Abstract
Cytokines are secreted from macrophages and other cells of the immune system in response to pathogens. Additionally, in autoinflammatory diseases cytokine secretion occurs in the absence of pathogenic stimuli. In the case of TRAPS [TNFR (tumour necrosis factor receptor)-associated periodic syndrome], inflammatory episodes result from mutations in the TNFRSF1A gene that encodes TNFR1. This work remains controversial, however, with at least three distinct separate mechanisms of receptor dysfunction having been proposed. Central to these hypotheses are the NF-κB (nuclear factor κB) and MAPK (mitogen-activated protein kinase) families of transcriptional activators that are able to up-regulate expression of a number of genes, including pro-inflammatory cytokines. The present review examines each proposed mechanism of TNFR1 dysfunction, and addresses how these processes might ultimately impact upon cytokine secretion and disease pathophysiology.
Keywords: mitogen-activated protein kinase (MAPK), nuclear factor κB (NF-κB), periodic syndrome, tumour necrosis factor (TNF), tumour necrosis factor receptor-associated periodic syndrome (TRAPS)
Abbreviations: ADAM, a disintegrin and metalloproteinase; AP, activator protein; APR, acute phase response; ATF6, activating transcription factor 6; CRD, cysteine-rich domain; CREBH, cAMP-responsive-element-binding protein H; CRP, C-reactive protein; ER, endoplasmic reticulum; IFN, interferon; IRF, IFN-regulatory factor; JNK, c-Jun N-terminal kinase; IL, interleukin; JAK/STAT, Janus kinase/signal transducer and activator of transcription; mAb, monoclonal antibody; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor κB; PBMC, peripheral blood mononuclear cell; ROS, reactive oxygen species; SAP, serum amyloid P; TNF, tumour necrosis factor; TACE, TNFα-converting enzyme; TNFR, TNF receptor; sTNFR1, soluble TNFR1; TRAPS, TNFR-associated periodic syndrome; UPR, unfolded protein response
INTRODUCTION
TRAPS {TNFR [TNF (tumour necrosis factor) receptor]-associated periodic syndrome} is an autosomal dominant autoinflammatory disease linked to chromosome 12p13 [1,2], and more specifically with mutations within the TNFRSF1A gene encoding TNFR1. Clinically, TRAPS is characterized by recurrent attacks of fever, abdominal pain, migratory rash, myalgia and periorbital oedema. Attacks are typically several days to several weeks in duration and often start in early childhood [3]. Critically, TRAPS patients are also susceptible to the development of potentially fatal secondary amyloidosis.
At present, 86 mutations (109 sequence variants) of the TNFRSF1A gene have been reported to lead to the development of TRAPS (INFEVERS TRAPS database http://fmf.igh.cnrs.fr/infevers). Of these, 78 are single nucleotide missense mutations occurring within exons 2, 3, 4 and 6; the exceptions are deletion (ΔD42) in exon 3, and a splicing mutation (c.472+1G→A) in intron 4. Thirty of the identified missense mutations affect extracellular cysteine residues (12 individual cysteine residues affected, some with multiple mutations per residue), with the majority of the mutations (91%) being located within CRDs (cysteine-rich domains) 1 and 2, with two mutations (C98Y and F112I) described in CRD3, none yet in CRD4, and I170N being the only mutation in close proximity to the transmembrane region (exon 6) that was described in a German family [4]. This novel mutation was, however, shown to cause defective receptor shedding, and is associated with reduced levels of sTNFR1 (soluble TNFR1).
Our understanding of TRAPS disease pathophysiology has been greatly aided by studies investigating intracellular transport of TNFR1. Although TNFR1 is found at the cell surface following pro-inflammatory stimuli, in the absence of any such stimulus it is instead primarily localized within Golgi storage pools [5–8]. A small fraction of TNFR1 is, however, normally trafficked to the cell surface. When circulating TNF levels become elevated, cell surface TNFR1 binds TNF, and the ligand–receptor complex subsequently triggers either cell survival/inflammation or apoptotic cell death pathways [9], with the cellular fate being determined through a complex balance of molecular switches and feedback mechanisms [10–14]. Crucially these cell signalling pathways are regulated by intracellular trafficking events, and subsequent TNFR1 compartmentalization [15,16]. Importantly, it is also becoming increasingly clear that a large number of different mutations in TNFRSF1A result in receptor mislocalization and/or ligand-independent activation. However, a consequence of most TRAPS mutations is the activation of the transcription factor, NF-κB (nuclear factor κB), although this is not always the case [17]. At least three distinct and separate mechanisms of receptor dysfunction have now been proposed (Figure 1). These hypotheses form the basis for this mini-review.
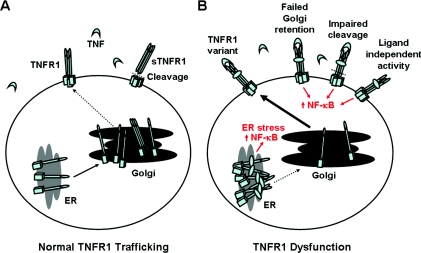
Figure 1. Modes of TNFR1 trafficking dysfunction associated with TRAPS.
(A) Normal transport of TNFR1 and (B) intracellular trafficking defects associated with TRAPS. TNFR1 is transported along the biosynthetic pathway through the ER to the Golgi storage pool, where the majority of the receptor is retained in cells of the immune system. TRAPS is thought to occur as a result of a number of possible mechanisms, namely defective TNFR1 oligomerization and ER misfolding-associated stress pathways, failed Golgi retention, impaired TNFR1 proteolytic cleavage and receptor shedding or ligand-independent NF-κB activation.
