Abstract
Heart failure is a risk factor for Alzheimer’s disease (AD) and cerebrovascular disease. In the absence of heart failure, we hypothesized that left ventricular ejection fraction (LVEF), an indicator of cardiac dysfunction, would be associated with pre-clinical brain magnetic resonance imaging (MRI) and neuropsychological markers of ischemia and AD in the community. Brain MRI, cardiac MRI, neuropsychological, and laboratory data were collected on 1114 Framingham Heart Study Offspring Cohort participants free from clinical stroke or dementia (40–89 years, 67±9; 54% women). Neuropsychological and neuroimaging markers of brain aging were related to cardiac MRI-assessed LVEF. In multivariable-adjusted linear regressions, LVEF was not associated with any brain aging variable (p-values>0.15). However, LVEF quintile analyses yielded several U-shape associations. Compared to the referent (Q2–Q4), the lowest quintile (Q1) LVEF was associated with a lower mean cognitive performance, including Visual Reproduction Delayed Recall (β= −0.27, p<0.001) and Hooper Visual Organization Test (β= −0.27, p<0.001). Compared to the referent, the highest quintile (Q5) LVEF values also were associated with lower mean cognitive performances, including Logical Memory Delayed Recall (β= −0.18, p=0.03), Visual Reproduction Delayed Recall (β= −0.17, p=0.03), Trail Making Test Part B-Part A (β= −0.22, p=0.02) and Hooper Visual Organization Test (Q5 β= −0.20, p=0.02). Findings were similar when analyses were repeated excluding prevalent cardiovascular disease. In conclusion, although our observational cross-sectional data cannot establish causality, they suggest a non-linear association between LVEF and measures of accelerated cognitive aging.
Introduction
Among patients with severe cardiomyopathies, left ventricular ejection fraction (LVEF) is related to abnormal brain aging, including cognitive impairment,1 structural neuroanatomical abnormalities,2 and an increased risk of Alzheimer’s disease (AD).3 Cognitive impairment improves4 and cerebral blood flow increases by more than 50% following heart transplantation,5 purportedly due to cardiac function improvement. Therefore, reduced LVEF may influence cerebral perfusion homeostasis and contribute to clinical brain injury. In the absence of end-stage heart disease, less is known about how LVEF affects or accelerates abnormal brain aging. Our cross-sectional investigation aims to better understand relations between LVEF and abnormal brain aging by extending prior work to a large, epidemiological cohort, assessing LVEF using sensitive cardiac MRI (CMR), and simultaneously considering shared vascular risks for brain and myocardial injury. Based on prior work, we hypothesized that lower LVEF would be associated with cognitive and neuroimaging markers of pre-clinical AD6,7 (learning and memory, brain volume, temporal horn volume, hippocampal volume) and cerebrovascular changes8,9 (executive functioning, white matter hyperintensities (WMH)) in a community-based cohort of adults free of clinical dementia or stroke.
