8 μmol NO/mg with half-lives from 0.3–4.9 h) should prove useful in the further study and utilization of these NO release materials for a broad range of fundamental and applied applications including pharmacological agents.
Supplementary Material
Acknowledgments
Financial support was provided by the National Institutes of Health (NIH EB000708). We are grateful to Professor Michel Gagne and Mr. Harlan Mangum at the University of North Carolina at Chapel Hill for providing and assisting in the use of the high-pressure reactor. We also thank Mr. Marc ter Horst for technical assistance and helpful discussion involving 1H NMR analysis of the dendrimers.
Footnotes
Supporting Information. Details of the synthesis of primary amine-functionalized PPI conjugates from first to fifth generation. This information is available free of charge via Internet at http://pubs.acs.org.
References
- 1.Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Nat Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 2.Mintzer MA, Grinstaff MW. Chem Soc Rev. 2011;40:173–190. doi: 10.1039/b901839p. [DOI] [PubMed] [Google Scholar]
- 3.Wolinsky JB, Grinstaff MW. Adv Drug Delivery Rev. 2008;60:1037–1055. doi: 10.1016/j.addr.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Joshi N, Grinstaff M. Curr Top Med Chem. 2008;8:1225–1236. doi: 10.2174/156802608785849067. [DOI] [PubMed] [Google Scholar]
- 5.Tomalia DA, Naylor AM, Goddard WA. Angew Chem, Int Ed. 1990;29:138–175. [Google Scholar]
- 6.Debrabandervandenberg EMM, Meijer EW. Angew Chem, Int Ed. 1993;32:1308–1311. [Google Scholar]
- 7.Sadler K, Tam JP. Rev Mol Biotechnol. 2002;90:195–229. doi: 10.1016/s1389-0352(01)00061-7. [DOI] [PubMed] [Google Scholar]
- 8.Ihre H, Hult A, Soderlind E. J Am Chem Soc. 1996;118:6388–6395. [Google Scholar]
- 9.Hawker CJ, Frechet JMJ. J Am Chem Soc. 1990;112:7638–7647. [Google Scholar]
- 10.Allen TM, Cullis PR. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 11.Malik N, Evagorou EG, Duncan R. Anti-Cancer Drugs. 1999;10:767–776. [PubMed] [Google Scholar]
- 12.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 13.Kukowska-Latallo JF, Candido KA, Cao ZY, Nigavekar SS, Majoros IJ, Thomas TP, Balogh LP, Khan MK, Baker JR. Cancer Res. 2005;65:5317–5324. doi: 10.1158/0008-5472.CAN-04-3921. [DOI] [PubMed] [Google Scholar]
- 14.Wooley KL, Hawker CJ, Frechet JMJ. J Am Chem Soc. 1993;115:11496–11505. [Google Scholar]
- 15.Gillies ER, Frechet JMJ. J Am Chem Soc. 2002;124:14137–14146. doi: 10.1021/ja028100n. [DOI] [PubMed] [Google Scholar]
- 16.Steffensen MB, Simanek EE. Angew Chem, Int Ed. 2004;43:5178–5180. doi: 10.1002/anie.200460031. [DOI] [PubMed] [Google Scholar]
- 17.Li YG, Cu YTH, Luo D. Nat Biotechnol. 2005;23:885–889. doi: 10.1038/nbt1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haensler J, Szoka FC. Bioconjugate Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 19.Tang MX, Redemann CT, Szoka FC. Bioconjugate Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 20.Vincent L, Varet J, Pille JY, Bompais H, Opolon P, Maksimenko A, Malvy C, Mirshahi M, Lu H, Soria JPVC, Li H. Int J Cancer. 2003;105:419–429. doi: 10.1002/ijc.11105. [DOI] [PubMed] [Google Scholar]
- 21.Thomas TP, Shukla R, Kotlyar A, Kukowska-Latallo J, Baker JR. Bioorg Med Chem Lett. 2010;20:700–703. doi: 10.1016/j.bmcl.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majoros IJ, Myc A, Thomas T, Mehta CB, Baker JR. Biomacromolecules. 2006;7:572–579. doi: 10.1021/bm0506142. [DOI] [PubMed] [Google Scholar]
- 23.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Magnet Reson Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 24.Margerum LD, Campion BK, Koo M, Shargill N, Lai JJ, Marumoto A, Sontum PC. J Alloy Compd. 1997;249:185–190. [Google Scholar]
- 25.Kobayashi H, Kawamoto S, Choyke PL, Sato N, Knopp MV, Star RA, Waldmann TA, Tagaya Y, Brechbiel MW. Magnet Reson Med. 2003;50:758–766. doi: 10.1002/mrm.10583. [DOI] [PubMed] [Google Scholar]
- 26.Ziemer LS, Lee WMF, Vinogradov SA, Sehgal C, Wilson DF. J Appl Physiol. 2005;98:1503–1510. doi: 10.1152/japplphysiol.01140.2004. [DOI] [PubMed] [Google Scholar]