SHEDDING HYPOTHESIS
TNFR1 is a member of the wider TNF receptor superfamily of 30 receptors and 19 associated ligands [18,19]. Although under resting conditions the majority of TNFR1 is stored within Golgi storage pools [5–8], a fraction of the global intracellular pool of TNFR1 is instead transported to the cell surface where it is localized within cholesterol- and sphingolipid-rich low density membrane lipid raft microdomains [20]. Here it undergoes metalloprotease-mediated cleavage in the receptors extracellular domain by the transmembrane glycoprotein, ADAM17 (a disintegrin and metalloproteinase 17) [21], also known as TACE (TNFα-converting enzyme). This then releases sTNFR1 into the bloodstream where it binds circulating free TNF and attenuates inflammation [22,23].
TRAPS disease pathophysiology was initially thought to be the result of unopposed action of TNF due to the reduced levels of sTNFR1 which normally acts as a physiological buffer. It was reported that patients with the C33Y, T50M, C52F and C88R mutations demonstrated significantly lower levels of sTNFR1 between attacks and disproportionately low levels during attacks when compared with appropriate controls [24]. This led to the development of the ‘shedding hypothesis’, where there is little or no physiological cleavage of TNFR1 by metalloproteases. Generation of a knock-in mouse with a non-sheddable form of TNFR1 gave further support to this hypothesis, since these mice showed increased TNF signalling and increased susceptibility to and increased severity of induced arthritis [25].
The likely cause of defective shedding is altered conformation in the extracellular domain of TNFR1. This can occur as a consequence of cysteine missense mutations which result in unpaired cysteine residues within the extracellular CRDs of TNFR1. In addition there are a number of non-cysteine mutations that have also been found to cause shedding defects. Notably, T50M disrupts a highly conserved threonine residue that is associated with an intrachain hydrogen bond. Other non-cysteine mutations have also been predicted to disrupt the receptors secondary structure, thereby altering protein folding and/or TNF-binding characteristics. Alternatively, reduced sTNFR1 might instead be a consequence of a lower concentration of cell surface TNFR1. Were this to be the case, the reduced cell surface TNF-binding capacity might also result in a failure to activate ADAM17/TACE and to induce downstream signalling cascades.
It is important to note that defective TNFR1 shedding is not universal in TRAPS [3], and some patients display lower levels of sTNFR1 during attacks compared with disease-matched controls, but not necessarily lower levels at baseline. In addition, it is important to recognize that as well as ADAM17/TACE-mediated sheddase generation of sTNFR1, full-length sTNFR1 can also be constitutively released into the bloodstream via exosome-like vesicles, via a mechanism that is independent of receptor cleavage [26]. Therefore, while a shedding defect remains the most likely cause of inflammation for patients carrying a number of TRAPS mutations, other mutations have been found not to result in defective shedding, and must therefore have a different underlying disease mechanism. This has led to the development of additional hypotheses.
LIGAND-INDEPENDENT SIGNALLING HYPOTHESIS
The conformational changes induced by TNF when initiating TNFR1 signal transduction cascades have been the subject of much debate. It was originally proposed that TNF homotrimers induce signalling by aggregation of TNFRs, although subsequent models proposed a minimal unit of receptor activation involving preformed receptor dimers on the cell surface which are allosterically modified by ligand binding [27]. This led to the development of the PLAD (preligand assembly domain) hypothesis, whereby the extracellular domains of both TNFR1, as well as TNFR6 (also known as Fas), associate in the absence of their ligands, namely TNF or FasL respectively [28–30]. Ligand binding then stabilizes the interaction between the receptor subunits, allowing the ligand–receptor complex to trigger a cascade of intracellular processes with diverse, and sometimes apparently contradictory, effects.
TNF-independent NF-κB activation is becoming increasingly implicated in TRAPS. Yousaf et al. [30] used a doxycycline-inducible expression system to analyse the relationship between wild-type receptors and the T50K variant of TNFR1. Interestingly, the authors found that modest up-regulation of the mutant receptor down-regulated cell surface expression of wild-type TNFR1 and vice versa. However, when the balance was tipped towards the mutant TNFRSF1A an increase in p65 NF-κB subunit activity was documented that was independent of TNF stimulation. By contrast, when wild-type expression was elevated, there was no increase in p65 in the absence of TNF stimulation. Cell surface cross-linking experiments also showed that T50K receptors are capable of oligomerizing with both T50K and wild-type receptors independent of TNF stimulation [30]. This work is also consistent with the observation that the T50M variant results in cell-surface expression of mutant receptor with much reduced TNF-binding capacity relative to wild-type receptor [31]. Taken together these findings suggest that, at least in some TRAPS mutations, TNFR1 signalling from the cell surface could be associated with an indirect route for NF-κB activation that does not involve TNF stimulation.
