SUMMARY
Subcutaneous (SQ) and visceral (VIS) obesity are associated with different risks of diabetes and the metabolic syndrome. To elucidate if these differences are due to anatomic location or intrinsic differences in adipose depots, we characterized mice after transplantation of SQ or VIS fat from donor mice into either SQ or VIS regions of recipient mice. In the group with SQ fat transplanted into the VIS cavity, there was decreased body weight, total fat mass, glucose and insulin levels. There was improved insulin sensitivity during hyperinsulinemic-euglycemic clamps with increased whole-body glucose uptake, glucose uptake into endogenous fat, and insulin suppression of hepatic glucose production. These effects were observed to a lesser extent with SQ transplanted to SQ areas, whereas VIS fat transplanted to VIS area was without effect. These data suggest that SQ fat is intrinsically different from VIS fat and produces substances that can act systemically to improve glucose metabolism.
INTRODUCTION
While the association between obesity and type 2 diabetes is well known, the site of fat accumulation in humans can play a pivotal role in these health risks. Central obesity, characterized by increased amounts of intra-abdominal fat, is associated with insulin resistance, high risk of type 2 diabetes, dyslipidemia, accelerated atherosclerosis, and mortality (Carey et al., 1997; Wang et al., 2005; Nicklas et al., 2006; Ross et al., 2007). By contrast, peripheral obesity, i.e., increased amounts of subcutaneous fat, especially in the gluteofemoral regions, is associated with improved insulin sensitivity and a lower risk of developing type 2 diabetes, dyslipidemia and atherosclerosis in comparison to levels associated with central obesity (Misra et al., 1997; Snijder et al., 2003; Tanko et al., 2003). Consistent with this notion that visceral fat produces adverse metabolic effects, omentectomy, i.e., removal of visceral fat, results in decreased insulin and glucose levels in humans (Thorne et al., 2002), whereas removal of subcutaneous fat by liposuction does not result in improvement in any aspect of the metabolic syndrome (Klein et al., 2004). Also, in general, diet and/or exercise cause improved insulin sensitivity, and this is associated with a greater loss of visceral fat than subcutaneous fat (Langendonk et al., 2006; Gan et al., 2003).
Rodents also show differences in fat distribution and insulin resistance. Mice on diets high in saturated fat, mice with genetic defects in leptin signaling, and mice during normal aging show increased amounts of fat, especially intra-abdominal depots, and are insulin resistant (Rebuffe-Scrive et al., 1993; Dubuc, 1976; Barzilai et al., 1998). As in humans, omentectomy in obese mice results in improved insulin action and reduced hepatic glucose production (Gabriely et al., 2002). However, removal of subcutaneous fat in obese Zucker rats results in no consistent beneficial changes in weight gain, serum glucose or insulin levels (Liszka et al., 1998). Thus, both human and rodent studies suggest that increased visceral fat, but not removal of peripheral fat, has adverse effects on metabolism and disease outcome.
The most likely mechanism for these metabolic differences between central and peripheral obesity is that adipokines, free fatty acids and other metabolites released from visceral fat drain into the portal circulation where they can exert adverse effects on hepatic and other tissue metabolism (Kabir et al., 2005). However, several findings suggest that this may be an over simplification. First, in lipoatrophic diabetes there is a loss of both visceral and subcutaneous fat, and this is associated with severe insulin resistance, hyperlipidemia and glucose intolerance (Capeau et al., 2005). Secondly, assessment of insulin sensitivity in humans with visceral obesity has shown lower levels of insulin resistance in those that also have subcutaneous obesity, suggesting perhaps some beneficial effect of subcutaneous fat in addition to the adverse effects of visceral fat (Misra et al., 1997; Snijder et al., 2003). This notion is also consistent with the effect of thiazolidinedione treatment which improves insulin sensitivity despite increasing total body fat mass by primarily increasing the subcutaneous fat depot (Miyazaki et al., 2002). Third, when obese ob/ob mice are engineered to overexpress adiponectin in adipose tissue, there is a massive further increase in subcutaneous fat, and this is associated with improved insulin sensitivity with decreased glucose and insulin levels, increased lipid clearance, improved diacylglycerol levels and histology of the liver and fully functional healthy β-cells (Kim et al., 2007). Likewise, increasing subcutaneous fat by transplanting visceral fat to the subcutaneous area of lipoatrophic mice improves their metabolic profile rather than making it worse (Gavrilova et al., 2000). Finally, studies of gene expression of subcutaneous and visceral fat in both rodents and humans show major differences in patterns of expression (Lefebvre et al., 1998; Tchkonia et al., 2007). Indeed, our own laboratory has shown that whole fat tissue, as well as adipocytes and preadipocytes from subcutaneous and intra-abdominal depots in both rodents and humans demonstrate major differences in expression of developmental and patterning genes, suggesting that adipose tissue in these depots may even have differing developmental lineage (Gesta et al., 2006). These observations suggest that, while visceral fat may have adverse effects, subcutaneous fat may actually have some beneficial effects on metabolism. In addition to intrinsic properties, fat cells are also exposed to different extrinsic factors, such as the effects of hormones and growth factors acting in a paracrine fashion, neuronal innervations, interaction with other cells in the surrounding tissue, sites of vascular drainage, supply of various nutrients and levels of oxygenation. This raises the question: Are the metabolic effects associated with visceral fat versus subcutaneous fat due to anatomic location or cell-autonomous differences between these adipose depots?
In the current study, we have explored these questions by using a fat transplantation strategy in which we have transplanted either visceral (intra-abdominal) fat or subcutaneous fat from donor mice into either visceral or peripheral subcutaneous regions of recipient mice, and examined the effects of this addition to these fat depots on both whole-body and cellular metabolism. Somewhat surprisingly, we find that the major effects on metabolism and body weight are beneficial effects of added subcutaneous fat rather than a detrimental effect of added visceral fat, and that this effect is greatest when the subcutaneous fat is placed in an intra-abdominal site. This suggests that fat cells in different depots have intrinsically different properties and that these may be detrimental as well as beneficial.