- 27.Dunphy I, Vinogradov SA, Wilson DF. Anal Biochem. 2002;310:191–198. doi: 10.1016/s0003-2697(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 28.Brinas RP, Troxler T, Hochstrasser RM, Vinogradov SA. J Am Chem Soc. 2005;127:11851–11862. doi: 10.1021/ja052947c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Floyd WC, Klemm PJ, Smiles DE, Kohlgruber AC, Pierre VC, Mynar JL, Frechet JMJ, Raymond KN. J Am Chem Soc. 2011;133:2390–2393. doi: 10.1021/ja110582e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozhkov V, Wilson D, Vinogradov S. Macromolecules. 2002;35:1991–1993. [Google Scholar]
- 31.Cloninger MJ. Curr Opin Chem Biol. 2002;6:742–748. doi: 10.1016/s1367-5931(02)00400-3. [DOI] [PubMed] [Google Scholar]
- 32.Wathier M, Jung PJ, Camahan MA, Kim T, Grinstaff MW. J Am Chem Soc. 2004;126:12744–12745. doi: 10.1021/ja045870l. [DOI] [PubMed] [Google Scholar]
- 33.Velazquez AJ, Carnahan MA, Kristinsson J, Stinnett S, Grinstaff MW, Kim T. Arch Ophthalmol-Chic. 2004;122:867–870. doi: 10.1001/archopht.122.6.867. [DOI] [PubMed] [Google Scholar]
- 34.Ma ML, Cheng YY, Xu ZH, Xu P, Qu H, Fang YJ, Xu TW, Wen LP. Eur J Med Chem. 2007;42:93–98. doi: 10.1016/j.ejmech.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Chen CZS, Beck-Tan NC, Dhurjati P, van Dyk TK, LaRossa RA, Cooper SL. Biomacromolecules. 2000;1:473–480. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 36.Meyers SR, Juhn FS, Griset AP, Luman NR, Grinstaff MW. J Am Chem Soc. 2008;130:14444–14445. doi: 10.1021/ja806912a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofseth LJ, Hussain SP, Wogan GN, Harris CC. Free Radical Bio Med. 2003;34:955–968. doi: 10.1016/s0891-5849(02)01363-1. [DOI] [PubMed] [Google Scholar]
- 38.Pacher P, Beckman JS, Liaudet L. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Bioorg Med Chem. 2008;16:2657–2664. doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Velazquez CA, Chen QH, Citro ML, Keefer LK, Knaus EE. J Med Chem. 2008;51:1954–1961. doi: 10.1021/jm701450q. [DOI] [PubMed] [Google Scholar]
- 41.Chakrapani H, Showalter BM, Kong L, Keefer LK, Saavedra JE. Org Lett. 2007;9:3409–3412. doi: 10.1021/ol701419a. [DOI] [PubMed] [Google Scholar]
- 42.Velazquez CA, Rao PNP, Citro ML, Keefer LK, Knaus EE. Bioorg Med Chem. 2007;15:4767–4774. doi: 10.1016/j.bmc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keefer LK, Flippen-Anderson JL, George C, Shanklin AP, Dunams TA, Christodoulou D, Saavedra JE, Sagan ES, Bohle DS. Nitric Oxide-Biol Chem. 2001;5:377–394. doi: 10.1006/niox.2001.0359. [DOI] [PubMed] [Google Scholar]
- 44.Davies KM, Wink DA, Saavedra JE, Keefer LK. J Am Chem Soc. 2001;123:5473–5481. doi: 10.1021/ja002899q. [DOI] [PubMed] [Google Scholar]
- 45.Keefer LK, Nims RW, Davies KM, Wink DA. Method Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 46.Hrabie JA, Klose JR, Wink DA, Keefer LK. J Org Chem. 1993;58:1472–1476. [Google Scholar]
- 47.Stasko NA, Schoenfisch MH. J Am Chem Soc. 2006;128:8265–8271. doi: 10.1021/ja060875z. [DOI] [PubMed] [Google Scholar]
- 48.Stasko NA, Fischer TH, Schoenfisch MH. Biomacromolecules. 2008;9:834–841. doi: 10.1021/bm7011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DeRosa F, Kibbe MR, Najjar SF, Citro ML, Keefer LK, Hrabie JA. J Am Chem Soc. 2007;129:3786–3787. doi: 10.1021/ja0686864. [DOI] [PubMed] [Google Scholar]
- 50.Arnold EV, Citro ML, Keefer LK, Hrabie JA. Org Lett. 2002;4:1323–1325. doi: 10.1021/ol025624j. [DOI] [PubMed] [Google Scholar]
- 51.Arnold EV, Keefer LK, Hrabie JA. Tetrahedron Lett. 2000;41:8421–8424. [Google Scholar]
- 52.Shin JH, Schoenfisch MH. Chem Mater. 2008;20:239–249. doi: 10.1021/cm702526q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim J, Lee Y, Singha K, Kim HY, Shin JH, Jo S, Han DK, Kim WJ. Bioconjugate Chem. 2011;22:1031–1038. doi: 10.1021/bc100405c. [DOI] [PubMed] [Google Scholar]
- 54.Schaefer FC, Peters GA. J Org Chem. 1961;26:412–418. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.