In addition to activation of NF-κB, TNF-mediated inflammation/cell survival can also result from additional pathways. MAPK (mitogen-activated protein kinase) signal transduction pathways have been implicated in multiple physiological processes including growth, differentiation, survival and cell death [32–34]. In humans, three groups of MAPK have been identified: the ERK (extracellular signal-regulated protein kinases), the JNK (c-Jun N-terminal kinase) and the p38 MAPK [35–37]. The p38 MAPK pathway plays an important role in cellular response to inflammatory stimuli and environmental stresses, and has recently been linked to the pathophysiology of several diseases including rheumatoid arthritis [38] and TRAPS [39]. A recent study reported that heterozygous T50M and C33Y mutations in macrophage TNFR1 led to enhanced activation of JNK and p38 MAPK, but that heterozygous mutations, and even more so homozygous mutations, diminished TNF-induced IL-6 (interleukin 6) production relative to controls [39]. Furthermore, LPS (lipopolysaccharide)-induced MAPK activation was found to be independent of TNF, as blockade with TNFR2-Fc failed to inhibit activation. Together these findings led the authors to speculate that TRAPS abnormalities might therefore result from intracellular ligand-independent TNFR1 signalling. Alternatively TRAPS-driven MAPK activation might instead be induced indirectly through other mediators, including ROS (reactive oxygen species) [40], which can sustain MAPK signalling through inactivation of MAPK phosphatases [41]. Indeed, ROS were found to be elevated in immune cells isolated from TRAPS patients, with altered mitochondrial function being shown to be responsible for the enhanced oxidative capacity and pro-inflammatory cytokine production [40]. It should also be noted that in TRAPS mutations that generate inactive TNFR1, TNF would signal solely through TNFR2. Under these circumstances there would then be an inability of cells to induce apoptosis via death domain activation pathways, potentially explaining the apoptosis defect found in many cells from TRAPS patients.
INTRACELLULAR TNFR1 TRAFFICKING DEFECTS ASSOCIATED WITH TRAPS
Molecular modelling of TNFR1 mutants predicts that TRAPS-associated mutations in TNFR1 might profoundly disrupt receptor trafficking [42]. Consistent with this prediction, a number of TNFR1 mutations have been reported to result in intracellular retention of mutant receptors within the ER (endoplasmic reticulum) [43,44], most likely due to abnormal oligomerization of mutant receptors through non-physiological disulfide bonds. It was thought that the intracellular increase in misfolded mutant TNFR1 in the ER might induce inflammation through the UPR (unfolded protein response), which in certain instances can induce production of pro-inflammatory cytokines [45]. Simon et al. [39] recently challenged this hypothesis, as they observed no spontaneous increase in the expression of classical ER stress-inducible genes in mice possessing the C33Y or T50M mutation in TNFRSF1A. It should be noted, however, that this evidence is not conclusive, as alternative UPR pathways also exist that were not investigated. Interestingly, Zhang et al. [45] delineated a molecular mechanism for activation of an ER-localized transcription factor, CREBH (cAMP-responsive-element-binding protein H), and revealed an unprecedented link by which the ER can initiate acute inflammatory response. Importantly CREBH does not contribute to the classical UPR induction, but is instead required for APR (acute phase response), regulating transcription of the CRP (C-reactive protein) and SAP (serum amyloid P) genes, CREBH and ATF6 (activating transcription factor 6) binding to a conserved promoter element [45]. CREBH and ATF6 thereby interact and synergistically regulate transcription of target genes in hepatocytes following ER stress. Pro-inflammatory cytokines induce cleavage of CREBH and activate both the APR and UPR in the liver in vivo, raising an intriguing notion that many physiological and pathological processes that induce ER stress, such as gene mutations that disturb protein folding as hypothesized in TRAPS, might induce an inflammatory response through CREBH cleavage, activating transcription of the APR genes encoding SAP and CRP.
While ER misfolding of TNFR1 clearly induces an inflammatory response, a cautionary note must be sounded with the ER misfolding hypothesis however, as this model is largely reliant upon data initially generated in transfected cell lines and ‘knock-in’ mice, rather than physiologically relevant immune cells taken from actual TRAPS patients. As vector-driven high protein overexpression levels have sometimes been found to trigger ER overload, and thereby result in the artefactual activation of ER stress-signalling pathways, care must be taken when interpreting these data. Indeed, in contrast to other studies where cysteine and threonine mutations in TNFR1 have been linked to failure of mutant TNFRs to elicit intracellular apoptotic signalling cascades in isolated neutrophils challenged with TNF and cycloheximide [46], or in PBMCs (peripheral blood mononuclear cells) challenged with infliximab [47], the mutants generated by Lobito et al. [43] failed to function as inhibitors of apoptosis. This would appear to bring into question the use of these particular vector constructs as a genuine reflection of the disease state. Therefore the misfolding hypothesis, while not disproven, should for the moment be viewed with an element of caution until confirmation can be provided using bona-fide patient-derived cells, although it is worth noting that more recent studies are now beginning to address this point.
Fundamental to our understanding of aberrant TNFR1 signalling is the compartmentation of the receptor. TNFR1, as with several cell surface receptors including TNFR6 (also known as Fas), traffics between the cell surface and Golgi storage pool [48,49], where TNFR1 predominantly localizes under resting conditions [5–8]. While the ability of cells to internalize TNF-bound TNFR1 and trigger apoptosis is well documented [15], the contribution of TNFR1 cycling dysfunction to inflammation pathophysiology is less well studied. The importance of this should not go unstated, however, as for instance there are profound implications for TNFR1 signalling should newly synthesized TNF and recycling TNFR1 meet in the same compartment of the endosomal system.
Elevated levels of cell surface TNFR1 have been reported in PBMCs isolated from TRAPS patients carrying a C73R mutation in TNFR1 in the absence of pro-inflammatory stimuli [50]. As C73R does not give rise to a TNFR1 shedding defect [51], this led the authors to speculate that the C73R mutation might instead result in failure to retain the receptor within the Golgi [50], with the C73R mutation either masking a Golgi retention motif within this region or in an area of the molecule that is influenced by mutations in this region. Alternatively the C73R mutation might instead abolish a Golgi retrieval signal, resulting in failure to internalize TNFR1 and return it to the Golgi storage pool. Unfortunately, however, the study in which this data appeared came from an investigation detailing the cell surface expression profiles of just three different TRAPS mutations. Moreover, of these TRAPS mutations only C73R was a fully penetrant disease mutation. Cell surface localization might therefore also be evident in other mutations that alter peptide sequence and/or protein conformation within CRD2. Clearly, more extensive investigation of Golgi retention/retrieval needs to be undertaken in cells with other TNFRSF1A mutations before we can confidently state whether this mechanism of induction of NF-κB activity is unique to the C73R mutation in TNFRSF1A, or is perhaps instead a more common mechanism of TNFR1 dysfunction in TRAPS.