RESULTS
Body Weight, Body Composition, and Energy Balance
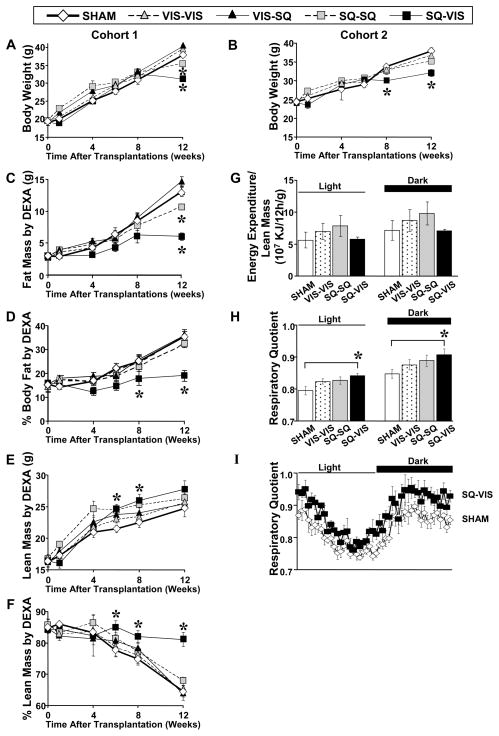
Fat transplantations were performed using subcutaneous (SQ) flank fat and visceral (VIS) epididymal fat of wild-type C57BL/6 donor mice (in cohort 1) or C57BL/6 donor mice transgenic for green fluorescent protein (GFP) expressed on the β-actin promoter (in cohort 2), and placing them into either the dorsal subcutaneous area or intra-abdominal area of 12-week-old wild-type C57BL/6 recipient mice (Fig. 1). During the 12 weeks after surgery, mice in the sham group had steady body weight gain in cohorts 1 and 2. In mice in which visceral fat was added to the visceral cavity (VIS-VIS group), total body weight gained at the end of the study was 108% of that in the sham group in cohort 1 and 88% of that in the sham group in 2 but was not significantly different from both sham groups (Fig. 2A,B). In the group with visceral fat transplanted to the SQ area (VIS-SQ group), body weight was not significantly different from the sham group and was not further studied. In contrast, when SQ fat was transplanted to the SQ area (SQ-SQ group), there was a significant slower increase in body weight, and by 12 weeks, this group had 85% and 84% of the weight gained by the sham group in cohorts 1 and 2 respectively. More striking was the effect of adding SQ fat to the VIS cavity (SQ-VIS group), which had the greatest impact on reducing the rate of weight gain. Thus, by the end of the 12-week study period, mice in the SQ-VIS group had gained on average 63% of the amount gained by the sham group in cohort 1 and 59% of the weight gained in the sham group in cohort 2 (p<0.05). Thus, increasing the amount of SQ fat by transplantation resulted in decreased subsequent weight gain, and this effect was greatest when the SQ fat was added to the VIS cavity. These differences in weight gain between the sham and transplantation groups were due primarily to differences in fat mass as measured by DEXA scan. Thus, total fat mass was similar among the sham, VIS-VIS, and VIS-SQ groups, was decreased by 18% in the SQ-SQ group (p<0.05), and reduced by about 54% in the SQ-VIS group (p<0.01) at the end of the study (Fig. 2C). Note that this pattern was similar to that of decreasing body weight in the same groups. Percent body fat, i.e. fat mass divided by total body weight, showed a similar decreasing pattern among the groups, with highest percent body fat in the sham, VIS-VIS, and VIS-SQ, and SQ-SQ groups, and a significant 46% reduction in the SQ-VIS cavity group (p<0.01) (Fig. 2D). Absolute lean body mass was significantly higher in the SQ-VIS group than the sham group at 6 and 8 weeks and tended to be higher at 12 weeks (Fig. 2E), but in relation to body weight, percent lean mass was highest in the SQ-VIS group in comparison to the sham group (Fig. 2F). The difference in body weight gain was not due to differences in food intake. Food intake measured at 10 weeks after transplantation by the CLAMS technique was not significantly different among groups (3.9±0.3, 3.9±0.1, 4.3±0.2, 4.2±0.2 g/day for the sham, VIS-VIS, SQ-SQ, and SQ-VIS groups respectively in cohort 2). The difference in body weight was also not due to levels of energy expenditure because these levels were not significantly different among the sham and all transplantation groups during the light and dark cycles (Fig. 2G). Simple regression analyses indicate that neither lean mass nor fat mass correlated with total energy expenditure (r2=0.0002 and r2=0.003 respectively) (StatView, SAS Institute Inc., version 5.0.1). Thus, lean mass and fat mass did not have an independent impact on energy expenditure. Respiratory quotient (RQ) is a ratio between the carbon dioxide production and the oxygen consumption, which is an indicator of the relative level of carbohydrate and fat oxidation in the whole body. RQ was significantly higher in the SQ-to-VIS group than in the sham group by 5.9% during the light cycle (p<0.05), by 7.0% during the dark cycle (p<0.05)(Fig. 2H), and almost all 30 minute-intervals during the 24-hour measurement (Fig. 2I), indicating a higher proportion of carbohydrate to fat metabolism in the SQ- to-VIS group. Otherwise, activity level, heat production, and water intake as measured by the CLAMS method were not significantly different among the sham and all the transplanted fat groups (data not shown). Overall, the SQ-VIS group had significantly improved metabolism in terms of decreased body weight, percent body fat, and increased percent lean mass, and this was associated with an increased proportion of carbohydrate to fat metabolism without significant changes in total energy expenditure or heat production.
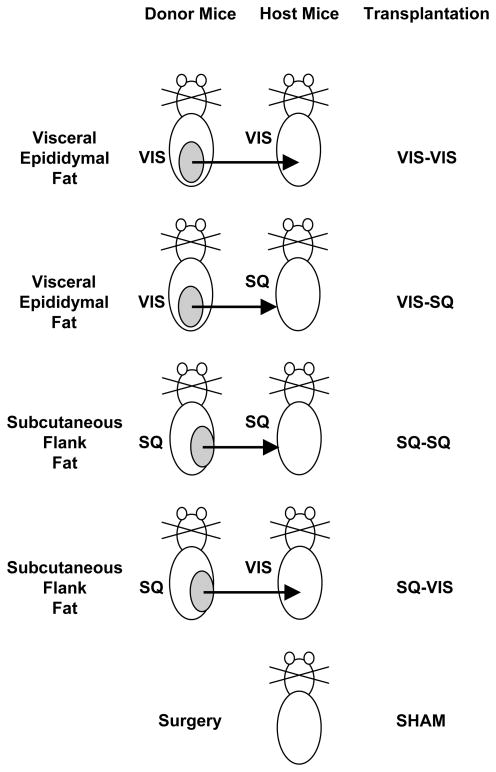
Figure 1.
Schematic of Fat Transplantation Groups. Visceral (VIS; epididymal) fat or subcutaneous (SQ; flank) fat from donor mice expressing whole-body GFP was transplanted into the visceral or subcutaneous area of wild-type C57BL/6 host mice. The sham group had surgery in the VIS or SQ area, but no fat was transplanted.
Figure 2.