Methods
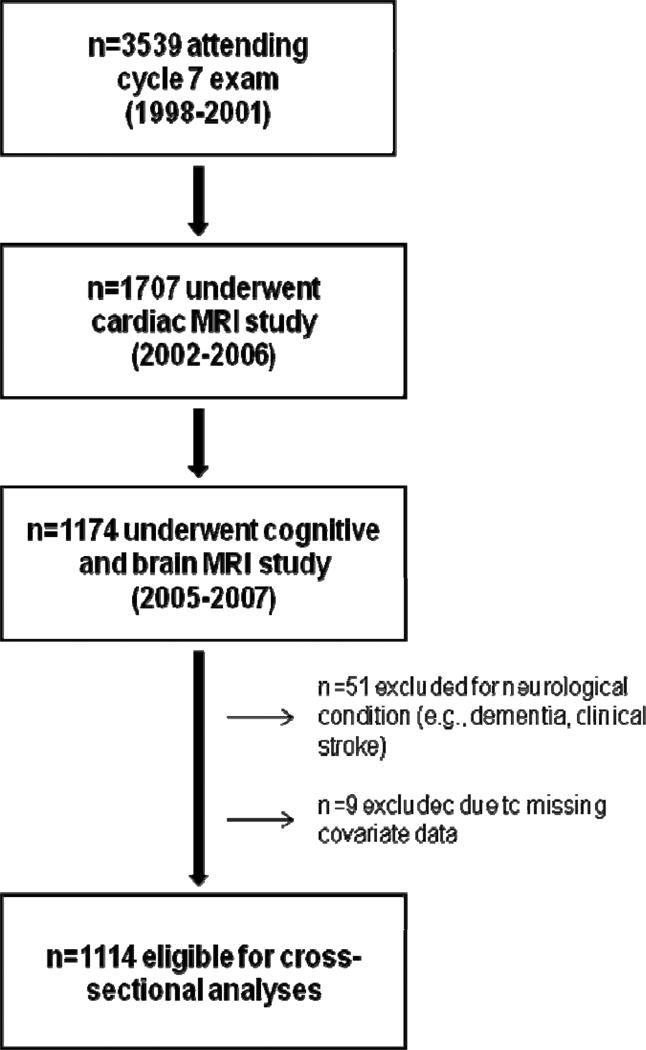
The Framingham Offspring Study design and selection criteria have been described elsewhere.10 From 1971 to 1975, 5124 participants were recruited and have been examined every 4–8 years since. Details on the derivation of the current sample are provided in Figure 1. The protocol was approved by the local IRBs. Participants provided written informed consent prior to assessments.
Figure 1.
Participant Enrollment and Exclusion Details
Participants completed the following cognitive protocol that was selected a priori to represent different cognitive systems: (1) Delayed Memory: Logical Memory Delayed Recall and Visual Reproduction Delayed Recall; (2) Language: Boston Naming Test-30 Item; (3) Executive Functioning: a difference score of Trail Making Test Part B minus Part A; (4) Verbal Reasoning: Similarities; and (5) Visuoperceptual Abilities: Hooper Visual Organization Test.
For brain imaging acquisition, participants were imaged on a Siemens 1T MR machine using a T2-weighted double spin-echo coronal imaging sequence. Digital information was post-processed by a central laboratory blinded to demographic and clinical information. A custom-written, semiautomatic segmentation protocol was used to quantify total cranial,11 total brain,12 frontal lobar,13 temporal horn,13 and hippocampal volumes14 and WMH segmentation.12 Inter-rater reliabilities for these methods have been published elsewhere.11,13,15,16 For this study, intra- and inter-rater reliabilities were consistently above 0.90. Hippocampal data were available for a subset of participants (n=423). For cardiac MRI acquisition, participants were imaged in the supine position using a Philips 1.5T MR system with a 5-element (3 anterior, 2 posterior) surface coil. Images were acquired at end-tidal breath-hold and analyzed by a single, experienced, blinded reviewer using a commercial workstation (EasyVision 4.0, Philips Medical Systems). End-systolic phase was determined as the minimal cross-sectional area of a mid-ventricular slice. The time delay from the QRS (phase) was analyzed for each contiguous slice and endocardial borders were segmented. End-diastolic volume (EDV) and end-systolic volume (ESV) were computed by summation of disks (i.e., modified Simpson’s Rule) to derive LVEF ([EDV-ESV]/EDV). Intra- and inter-observer coefficients of variation for these methods have been published elsewhere.17 For this study, inter-rater reliabilities were consistently above 0.92.18
Total brain, frontal lobe, temporal horn, and hippocampal volumes, and WMH were expressed as percent of total cranial volume. WMH, Trail Making Test Part B-Part A, and Hooper Visual Organization Test were natural log-transformed to normalize distributions. As previously described,18 neuropsychological scores were adjusted for age and education, separately by sex, to enable comparison across measures. Resulting values were standardized, separately by sex, to a mean of 0 and a standard deviation of 1 (i.e., values were transformed to represent standard deviation units from the mean).