CONSEQUENCES OF TNFR1 TRAFFICKING DYSFUNCTION UPON CYTOKINE SECRETION
Measurements of serum cytokine levels from a cohort of TRAPS patients not having a clinical flare at the time of sample collection revealed elevated levels of IL-6, TNF and the pro-inflammatory chemokine IL-8 [39,52]. Many patients with TNFR1 mutations were also found to have elevated CRP levels. Together, these findings illustrate that even under resting conditions, cells from TRAPS patients experience ongoing inflammatory activity. In addition it is also known that the acute-phase inflammatory response in TRAPS patients is also associated with increased production of pyrogenic cytokines, namely IL-6, IL-1β and TNF, as well as CRP and serum amyloid A [53]. Increased secretion of pyrogenic cytokines is also observed after infliximab stimulation of PBMCs isolated from TRAPS patients with the T50M mutation in TNFRSF1A [47], as well as decreased secretion of the anti-inflammatory cytokine IL-4 [54]. The mechanism of TRAPS-associated cytokine release is, however, more complex than would first appear, as recent work has shown that different TRAPS mutations stimulate distinct NF-κB family subunit activities. Specifically, mutations resulting in high NF-κB p65 subunit activity triggered increased IL-8 secretion, whereas those resulting in high c-Rel activity increased IL-1β and IL-12 secretion [55]. Thus different NF-κB subunits may serve as independent inflammatory stimuli, with each NF-κB subunit activity being dependent on the nature of the specific TNFR1 mutation. However, together these distinct NF-κB subunit actions are complementary in generating the inflammatory features of TRAPS.
IL-1β is a pro-inflammatory cytokine that induces the pro-duction of several cytokines, including itself, TNF, IL-6, IL-8 and IL-12. It also can be induced by acute-phase proteins such as CRP [56]. Early evidence suggests that anti-IL-1 drugs such as Anakinra provide encouraging prognoses in patients with TRAPS, suggesting that IL-1 might also serve to amplify the pro-inflammatory background in TRAPS patients. Another potential candidate that might also induce cytokine activation is IL-6, as we know that CRP is modulated by IL-6 [57] and that IL-6 secretion is elevated in isolated PBMCs with certain TRAPS mutations in response to pro-inflammatory stimulation [47,55]. Indeed, recent preliminary findings indicate that the administration of anti-IL-6 drug tocilizumab could prove a useful tool to successfully attenuate and subsequently prevent acute inflammatory TRAPS attacks, although the underlying disease pathogenesis still remains [58]. IL-8 also plays an important regulatory role, with monocytes and fibroblasts producing IL-8, a chemoattractant for neutrophils, during inflammation. This chemokine might also have a role in the dermatological manifestations notable in TRAPS, and in addition monocytic fasciitis has also been observed in affected muscles during attacks of TRAPS [3].
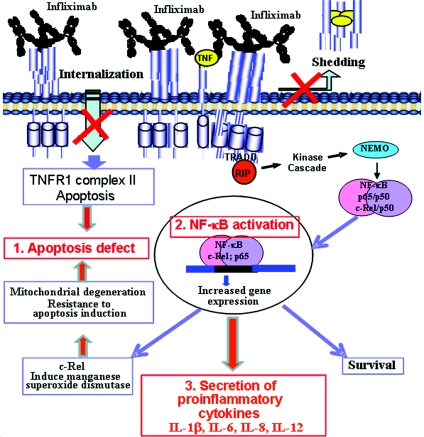
Upon binding TNF at the cell surface, two spatially distinct TNFR1 signalling complexes are formed which have the capacity to signal either NF-κB activation from the cell surface or apoptosis from internalized receptosomes [12–14], thereby indicating that TNFR1 compartmentalization has an important role in driving TNF-mediated biological responses [15,16]. Furthermore, it is known that conformational changes in the extracellular portion of TNFR1 interfere with receptor internalization and subsequent activation of apoptotic pathways [59]. In addition, inhibition of TNFR1 internalization either by deletion of the TNFR1 internalization domain [59] or by infection with an adenovirus [60] blocked apoptotic signalling complex recruitment, while still allowing recruitment of signalling molecules associated with NF-κB activation. Thus an internalization defect would likely result in an accumulation of TNFR1 at the cell surface, thereby leading to a constitutive activation of the receptor and the subsequent activation of NF-κB, as has been reported for C73R cells [50]. In addition, the absence of internalization is synonymous with defective formation of TNFR1 complex II that is required for activation of apoptosis. This would also explain the observation that T50M cells similarly failed to trigger apoptosis following incubation with the anti-TNF mAb (monoclonal antibody), infliximab [47], or similarly that C43S cells were found to shed normally yet be insensitive to TNF-induced apoptosis [17] and other cysteine and threonine mutations showed a modest shedding defect and yet were also insensitive to TNF-induced apoptosis [46]. In addition, failure to internalize TNFR1 receptors is also likely to be further compounded by the activation of anti-apoptotic c-Rel NF-κB subunits [47]. These pathways are summarized in Figure 2.