Body Weight, Body Composition, Energy Expenditure and Respiratory Quotient After Fat Transplantation. (A, B) In cohorts 1 and 2, body weight gained was similar or higher after VIS fat transplantations, but lower after SQ-SQ fat transplantation and lowest in the SQ-VIS group in comparison to the sham group. All data are presented as mean ± SEM. (C) Fat mass was similar between the sham and VIS transplantation groups, decreased in the SQ-SQ group, and most reduced in the SQ-VIS group. (D) Percent body fat was significantly lower only in the SQ-VIS group as compared to the sham group. (E) Lean mass and (F) percent lean mass were significantly higher in the SQ-VIS in comparison to the sham group. (G) Total energy expenditure (TEE) (not shown) and TEE divided by lean mass were not significantly different among all sham and transplantation groups during the light and dark cycles. (H) Respiratory Quotient (RQ) was significantly higher in the SQ-VIS group than in the sham group during the light and dark cycles and (I) almost all 30 minute-intervals during the 24-hour measurement, thereby indicating a higher proportion of carbohydrate to fat oxidation in the SQ-VIS group.
Plasma Levels, Glucose and Insulin Tolerance Tests (GTT, ITT)
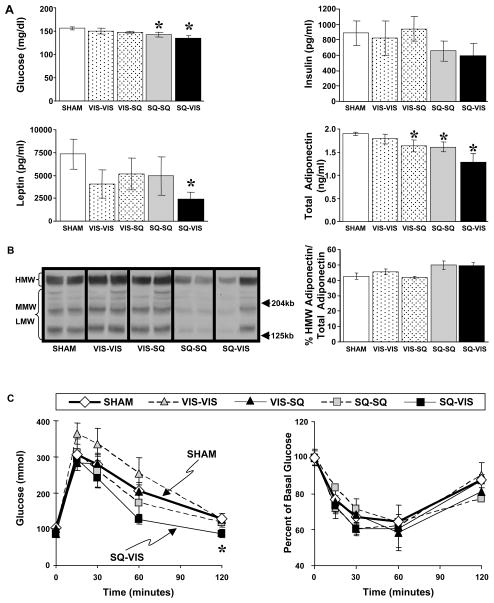
At nine weeks after transplantation, the levels of plasma glucose, insulin, leptin and adiponectin were assessed, and these showed a similar pattern to that of body weight and fat mass. Basal plasma glucose levels were similar among the sham, VIS-VIS, and VIS-SQ groups at 145–150 mg/dl, were slightly but significantly decreased (~6%) in the SQ-SQ group (p<0.05), and was even more significantly decreased in these lean animals by 15% when SQ fat was transplanted to the VIS cavity (p<0.01)(Fig. 3A). Plasma insulin levels paralleled the glucose levels and were similar among the sham, VIS-VIS, and VIS-SQ groups (about 850 pg/ml), decreased by 26% in the SQ-SQ group and decreased by 33% in the SQ-VIS group when compared to the sham group, although these differences did not quite reach statistical significance. Thus, adding SQ fat to a normal mouse resulted in decreased glucose levels with normal or decreased insulin levels suggesting improved insulin sensitivity. These metabolic improvements were greatest when the SQ fat was transplanted to the VIS cavity and were specific to the addition of SQ fat. Adding VIS fat to the VIS cavity had no effect on body weight gain, body composition, glucose or insulin levels. Plasma leptin levels were not significantly different among the sham, VIS-VIS, VIS-SQ and SQ-SQ groups, but were significantly decreased by 70% in the SQ-VIS in comparison to that of the sham controls (p<0.05). The low leptin levels in the SQ-VIS group correlate with the lower fat mass in this group. Total plasma adiponectin levels were similar between the sham and VIS-VIS groups, significantly decreased by 12% in the VIS-SQ and SQ-SQ groups (p<0.05) and decreased by 25% in the SQ-VIS group (p<0.01) in comparison to the sham group. Higher levels of high molecular weight (HMW) adiponectin to total adiponectin ratio, but not absolute total adiponectin levels, correlate better with improved insulin sensitivity (Pajvani et al., 2004). However, percent HMW adiponectin to total adiponectin was not significantly different among the sham and all transplantation groups (Fig. 3B). Since adiponectin levels in the SQ-VIS group were either decreased or similar to that of the sham group in the SQ-VIS group, it is unlikely that adiponectin can account for the decreased body weight or fat mass in this group.
Figure 3.
Basal Plasma Levels of Hormones and Substrates and Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT) After Fat Transplantations in Cohort 1. (A) Basal plasma glucose and insulin levels were not significantly different among the VIS transplantation groups and sham groups, were lower in the SQ-SQ group, and lowest in the SQ-VIS group. Plasma leptin and total adiponectin levels also had a decreasing pattern with levels highest in the sham group and significantly lowest in the SQ-VIS group. (B) Plasma samples were electrophoresed through nonreducing SDS gels, transferred to membranes, and probed with rabbit anti-mouse adiponectin antibody. Percent of high molecular weight (HMW) adiponectin to total (i.e. HMW + medium molecular weight (MMW) + low molecular weight (LMW)) adiponectin was not significantly different among all groups. (C) After a two hour fast for the GTT, glucose was given i.p. Glucose levels at 120 min were significantly lower only in the SQ-VIS group as compared to sham group. After an overnight fast for the ITT, insulin was injected i.p. No significant difference in blood glucose levels was observed among all groups.
At 10 weeks after transplantation, intraperitoneal glucose tolerance tests were performed in the mice. Most notable was the SQ-VIS group which had the lowest glucose levels in comparison to levels in the sham group, and this reached statistically significance at 120 minutes after the glucose load (p<0.05) (Fig. 3C). In the remaining groups, blood glucose levels during the 120-minute period tended to be highest in the VIS-VIS group, similar to the sham group for the VIS-SQ group, and lower in the SQ-SQ group, although these differences did not reach statistical significance. Thus, adding SQ fat to the VIS cavity significantly improved insulin glucose tolerance, whereas adding VIS fat to the VIS cavity, if anything, tended to cause a deterioration of glucose tolerance. Intraperitoneal insulin tolerance tests performed at 11 weeks after transplantation showed no significant difference among the groups, although at 120 min, the SQ-SQ groups tended to have lower glucose levels than the other groups.
Insulin Sensitivity by Hyperinsulinemic-Euglycemic Clamp
To more precisely and directly assess insulin sensitivity, we utilized the hyperinsulinemic-euglycemic clamp coupled with D-[3-3H]-glucose and 14C-deoxyglucose infusions. This allowed assessment of three different parameters of glucose metabolism: 1) whole body insulin sensitivity; 2) glucose uptake into muscle and endogenous and transplanted fat; and 3) effects of insulin on hepatic glucose output. These hyperinsulinemic-euglycemic clamps were performed at 12 weeks after transplantations in cohort 2.