Figure 2. Anti-TNF drug infliximab activates NF-κB c-Rel and p65 subunits, and triggers secretion of the pro-inflammatory cytokines IL-1β, IL-6, IL-8 and IL-12 in PBMCs isolated from TRAPS patients.
(1) The mAb anti-TNF drug infliximab triggers pro-inflammatory cytokine secretion due to failure to internalize or shed cell surface TNFR1, (2) resulting in TNF-stimulated NF-κB activation through the canonical pathway or ligand-independent TNFR1 signalling. This in turn results in nuclear translocation of p65 and c-Rel subunits of NF-κB, c-Rel-mediated reinforcement of the inhibition of apoptosis, and (3) transcriptional activation of cell survival pathways, increased pro-inflammatory cytokine secretion and the onset of TRAPS.
Synthesis of cytokines, such as TNF-α, IL-1β, IL-6 and IL-8, is mediated by NF-κB. NF-κB is also able to function in concert with other transcription factors, including AP-1 (activator protein 1). For example, NF-κB translocation enhances transcription of the collagenase-3 gene, also known as MMP13 (matrix metalloproteinase 13), in IL-1-stimulated synoviocytes [61]. However, expression of collagenase gene transcription also depends on the participation of AP-1, whose transcriptional induction follows a distinct pathway involving phosphorylation of the MAPK, JNK and subsequent phosphorylation of c-Jun. Therefore, as AP-1 and NF-κB are simultaneously activated in IL-1 or TNF-stimulated PBMC, as well endothelial cells from TRAPS patients, TNFR1 trafficking dysfunction is likely to involve coordinated stimulation of both NF-κB and AP-1.
Additional insight into the complexity of TRAPS biology came from findings that revealed a regulatory role of IRFs [IFN (interferon)-regulatory factors] in TNFR1 signalling. The investigation of signal transduction has typically focused on direct early events, but analysis of the mechanisms by which signals are propagated over time and the importance of autocrine loops in regulating cellular responses has started to gain more attention [62–65]. It was recently found that in myeloid cells, TNF initiated a type I IFN-mediated autocrine loop that sustained the expression of inflammation-related genes such as Cxcl9, Cxcl10 and Cxcl11 and led to the expression of genes encoding classic IFN-response molecules, including those known to mediate antiviral responses and prime macrophages for enhanced subsequent responses to cytokines and microbial products [66]. TNF-mediated production of IFN-β and subsequent autocrine regulation of gene expression was dependent on IRF1 and on synergy between small amounts of IFN produced and additional TNF-induced signals, such as activation of NF-κB. These findings identified a hitherto unknown TNF-activated IRF1-IFN JAK/STAT (Janus kinase/signal transducer and activator of transcription) signalling pathway that not only contributes to the pro-inflammatory functions of TNF, and provided evidence that induction of IFN-mediated autocrine loops is not limited to pattern-recognition receptors but also contributes to macrophage response to endogenous inflammatory factors such as TNF [67].
Defective TNFR1 internalization might also explain some of the chronic inflammation and flare symptoms observed in TRAPS patients. Indeed a model in which persistent stimulation with TNF activates NF-κB and MAPK pathways would lead to rapid expression of inflammatory genes and to higher expression of IRF1. As detailed above, this would in turn activate JAK/STAT signalling that acts in synergy with other TNF-induced signals to sustain expression of inflammatory chemokines and IFN-β, inducing slow and delayed accumulation of mRNA molecules encoded by canonical IFN-response genes, increased expression of signalling components such as IKK-ϵ (inhibitor of NF-κB kinase ϵ), IRF7 and STAT1 that are known to further amplify the activation of genes by low concentrations of IFN, and shift the balance of macrophage responses in an inflammatory direction [62,68–70]. Thus, TNF-induced gene expression could be sustained and amplified by the sequential induction of IRF1, IFN-β and STAT1. TNF would thereby activate a ‘feed-forward’ loop that sustains inflammation but avoids the potential toxicity associated with the high IFN production induced by stimulation of Toll-like receptors.
CONCLUSIONS
As our understanding of TNFR1 biology continues to grow, so does the complexity of TNF signalling and its cross-talk with other signalling mediators. Similarly, the biology underpinning TNFR1 dysfunction in TRAPS is also far from straightforward, with multiple models of trafficking dysfunction having been linked to various respective TNFR mutations. In addition, ligand-independent signalling adds an extra dimension to the underlying disease pathophysiology. Despite the existence of these diverse models of TNFR1 dysfunction, there is, however, one unifying factor common to most of these mechanisms, namely enhanced NF-κB activity. Even here, however, the situation is far from straightforward, as in addition to transcriptional activation through p65/p50 heterodimers, recent studies indicate a growing role for c-Rel in inflammatory disease pathogenesis, including TRAPS [55]. Interestingly, c-Rel is also activated by the anti-TNF drug infliximab in PBMCs isolated from TRAPS patients [47], and this has been proposed to be the result of defective TNFR1 internalization, and/or cell surface activation of TNFR1 that is unable to be shed (Figure 2). Given that there is also now increasing evidence for additional transcriptional regulation through MAPK activation of AP-1, no doubt there will be further bumps in the road ahead as we continue to develop a better understanding of TRAPS disease pathophysiology. However, it is only through more detailed knowledge of TRAPS-associated dysfunction of TNFR1 biochemistry and cell biology that we can hope to develop more effective targeted therapeutic treatment options.
FUNDING
The related TNFR1 studies within the Turner laboratory were funded by Barts and The London Charitable Foundation.
References
- 1.Mcdermott M. F., Ogunkolade B. W., McDermott E. M., Jones L. C., Wan Y., Quane K. A., McCarthy J., Phelan M., Molloy M. G., Powell R. J., et al. Linkage of familial Hibernian fever to chromosome 12p13. Am. J. Hum. Genet. 1998;65:1446–1451. doi: 10.1086/301886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulley J., Saar K., Hewitt G., Rüschendorf F., Phillips H., Colley A., Sillence D., Reis A., Wilson M. Gene localization for an autosomal dominant familial periodic fever to 12p13. Am. J. Hum. Genet. 1998;62:884–889. doi: 10.1086/301793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hull K. M., Drewe E., Aksentijevich I., Singh H. K., Wong K., McDermott E. M., Dean J., Powell R. J., Kastner D. L. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine. 2002;81:349–368. doi: 10.1097/00005792-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Kriegel M. A., Huffmeier U., Scherb E., Scheidig C., Geiler T., Kalden J. R., Reis A., Lorenz H. M. Tumor necrosis factor receptor-associated periodic syndrome characterized by a mutation affecting the cleavage site of the receptor: implications for pathogenesis. Arthritis Rheum. 2003;48:2386–2388. doi: 10.1002/art.11169. [DOI] [PubMed] [Google Scholar]
- 5.Bradley J. R., Thiru S., Pober J. S. Disparate localization of 55-kd and 75-kd tumor necrosis factor receptors in human endothelial cells. Am. J. Pathol. 1995;146:27–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Jones S. J., Ledgerwood E. C., Prins J. B., Galbraith J., Johnson D. R., Pober J. S., Bradley J. R. TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 1999;162:1042–1048. [PubMed] [Google Scholar]
- 7.Gaeta M. L., Johnson D. R., Kluger M. S., Pober J. S. The death domain of tumor necrosis factor receptor 1 is necessary but not sufficient for Golgi retention of the receptor and mediates receptor desensitization. Lab. Invest. 2000;80:1185–1194. doi: 10.1038/labinvest.3780126. [DOI] [PubMed] [Google Scholar]
- 8.Storey H., Stewart A., Vandenabeele P., Luzio J. P. The p55 tumour necrosis factor receptor TNFR1 contains a trans-Golgi network localization signal in the C-terminal region of the cytoplasmic tail. Biochem. J. 2002;366:15–22. doi: 10.1042/BJ20020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Micheau O., Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 10.MacEwan D. J. TNF receptor subtype signalling: differences and cellular consequences. Cell. Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 11.Hehlgans T., Männel D. N. The TNF–TNF receptor system. Biol. Chem. 2002;383:1581–1585. doi: 10.1515/BC.2002.178. [DOI] [PubMed] [Google Scholar]
- 12.Heyninck K., Beyaert R. Crosstalk between NF-κB-activating and apoptosis-inducing proteins of the TNF-receptor complex. Mol. Cell Biol. Res. Commun. 2001;4:259–265. doi: 10.1006/mcbr.2001.0295. [DOI] [PubMed] [Google Scholar]
- 13.Van Herreweghe F., Festjens N., Declercq W., Vandenabeele P. Tumor necrosis factor-mediated cell death: to break or bust, that's the question. Cell. Mol. Life Sci. 2010;67:1567–1579. doi: 10.1007/s00018-010-0283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethu S., Melendez A. J. New developments on the TNFα-mediated signalling pathways. Biosci. Rep. 2010;31:63–76. doi: 10.1042/BSR20100040. [DOI] [PubMed] [Google Scholar]
- 15.Schutze S., Tchikov V., Schneider-Brachert W. Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 2008;9:655–662. doi: 10.1038/nrm2430. [DOI] [PubMed] [Google Scholar]
- 16.Tchikov V., Bertsch U., Fritsch J., Edelmann B., Schutze S. Subcellular compartmentalization of TNF receptor-1 and CD95 signaling. Eur. J. Cell Biol. 2011;90:467–475. doi: 10.1016/j.ejcb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Siebert S., Amos N., Fielding C. A., Wang E. C., Aksentijevich I., Williams B. D., Brennan P. Reduced tumor necrosis factor signaling in primary human fibroblasts containing a tumor necrosis factor receptor superfamily 1A mutant. Arthritis Rheum. 2005;52:1287–1292. doi: 10.1002/art.20955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idriss H. T., Naismith J. H. TNFα and the TNF receptor superfamily: structure–function relationship(s) Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 19.Hehlgans T., Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115:1–20. doi: 10.1111/j.1365-2567.2005.02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Alessio A., Al-Lamki R. S., Bradley J. R., Pober J. S. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am. J. Pathol. 2005;166:1273–1282. doi: 10.1016/S0002-9440(10)62346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Alessio A., Esposito B., Giampietri C., Ziparo E., Pober J. S., Filippini A. Plasma membrane micro domains regulate TACE-dependent TNFR1 shedding in human endothelial cells. J. Cell Mol. Med. 2011 doi: 10.1111/j.1582-4934.2011.01353.x. doi:10.1111/j.1582-4934.2011.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porteu F., Nathan C. Shedding of tumor necrosis factor receptors by activated human neutrophils. J. Exp. Med. 1990;172:599–607. doi: 10.1084/jem.172.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müllberg J., Althoff K., Jostock T., Rose-John S. The importance of shedding of membrane proteins for cytokine biology. Eur. Cytokine Netw. 2000;11:27–38. [PubMed] [Google Scholar]