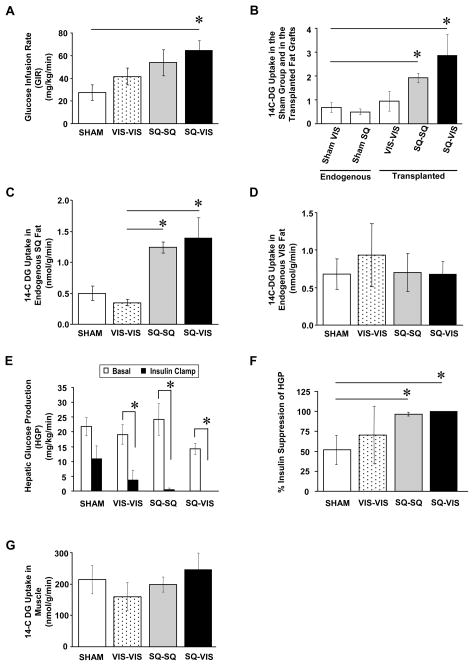
Direct measurement of whole-body insulin sensitivity was quantified during the hyperinsulinemic-euglycemic clamp as the amount of exogenous glucose infusion required to maintain blood glucose levels at initial fasting levels during the hyperinsulinemic infusion and expressed as glucose infusion rate (GIR). GIR was not significantly different between the sham and VIS-VIS groups (Fig. 4A). However, the GIR was significantly increased by 2.0-fold when SQ fat was transplanted to the SQ area and had an even greater increase to 2.4-fold when SQ fat was transplanted to the VIS area in comparison to the GIR in the sham group (p<0.05). Hence, increasing SQ fat in the SQ depot and, more strikingly, increasing SQ fat in the VIS depot improved whole-body insulin sensitivity as measured by glucose infusion rate in response to a stable insulin infusion.
Figure 4.
Direct Measures of Insulin Sensitivity by Hyperinsulinemic- Euglycemic Clamp After Fat Transplantations in Cohort 2. (A) Whole-body insulin sensitivity (quantified by glucose infusion rate; GIR) was not significantly different among the sham, VIS-VIS, and SQ-SQ groups, but was significantly higher in the SQ-VIS group. (B) Insulin-stimulated 14C-deoxyglucose (14C-DG) uptake was similar between the endogenous VIS fat and the endogenous SQ fat in the sham group. 14C-DG uptake in the fat grafts was not significantly different in all three transplantation groups, and had at least the same level of 14C-DG uptake as the endogenous fat. (C) 14C-DG uptake into endogenous SQ fat of the host mice was greater in the SQ-SQ and SQ-VIS groups in comparison to the sham and VIS-VIS groups. (D) 14C-DG uptake into endogenous VIS fat of the sham and all transplantation groups was not significantly different. (E) Hepatic glucose production (HGP) at basal time point was not significantly different among all groups, with a trend for lowest levels in the SQ-VIS group in comparison to the sham group. As expected during the clamp, insulin decreased HGP in the sham and VIS-VIS groups in comparison to their corresponding basal HGP levels, and more importantly, further decreased HGP in the SQ-SQ and SQ-VIS groups. (F) The greatest percent suppression of HGP during the hyperinsulinemic clamp with respect to the basal level was in the SQ-SQ and SQ-VIS groups. (G) Glucose uptake in muscle was not significantly different among all groups.
Insulin-stimulated glucose uptake into transplanted fat depots and endogenous fat and muscle was assessed during the final 45 minutes of the hyperinsulinemic-euglycemic clamp using 14C-deoxyglucose. In the sham-operated group, 14C-deoxyglucose uptake was similar in endogenous SQ fat and VIS fat (Fig. 4B), i.e., there was no intrinsic difference in glucose uptake between the SQ and VIS fat depots in the control animals of this study. All fat grafts in the three transplantation groups had at least the same level of glucose uptake as the endogenous SQ and VIS fat in the sham group (Fig. 4B). In the transplanted fat grafts themselves, 14C-deoxyglucose uptake appeared to be lowest in the VIS-VIS group and tended to be higher in the SQ-SQ and SQ-VIS groups, although these changes were not statistically significant. In fact, the glucose uptake in the fat grafts averaged 1.4- to 5.7-fold higher than that of the fat in the sham group, further indicating good vascularization and function of the transplanted fat grafts (Fig. 4B), and thereby confirming the success of the fat transplantation. Most interestingly, 14C-deoxyglucose uptake into endogenous SQ fat of the recipient host mice was significantly increased by about 2.5- to 2.8-fold in the groups with SQ fat transplanted into either the SQ or VIS depots (SQ-SQ and SQ-VIS groups) in comparison to the sham group (p<0.05), whereas endogenous SQ fat in the group with VIS fat transplanted into the VIS cavity had similar levels of glucose uptake as the fat in the sham group (Fig 4C). These results suggest cross-talk between SQ fat grafts and endogenous SQ fat, regardless of whether the SQ fat graft is transplanted to the SQ area or VIS cavity. By contrast, 14C-deoxyglucose uptake into endogenous VIS fat was not different among the sham and three transplantation groups (Fig. 4D).
Hepatic glucose production (HGP) was assessed in both the basal state and during the insulin clamp (Fig 4E). Basal HGP was not significantly different between all groups, although it tended to be decreased in the SQ-VIS group in comparison to the sham group. Insulin infusion during the clamps decreased HGP in the sham and VIS-VIS group as expected, but more interestingly, the decrease was greatest in the SQ-SQ and SQ-VIS groups. In the sham and VIS-VIS groups the insulin suppression of HGP was 52±18% and 71±36%, respectively, as compared to their own corresponding basal HGP levels (Fig. 4F). Consistent with the improved insulin sensitivity, insulin suppression of HGP was greatest in the groups with SQ fat transplantation with 97±2% suppression in the SQ-SQ group and 100±0% suppression in the SQ-VIS group. 14C-deoxyglucose uptake in muscle was not significantly different among all groups (Fig 4G). Thus, the addition of SQ fat to a normal mouse via transplantation enhanced insulin sensitivity in the liver, as well as on endogenous SQ fat. In all cases, the effect was observed when SQ fat was added to the SQ area but was most pronounced when SQ fat was added to the VIS cavity. By contrast, addition of VIS fat to the VIS cavity did not improve nor worsen insulin sensitivity.
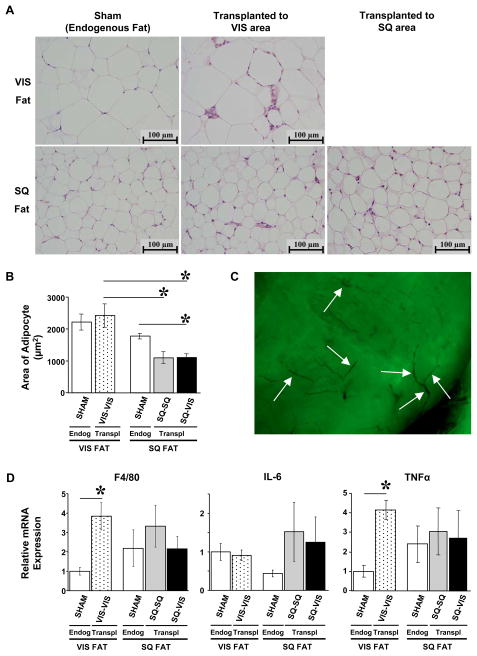
Histology and Markers of Inflammation
At 12 weeks after fat transplantation, the recipient mice were sacrificed, the endogenous fat and exogenous fat grafts, as identified by presence of GFP, were removed for histological examination. In cohort 2, hematoxylin and eosin staining of endogenous visceral/epididymal and subcutaneous/flank fat pads of the recipient mice showed normal appearing signet rings of fat cells, with occasional interspersed macrophages and vascular cells. The transplanted fat allografts had normal histology in comparison to endogenous SQ and VIS fat in the sham-operated group (Fig. 5A). The mean area of the adipocytes in the visceral depot appeared to be larger than that of the subcutaneous depot in the sham group by 20%, but this was not statistically significant (i.e. first and third bars in Fig. 5B). Adding visceral fat to the visceral cavity did not significantly change the mean area of the transplanted adipocytes in comparison to that of the endogenous visceral fat in the sham group (i.e. first two bars in Fig. 5B). Adding subcutaneous fat to the subcutaneous area tended to decrease average adipocyte size in comparison to the endogenous SQ fat in the sham group, but this also did not reach statistical significance. Remarkably, transplantation of subcutaneous fat to the visceral cavity (SQ-VIS group) significantly decreased average adipocyte area by 38% when compared to cells in the endogenous subcutaneous fat depot, and more importantly, did not increase in size to that of the surrounding endogenous VIS fat. Vascularization in the transplanted GFP fat grafts was observed in whole-mounts of fat, thereby further confirming the viability of the fat grafts (Fig. 5C). Levels of macrophages and inflammation were quantitated by mRNA levels of macrophage cell surface marker F4/80 and the cytokines IL-6 and TNF-α using quantitative real-time qRT-PCR. While all of the transplanted fat had minimally increased levels of mRNA levels for F4/80, IL-6 and TNF-α, this was significant only in the visceral fat transplanted into the visceral depot (Fig. 5D). Thus, as expected, the syngenic transplant of fat resulted in very little, if any, rejection or inflammatory reaction.
Figure 5.
Histology and Inflammatory Markers in Transplanted and Endogenous Fat in Cohort 2. (A) H&E staining of VIS and SQ fat pads were performed in all groups of cohort 2. Transplanted VIS and SQ fat pads had normal histology in comparison to endogenous VIS and SQ fat pads in the sham group as shown at 400X magnification. (B) Area of adipocytes was measured on the H&E slides with ImageJ software. Transplanting SQ fat to the visceral cavity (SQ-VIS group) significantly decreased the mean area of the adipocytes in comparison to that of the endogenous SQ fat in the sham group. (C) Whole-mount of transplanted fat graft expressing GFP was viewed under UV light. Arrows indicate sites of vascularization in the fat graft. (D) Presence of macrophages and inflammatory markers was assessed by quantifying mRNA expression levels of macrophage cell surface receptors F4/80 and inflammatory cytokines interleukin-6 and TNF-α by real-time qRT-PCR. Levels in the VIS-VIS fat grafts were significantly higher than that of the endogenous VIS fat in the sham group. There were no significant differences in macrophages, IL-6, or TNF-α among the SQ-SQ and SQ-VIS fat grafts and the endogenous fat pads.
Gene Expression
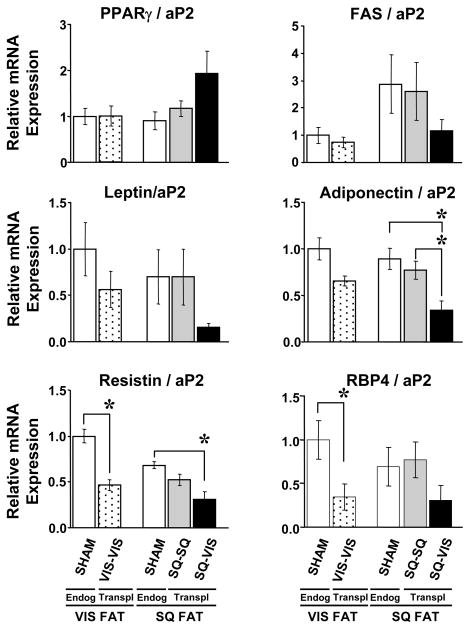
To further assess the status of the adipose tissue graft and explore the potential mechanisms by which the adipose graft might affect whole-body metabolism, the expression of several fat-related molecules, specifically PPARγ, FAS, leptin, adiponectin, resistin, and retinol-binding protein (RBP4), was analyzed. Since it was impossible to isolate enough fat cells from the transplanted fat in each mouse for this analysis, the entire transplant or endogenous fat pad was used, and the data were expressed both in terms of absolute mRNA levels and mRNA levels relative to aP2 levels, as a marker for amount of differentiated fat cell present in the tissue. The patterns for absolute gene expression levels and gene expression levels relative to aP2 levels were similar.
This analysis revealed that adding more VIS fat to the VIS area (VIS-VIS group) did not significantly change gene expression levels of PPARγ, FAS, leptin, or adiponectin in the fat graft as compared to levels in the endogenous VIS fat in the sham group, with the exceptions of resistin and RBP4 levels which were 52% and 65% lower, respectively, in the fat grafts than that in the endogenous VIS fat in the sham group (p<0.01) (i.e. comparison of the first and second columns in graphs in Fig. 6). Also, adding more SQ fat to the SQ area (SQ-SQ group) did not significantly change gene expression levels of the six adipocyte marker genes when compared to endogenous SQ fat in the sham group (i.e. comparison of the third and fourth columns in each graph in Fig. 6). Thus, adding more fat of the same depot did not change relative gene expression of PPARγ, FAS, leptin, and adiponectin with either the SQ and VIS fat grafts, but did have an effect to lower resistin and RBP4 levels when VIS fat was transplanted to the VIS area but not when SQ fat was transplanted to the SQ area. When SQ fat was transplanted to the VIS area (SQ-VIS group), gene expression levels for PPARγ, FAS, leptin, and RBP4 were also not significantly different from any other group, although the gene expression levels for the fat graft in the SQ-VIS group showed considerable variability. Interestingly, adiponectin and resistin levels in the SQ fat graft that was transplanted to the VIS cavity (SQ-VIS group) were significantly decreased by 2.6- and 2.2-fold, respectively, when compared to the endogenous SQ fat in the sham group (p<0.01 and p<0.05). These low gene expression levels for leptin and adiponectin are consistent with the lower levels of leptin and adiponectin in the plasma of the SQ-VIS group.