- 24.McDermott M. F., Aksentijevich I., Galon J., McDermott E. M., Ogunkolade B. W., Centola M., Mansfield E., Gadina M., Karenko L., Pettersson T., et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 25.Xanthoulea S., Pasparakis M., Kousteni S., Brakebusch C., Wallach D., Bauer J., Lassmann H., Kollias G. Tumor necrosis factor (TNF) receptor shedding controls thresholds of innate immune activation that balance opposing TNF functions in infectious and inflammatory diseases. J. Exp. Med. 2004;200:367–376. doi: 10.1084/jem.20040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawari F. I., Rouhani F. N., Cui X., Yu Z. X., Buckley C., Kaler M., Levine S. J. Release of full-length 55-kDa TNF receptor 1 in exosome-like vesicles: a mechanism for generation of soluble cytokine receptors. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1297–1302. doi: 10.1073/pnas.0307981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beutler B., Bazzoni F. TNF, apoptosis and autoimmunity: a common thread? Blood Cells Mol. Dis. 1998;24:216–230. doi: 10.1006/bcmd.1998.0187. [DOI] [PubMed] [Google Scholar]
- 28.Chan F. K., Chun H. J., Zheng L., Siegel R. M., Bui K. L., Lenardo M. J. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 29.Siegel R. M., Frederiksen J. K., Zacharias D. A., Chan F. K., Johnson M., Lynch D., Tsien R. Y., Lenardo M. J. Fas preassociation required for apoptosis signalling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 30.Yousaf N., Gould D. J., Aganna E., Hammond L., Mirakian R. M., Turner M. D., Hitman G. A., McDermott M., Chernajovsky Y. Tumor necrosis factor receptor I from patients with tumor necrosis factor receptor-associated periodic syndrome interacts with wild-type tumor necrosis factor receptor I and induces ligand-independent. Arthritis Rheum. 2005;52:2906–2916. doi: 10.1002/art.21268. [DOI] [PubMed] [Google Scholar]
- 31.Huggins M. L., Radford P. M., McIntosh R. S. Shedding of mutant tumor necrosis factor receptor superfamily 1A associated with tumor necrosis factor associated periodic syndrome (TRAPS): differences between cell types. Arthritis Rheum. 2004;50:2651–2659. doi: 10.1002/art.20380. [DOI] [PubMed] [Google Scholar]
- 32.Whitmarsh A. J., Davis R. J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 33.Robinson M. J., Cobb M. H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 34.Ip Y. T., Davis R. J. Signal transduction by the c-Jun N-terminal kinase (JNK): from inflammation to development. Curr. Opin. Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 35.Kyriakis J. M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 36.Widmann C., Gibson S., Jarpe M. B., Johnson G. L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol. Rev. 1999;79:143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 37.Johnson G. L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 38.Biologics in Rheumatoid Arthritis Genetics and Genomics. Coulthard L. R., Taylor J. C., Eyre S., Robinson J. I., Wilson A. G., Isaacs J. D., Hyrich K., Emery P., Barton A., et al. Genetic variants within the MAP kinase signalling network and anti-TNF treatment response in rheumatoid arthritis patients. Ann. Rheum. Dis. 2011;70:98–103. doi: 10.1136/ard.2010.133249. [DOI] [PubMed] [Google Scholar]
- 39.Simon A., Park H., Maddipati R., Lobito A. A., Bulua A. C., Jackson A. J., Chae J. J., Ettinger R., de Koning H. D., Cruz A. C., et al. Concerted action of wild-type and mutant TNF receptors enhances inflammation in TNF receptor 1-associated periodic fever syndrome. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9801–9806. doi: 10.1073/pnas.0914118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bulua A. C., Simon A., Maddipati R., Pelletier M., Park H., Kim K. Y., Sack M. N., Kastner D. L., Siegel R. M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J. Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamata H., Honda S., Maeda S., Chang L., Hirata H., Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 42.Rebelo S. L., Bainbridge S. E., Amel-Kashipaz M. R., Radford P. M., Powell R. J., Todd I., Tighe P. J. Modeling of tumor necrosis factor receptor superfamily 1A mutants associated with tumor necrosis factor receptor-associated periodic syndrome indicates misfolding consistent with abnormal function. Arthritis Rheum. 2006;54:2674–2687. doi: 10.1002/art.21964. [DOI] [PubMed] [Google Scholar]
- 43.Lobito A. A., Kimberley F. C., Muppidi J. R., Komarow H., Jackson A. J., Hull K. M., Kastner D. L., Screaton G. R., Siegel R. M. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS) Blood. 2006;108:1320–1327. doi: 10.1182/blood-2005-11-006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todd I., Radford P. M., Daffa N., Bainbridge S. E., Powell R. J., Tighe P. J. Mutant tumor necrosis factor receptor associated with tumor necrosis factor receptor-associated periodic syndrome is altered antigenically and is retained within patients' leukocytes. Arthritis Rheum. 2007;56:2765–2773. doi: 10.1002/art.22740. [DOI] [PubMed] [Google Scholar]
- 45.Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D. T., Back S. H., Kaufman R. J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 46.D'Osualdo A., Ferlito F., Prigione I., Obici L., Meini A., Zulian F., Pontillo A., Corona F., Barcellona R., Di Duca M., et al. Neutrophils from patients with TNFRSF1A mutations display resistance to tumor necrosis factor-induced apoptosis: pathogenetic and clinical implications. Arthritis Rheum. 2006;54:998–1008. doi: 10.1002/art.21657. [DOI] [PubMed] [Google Scholar]