Figure 6.
Gene Expression in Endogenous and Transplanted fat was measured by real-time qRT-PCR. Analyses of fat-related molecules, such as PPARγ, FAS, leptin, adiponectin, resistin and RBP4, showed that: 1) all transplanted fat grafts (in the VIS-VIS, SQ-SQ, and SQ-VIS transplantation groups) express PPARγ and FAS mRNA levels that are not significantly different from that of endogenous VIS or endogenous SQ fats in the sham group; 2) adding more fat of the same depot (VIS-VIS or SQ-SQ) does not change gene expression levels of the first four fat genes analyzed except for resistin and RBP4 in the VIS-VIS group; and 3) adding SQ fat to the VIS cavity lowers mRNA levels of leptin, adiponectin, resistin and RBP4 in comparison to levels in the endogenous VIS and SQ fats in the sham group.
Overall, these analyses of gene expression levels showed that: 1) the transplanted fat grafts in the VIS-VIS, SQ-SQ, and SQ-VIS transplantation groups expressed PPARγ and FAS mRNA levels that were not significantly different from those of endogenous VIS or endogenous SQ fats in the sham group; 2) adding more fat of the same depot (VIS-VIS or SQ-SQ) did not change gene expression levels of PPARγ, FAS, leptin, and adiponectin, except for resistin and RBP4 when VIS fat was added to the VIS cavity; and 3) adding SQ fat to the VIS cavity lowered mRNA levels of leptin, adiponectin and resistin, but not PPARγ, FAS or RBP4, in comparison to levels in the endogenous SQ fat.
DISCUSSION
The differential physiological effects and risk of metabolic disease of visceral (central) versus subcutaneous (peripheral) obesity have been documented in multiple epidemiological and physiological studies (Carey et al., 1997; Wang et al., 2005; Nicklas et al., 2006; Ross et al, 2007; Misra et al, 1997; Snijder et al, 2003; Tanko et al., 2003). We have further investigated mechanisms by which subcutaneous and visceral fat cause contrasting metabolic profiles by performing a novel technique of transplanting fat from both SQ and VIS depots to both SQ and VIS areas. The fat transplantations were successful as confirmed by normal histology of fat and no significant differences in number of macrophages or levels of inflammatory molecules IL-6 and TNF-α, except for the group with visceral fat added to the visceral cavity. Furthermore, the fat grafts had a high uptake of 14C-deoxyglucose indicating re-established vascularization as well as no significant changes in mRNA levels of PPARγ and fatty acid synthase in comparison to those of the endogenous SQ and VIS fat in the sham-operated mice.
Remarkably, we find that when SQ fat is transplanted into the VIS cavity in mice (SQ-VIS group), there is decreased body weight, total fat mass accumulation and adipocyte cell size, despite no change in food intake, total energy expenditure, activity level, or heat production. However, small effects on body weight, fat and lean mass in the SQ-VIS group, which took from 8 to 12 weeks to become observable, may have made the mechanisms difficult to detect by the CLAMS technique. The SQ to VIS transplant group also showed improved glucose homeostasis, including decreased plasma glucose and insulin levels, and improved glucose tolerance when compared to sham-operated mice. The improved metabolic effects in the SQ-VIS group were confirmed by hyperinsulinemic-euglycemic clamp, which revealed increased insulin sensitivity as measured by three parameters, namely increased whole-body glucose uptake as measured by glucose infusion rate, increased glucose uptake in endogenous SQ fat, and increased insulin suppression of hepatic glucose production. These metabolic effects were specific to transplantation of SQ fat and were greatest when SQ fat was transplanted into the visceral cavity, but were not observed following transplantation of VIS fat to the VIS cavity. Thus, there are specific beneficial metabolic effects of transplanting SQ fat to the VIS area.
Successful transplantations of adipocytes and whole fat pads to the SQ area in mice have previously been reported (Green and Kehinde, 1979; Gavrilova et al., 2000). However, transplantations of mature adipocytes into the VIS cavity has been less successful (Rieck and Schlaak, 2003). Recently, Konrad et al have successfully transplanted the whole visceral fat pad to the visceral cavity by stitching epididymal fat to the side of the peritoneal cavity (Konrad et al., 2007). However, in contrast to our results and those of epidemiological studies, in their hands, this appeared to improve insulin sensitivity. Visceral fat in humans drains portally, but the intra-abdominal fat grafts in Konrad’s study likely drained into the adjacent systemic circulation. In our study, when visceral fat was added to the visceral cavity (VIS-VIS group), there were multiple sites of engraftment, in which the graft was mixed with epididymal fat, next to mesenteric fat and under the liver. Thus, in our case, there was likely both portal and systemic drainage. Further studies are needed to determine whether the amount of visceral fat or the balance between portal and systemic drainage is more important for insulin sensitivity. A recent online letter publication by Hocking et al confirmed some of our results in which transplantation of SQ fat into the intra-abdominal space by suturing to the visceral side of the peritoneum had a protective effect on adiposity and glucose tolerance by ipGTT.
The beneficial metabolic effects of SQ fat transplantation into the VIS depot versus VIS fat transplantation into the VIS depot indicates that SQ fat tissue must have some cell-autonomous properties that can act on other tissues to improve insulin sensitivity and metabolic state. This effect was observed regardless of whether SQ fat was transplanted to the SQ area or placed in the VIS cavity, but was greatest in the intra-abdominal transplant group. These findings are consistent with other studies, which have shown persistent differences in gene expression profiles, degree of cell proliferation, capacity to differentiate, and lipid content during in vitro culture of SQ versus VIS fat cells obtained from humans and mice, and thereby indicating cell-autonomous properties of SQ and VIS fat cells (Tchkonia et al., 2007; Gesta et al., 2006). Exactly what these cell-autonomous properties of SQ fat are will require further study. Furthermore, evidence for cross-talk between various organs and adipose tissue has been exemplified by several studies. For example, in mice in which there is a muscle-specific inactivation of the insulin receptor, there is an increase in glucose uptake in fat and an increase in fat pad size (Bruning et al., 1998). Conversely, specific impairment of GLUT4 in adipose tissue results in decreased glucose uptake into muscle, possibly as a result of altered release of specific molecules from fat (Abel et al., 2001). Thus, our findings of cross-talk specifically between SQ fat graft and the liver and other adipose tissue depots are novel, and its possible mechanisms need to be elucidated.
What is clear is that these effects cannot be explained by differences in inflammatory cytokines or the best known adipokines. Inflammatory cells and molecules, such as macrophages, TNF-α and IL6, have been observed at high levels in obese subjects and have been associated with insulin resistance and development of type 2 diabetes (Weisberg et al., 2003). However, in our fat transplantation model, levels of macrophages, IL-6, and TNF-α in the transplanted SQ fat grafts were not significantly different from that of endogenous fats in the sham group. Whether other inflammatory pathways and molecules play an important role in the improved metabolic effect in this model has not been ruled out yet. Second, adipokines, such as high levels of leptin and adiponectin or low levels of RBP4, can favorably affect food intake, insulin sensitivity, glucose and lipid metabolisms and inflammation. However, our results indicate that both plasma levels and gene expression in endogenous and transplanted fat of leptin, total adiponectin and percent HMW adiponectin were lower or not changed in the SQ-VIS transplantation group, which had improved metabolic effects when compared to that of the sham group. Thus, although these adipokines may be important in models of obesity, they are not likely candidates to explain the improved metabolic effects in our transplantation model. Interestingly, gene expression levels of the adipokines resistin and RBP4 were lower in the fat graft of the SQ-VIS transplantation group, which is in agreement with studies that report an inverse relationship between resistin or RBP4 and insulin sensitivity. However, the mechanism by which low levels of resistin levels could lead to improved metabolic effects requires further investigation. Gene expression levels of RBP4 in the fat graft of the SQ-VIS group was not significantly different from that of the sham group but appeared to be lower and could potentially play a role in the increased insulin sensitivity. Overall, mechanisms to explain the beneficial metabolic effects of transplanting SQ fat to the VIS cavity do not seem to involve inflammatory molecules such as macrophages, IL-6 and TNF-α, or known adipokines such as leptin and adiponectin, nevertheless, resistin, RBP4 and other still unrecognized adipokines are possible routes of further exploration..
In conclusion, using an in vivo fat transplantation strategy, we have demonstrated that SQ adipose tissue can have direct and beneficial effects on control of body weight and metabolism. These effects appear to be due to cell-autonomous property of the SQ fat, most likely secretion of some factor(s) that can mediate improvements in the metabolic profile. Our results showed that SQ fat can cross-talk with the other fat tissues and liver to bring about improved metabolic effects. The degree to which SQ fat ameliorates metabolic improvement is greatest when SQ fat was placed in the visceral cavity, suggesting that it is important for secreted factors or other factors associated with the SQ fat to be close to or at high enough concentration next to the VIS fat or liver in order to mediate the greatest metabolic effects. Identification and investigation of these secreted factors or other factors associated specifically with SQ fat may provide new targets for the treatment and/or prevention of obesity-related complications and diseases such as insulin resistance, metabolic syndrome, cardiovascular disease and diabetes.
EXPERIMENTAL PROCEDURES
Mice and Fat Transplantation
12-week old male C57BL/6 mice were used as recipients. In cohort 1, 10-week old male mice of the same strain were used as donors. In cohort 2, C57BL/6 mice carrying the green fluorescent protein (GFP) as a transgene on the β-actin promoter (ACTB) were used as donors (stock # 003291, Jackson Labs, Bar Harbor, ME). Since this transgene was expressed in all tissues of the donor mice, it was possible to identify the transplanted fat in the recipient mice using U.V. illumination. All animals were kept on a standard mouse diet (9F 5020 Lab Diet, PharmaServ, Inc., Framingham, MA) and a standard 12-hour light-dark cycle. The Joslin Diabetes Center Institutional Animal Care and Use Committee approved all experiments.
Fat transplantation was performed using fat pads that were removed from either subcutaneous flank area or the intra-abdominal perigonadal (epididymal) area. After cervical dislocation of donor mice, the fat pads were removed, cut into approximately 200 mg slices, and kept in saline in 50 ml tubes placed in 37°C water bath until transplantation. The recipient mice were anesthetized by intraperitoneal injection with 0.015 ml/kg of a 2.4% solution of 1:1 mixture of 2,2,2-tribromoethanol and t-amyl alcohol. For each recipient mouse, a total of 1.0 g of the resulting slices of fat were transplanted into the visceral (VIS) area, i.e., the maximum amount of fat graft that could fit in the intra-abdominal cavity, was carefully lodged deep between folds within sliced portions of endogenous epididymal fat of the recipient mice and lodged next to the mesenteric fat just below the liver, or subcutaneously (SQ), i.e. below the skin on the back of host mice. Five groups of mice were studied in which VIS epididymal fat was transplanted into the VIS cavity (VIS-VIS group) or SQ area (VIS-SQ group), SQ flank fat was placed in the SQ area (SQ-SQ group) or VIS cavity (SQ-VIS group), or mice underwent surgery, but no fat was added (sham group)(Fig. 1). Cohort 1 of mice was used to measure body weight, body composition, basal plasma levels, GTT and ITT and the sample size was 8, 5, 7, 7, and 8 mice in the respective groups above. Cohort 2 (without the VIS-SQ group) was used for the CLAMS technique, hyperinsulinemic-euglycemic clamp studies, histological analyses, and gene expression data and the sample size was 7, 3, 5 and 5 mice respectively.
Biochemical and Physiological Methods
In cohort 2, histological hematoxylin and eosin (H&E) stained sections of fat were made after fixation in 10% buffered phosphate formalin. To measure the size of the adipocytes, at least 300 cells per H&E-stained sample were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). To assess presence of macrophages and inflammatory markers, mRNA expression of F4/80 cell surface receptors of macrophages, interleukin 6 (IL-6) and tumor necrosis factor (TNF)-α, were measured by real-time qRT-PCR as described (Gesta et al., 2006). Gene expression levels of several fat-related genes, specifically aP2, peroxisome proliferator-activated receptor γ (PPARγ), fatty acid synthase (FAS), leptin, adiponectin, resistin, and retinol-binding protein (RBP)-4 in the endogenous and transplanted fat grafts were also measured by real-time qRT-PCR.