- 47.Nedjai B., Hitman G. A., Quillinan N., Coughlan R. J., Church L. D., McDermott M. F., Turner M. D. Proinflammatory action of the antiinflammatory drug infliximab in TNF-receptor associated periodic syndrome. Arthritis Rheum. 2009;60:619–625. doi: 10.1002/art.24294. [DOI] [PubMed] [Google Scholar]
- 48.Schutze S., Machleidt T., Adam D., Schwandner R., Wiegmann K., Kruse M. L., Heinrich M., Wickel M., Krönke M. Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J. Biol. Chem. 1999;274:10203–10212. doi: 10.1074/jbc.274.15.10203. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Al-Lamki R. S., Zhang H., Kirkiles-Smith N., Gaeta M. L., Thiru S., Pober J. S., Bradley J. R. Histamine antagonizes tumor necrosis factor (TNF) signaling by stimulating TNF receptor shedding from the cell surface and Golgi storage pool. J. Biol. Chem. 2003;278:21751–21760. doi: 10.1074/jbc.M212662200. [DOI] [PubMed] [Google Scholar]
- 50.Nedjai B., Hitman G. A., Yousaf N., Chernajovsky Y., Stjernberg S., Pettersson T., Ranki A., Hawkins P., Arkwright P. D., McDermott M. F., Turner M. D. Abnormal tumor necrosis factor receptor I cell surface expression and NF-κB activation in tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum. 2008;58:273–283. doi: 10.1002/art.23123. [DOI] [PubMed] [Google Scholar]
- 51.Stjernberg-Salmela S., Pettersson T., Karenko L., Blazevic V., Nevala H., Pitkänen S., Peterson P., Ranki A. A novel tumour necrosis factor receptor mutation in a Finnish family with periodic fever syndrome. Scand. J. Rheumatol. 2004;33:140–144. doi: 10.1080/03009740310004892. [DOI] [PubMed] [Google Scholar]
- 52.Nowlan M. L., Drewe E., Bulsara H., Esposito N., Robins R. A., Tighe P. J., Powell R. J., Todd I. Systemic cytokine levels and the effects of etanercept in TNF receptor-associated periodic syndrome (TRAPS) involving a C33Y mutation in TNFRSF1A. Rheumatology. 2006;45:31–37. doi: 10.1093/rheumatology/kei090. [DOI] [PubMed] [Google Scholar]
- 53.Dinarello C. A. Thermoregulation and the pathogenesis of fever. Infect. Dis. Clin. North Am. 1996;10:433–449. doi: 10.1016/s0891-5520(05)70306-8. [DOI] [PubMed] [Google Scholar]
- 54.Nedjai B., Quillinan N., Coughlan R. J., Church L., McDermott M. F., Hitman G. A., Turner M. D. Lessons from anti-TNF biologics: infliximab failure in a TRAPS family with the T50M mutation in TNFRSF1A. Adv. Exp. Med. Biol. 2011;691:409–419. doi: 10.1007/978-1-4419-6612-4_43. [DOI] [PubMed] [Google Scholar]
- 55.Nedjai B., Hitman G. A., Church L. D., Minden K., Whiteford M. L., McKee S., Stjernberg S., Pettersson T., Ranki A., Hawkins P., et al. Differential cytokine secretion resulting from p65 and c-Rel NF-κB subunit signaling in peripheral blood mononuclear cells of TNFR-associated periodic fever syndrome patients. Cell. Immunol. 2011;268:55–59. doi: 10.1016/j.cellimm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Dinarello C. A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 57.Ganter U., Arcone R., Toniatti C., Morrone G., Ciliberto G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 1989;8:3773–3779. doi: 10.1002/j.1460-2075.1989.tb08554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaitla P. M., Radford P. M., Tighe P. J., Powell R. J., McDermott E. M., Todd I., Drewe E. Role of interleukin-6 in a patient with tumor necrosis factor receptor-associated periodic syndrome: assessment of outcomes following treatment with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum. 2011;63:1151–1155. doi: 10.1002/art.30215. [DOI] [PubMed] [Google Scholar]
- 59.Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Schneider-Brachert W., Tchikov V., Merkel O., Jakob M., Hallas C., Kruse M. L., Groitl P., Lehn A., Hildt E., Held-Feindt J., et al. Inhibition of TNF receptor 1 internalization by adenovirus 14.7K as a novel immune escape mechanism. J. Clin. Invest. 2006;116:2901–2913. doi: 10.1172/JCI23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mengshol J. A., Vincenti M. P., Coon C. I., Barchowsky A., Brinckerhoff C. E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 62.Marie I., Durbin J. E., Levy D. E. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doyle S., Vaidya S., O'Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 64.Covert M. W., Leung T. H., Gaston J. E., Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 65.Werner S. L., Barken D., Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- 66.Yarilina A., Ivashkiv L. B. Type I interferon: a new player in TNF signaling. Curr. Dir. Autoimmun. 2010;11:94–104. doi: 10.1159/000289199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yarilina A., Park-Min K. H., Antoniv T., Hu X., Ivashkiv L. B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1dependent type I interferon-response genes. Nat. Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- 68.Hu X., Herrero C., Li W. P., Antoniv T. T., Falck-Pedersen E., Koch A. E., Woods J. M., Haines G. K., Ivashkiv L. B. Sensitization of IFN-gamma JAK-STAT signaling during macrophage activation. Nat. Immunol. 2002;3:859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- 69.Tassiulas I., Hu X., Ho H., Kashyap Y., Paik P., Hu Y., Lowell C. A., Ivashkiv L. B. Amplification of IFN-α-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat. Immunol. 2004;5:1181–1189. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 70.Honda K., Yanai H., Negishi H., Asagiri M., Sato M., Mizutani T., Shimada N., Ohba Y., Takaoka A., Yoshida N., Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]