To assess metabolic effects of fat transplantation, body weight and body composition were measured. Fat and lean mass were measured by DEXA (dual energy X-ray absorptiometry) scanning. The Comprehensive Lab Animal Monitoring System (CLAMS) method was used to measure activity level, food and water intakes, volume of O2 consumption, volume of CO2 production, and heat production at 10 weeks after fat transplantation (Oxymax OPTO-M3 system; Columbus Instruments, Columbus, OH). Total energy expenditure for mice was calculated as previously described (Albarado et al., 2004). Plasma levels of hormones and metabolites were assessed by blood obtained by tail vein sampling after a two-hour fast from 9 to 11 AM. Basal glucose was measured with an Elite one-touch glucometer (Bayer, Mishawaka, IN). Plasma insulin, leptin and total adiponectin were measured with mouse ELISA kits (Crystal Chem Inc., Downers Grove, IL; Alpco Diagnostics Inc., Salem, NH). HMW adiponectin levels were measured as previously described (Coenen et al., 2007) with a 6% SDS nonreducing gel and rabbit anti-adiponectin antibody (BioVendor, Candler, NC) at a 1:2000 dilution, and secondary antibody at a 1:10,000 dilution. Glucose tolerance test (GTT) was performed after an overnight fast from 6 PM to 9 AM, and an intraperitoneal injection of 20% dextrose solution at a dose of 2.0g/kg body weight. An insulin tolerance test (ITT) was performed after a two-hour fast from 9 to 11 AM, and an intraperitoneal injection of 1.25 U of insulin (Novolin regular human insulin, Novo Nordisk, Princeton, NJ) per kg body weight. Insulin sensitivity was directly and more precisely assessed by performing hyperinsulinemic-euglycemic clamps to measure whole body insulin sensitivity and fat and muscle specific glucose uptake as described by Norris et al. (2003) except that a continuous insulin infusion dose of 5 mU/kg/min was used and blood glucose was maintained at 115 mg/dl during the clamp. Hepatic glucose production (HGP) was assessed by subtraction of the glucose infusion rate from whole-body glucose turnover as measured with D-[3-3H]-glucose (Finegood et al., 1988).
Acknowledgments
The authors thank Laureen Mazzola and Michael Rourk for excellent care of the animals and also thank the Joslin DERC Physiology and Microscopy Cores. The authors greatly appreciate Vlad Chupis’ work with the ImageJ software, and Kevin A. Gosselin’s advice with the cover image for Cell Metabolism. This work was supported by NIH grants DK33201 and DK31036.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am J Epidemiol. 1997 Apr 1;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–563. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- Ross R, Berentzen T, Bradshaw AJ, Janssen I, Kahn HS, Katzmarzyk PT, Kuk JL, Seidell JC, Snijder MB, Sorensen TI, Despres JP. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2007 doi: 10.1111/j.1467-789X.2007.00411.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Misra A, Garg A, Abate N, Peshock RM, Stray-Gundersen J, Grundy SM. Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res. 1997;5:93–99. doi: 10.1002/j.1550-8528.1997.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr. 2003;77:1192–1197. doi: 10.1093/ajcn/77.5.1192. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. Int J Obes Relat Metab Disord. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Langendonk JG, Kok P, Frolich M, Pijl H, Meinders AE. Decrease in visceral fat following diet-induced weight loss in upper body compared to lower body obese premenopausal women. Eur J Intern Med. 2006;17:465–469. doi: 10.1016/j.ejim.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Gan SK, Kriketos AD, Ellis BA, Thompson CH, Kraegen EW, Chisholm DJ. Changes in aerobic capacity and visceral fat but not myocyte lipid levels predict increased insulin action after exercise in overweight and obese men. Diabetes Care. 2003;26:1706–1713. doi: 10.2337/diacare.26.6.1706. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42:1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- Dubuc PU. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976;25:1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Barzilai N, Banerjee S, Hawkins M, Chang CJ, Chen W, Rossetti L. The effect of age-dependent increase in fat mass on peripheral insulin action is saturable. J Gerontol A Biol Sci Med Sci. 1998;53:B141–B146. doi: 10.1093/gerona/53a.2.b141. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, Scherer P, Rossetti L, Barzilai N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Liszka TG, Dellon AL, Im M, Angel MF, Plotnick L. Effect of lipectomy on growth and development of hyperinsulinemia and hyperlipidemia in the Zucker rat. Plast Reconstr Surg. 1998;102:1122–1127. doi: 10.1097/00006534-199809040-00031. [DOI] [PubMed] [Google Scholar]
- Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005;288:E454–E461. doi: 10.1152/ajpendo.00203.2004. [DOI] [PubMed] [Google Scholar]
- Capeau J, Magre J, Lascols O, Caron M, Bereziat V, Vigouroux C, Bastard JP. Diseases of adipose tissue: genetic and acquired lipodystrophies. Biochem Soc Trans. 2005;33:1073–1077. doi: 10.1042/BST0331073. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784–2791. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- Kim JY, vandeWall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durland JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Sherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre AM, Laville M, Vega N, Riou JP, Van Gaal L, Auwerx J, Vidal H. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes. 1998;47:98–103. doi: 10.2337/diab.47.1.98. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Lenburg M, Thomou T, Giorgadze N, Frampton G, Pirtskhalava T, Cartwright A, Cartwright M, Flanagan J, Karagiannides I, Gerry N, Forse RA, Tchoukalova Y, Jensen MD, Pothoulakis C, Kirkland JL. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- Green H, Kehinde O. Formation of normally differentiated subcutaneous fat pads by an established preadipose cell line. J Cell Physiol. 1979;101:169–171. doi: 10.1002/jcp.1041010119. [DOI] [PubMed] [Google Scholar]
- Rieck B, Schlaak S. In vivo tracking of rat preadipocytes after autologous transplantation. Ann Plast Surg. 2003;51:294–300. doi: 10.1097/01.SAP.0000063758.16488.A9. [DOI] [PubMed] [Google Scholar]
- Konrad D, Rudich A, Schoenle EJ. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia. 2007;50:833–839. doi: 10.1007/s00125-007-0596-1. [DOI] [PubMed] [Google Scholar]
- Hocking SL, Chisholm DJ, James DE. Studies of regional adipose transplantation reveal a unique and beneficial interaction between subcutaneous adipose tissue and the intra-abdoominal compartment. Diabetologia. 2008 doi: 10.1007/s00125-008-0969-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Michael MD, Winnay JN, Hayashi T, Horsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology. 2004;145:243–252. doi: 10.1210/en.2003-0452. [DOI] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M. Modelling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes. 1988;37:1025–1034. doi: 10.2337/diab.37.8.1025. [DOI] [PubMed] [Google Scholar]