We used regression to assess linear relations between LVEF and each brain aging variable. Next, we compared brain aging variables among participants classified by LVEF quintile and noted U-shaped associations. We therefore compared the lower (Q1) and upper quintile (Q5) to the referent (Q2–Q4) for each brain aging variable. Based on prior work,18 we adjusted for covariates defined at the 7th examination cycle, including: age, sex, systolic blood pressure, smoking status, diabetes mellitus (i.e., history of fasting blood glucose ≥126mg/dl or use of oral hypoglycemic/insulin), hypertension treatment, atrial fibrillation, and prevalent CVD, including coronary heart disease, heart failure, and intermittent claudication.19 Secondary analyses were performed: (1) excluding prevalent CVD (n=77), (2) using the categorical LVEF variable (i.e., Q1, Q5, Q2–Q4 referent) assessing effect modification by sex, age (<60 vs. ≥60 years), and APOE status20 (ε4 − vs. ε4+) and stratifying analyses by subgroups as indicated. Significance was set at p<0.05 for all models. Data were analyzed using SAS version 9.1 (Cary, NC).
Results
Clinical characteristics are provided in Table 1. Cardiac MRI, brain MRI, and neuropsychological descriptives are provided in Table 2. As a continuous variable, LVEF was unrelated to any brain MRI or neuropsychological variable (Table 3). Findings were not altered when participants with CVD were excluded (Table 4).
Table 1.
Clinical & imaging characteristics
| Variable | n=1114 |
|---|---|
| Age at brain MRI (years) | 67±9 |
| Women | 602 (54%) |
| Systolic blood pressure (mm Hg) | 124±17 |
| Cigarette smoker | 102 (9%) |
| Diabetes mellitus | 93 (8%) |
| Atrial fibrillation | 20 (2%) |
| Hypertension treatment | 293 (26%) |
| Prevalent cardiovascular disease | 77 (7%) |
| Time to brain MRI (years) | 6.9±0.9 |
| Time from CMR to brain MRI (years) | 2.5±1.1 |
| LVEF (%) | 67.3±6.7 |
| Quintile 1 | 225 (<62.0%) |
| Quintile 2 | 217 (62.0% to 65.9%) |
| Quintile 3 | 226 (65.9% to 68.8%) |
| Quintile 4 | 226 (68.8% to 73.2%) |
| Quintile 5 | 220 (≥73.2%) |
Note: Values denoted as percentages or mean±standard deviation; LVEF=left ventricular ejection fraction
Table 2.
Left ventricular ejection fraction and brain aging data
| Brain MRI Data (% of total cranial volume) | Mean±SD | |||
|---|---|---|---|---|
| Total Sample n=1114 |
Q1 n=225 |
Q2–Q4 n=669 |
Q5 n=220 |
|
| WMH* | −2.38±1.13 | −2.42±1.17 | −2.45±1.10 | −2.15±1.15 |
| Total brain volume£ | 79.02±3.81 | 79.35±3.66 | 79.14±3.82 | 78.32±3.87 |
| Frontal lobar volume£ | 36.07±3.37 | 36.23±3.40 | 36.25±3.30 | 35.35±3.49 |
| Temporal horn volume*,£ | −3.08±0.88 | −3.10±0.84 | −3.10±0.92 | −3.00±0.80 |
| Hippocampal volume£ | 0.37±0.06† | 0.37±0.06‡ | 0.37±0.06^ | 0.37±0.06¥ |
| Neuropsychological Data | Median (minimum, maximum) | |||
| Total Sample | Q1 | Q2–Q4 | Q5 | |
| n=1114 | n=222 | n=665 | n=217 | |
| Logical Memory Delayed, total | 12 (0, 22) | 12 (0, 22) | 12 (0, 22) | 11 (0, 19) |
| Visual Reproduction Delayed, total | 9 (0, 14) | 8 (0, 14) | 9 (0, 14) | 8 (1, 14) |
| Boston Naming Test-30 Item, total | 28 (12, 30) | 28 (15, 30) | 28 (16, 30) | 28 (12, 30) |
| Trail Making Test Part B-Part A | 0.77 (0.08, 9.62) | 0.77 (0.15, 9.30) | 0.74 (0.08, 9.62) | 0.84 (0.10, 9.55) |
| Hooper Visual Organization Test, total | 25.5 (11.5, 30.0) | 25.25 (14.5, 30.0) | 26 (12.5, 30.0) | 25 (11.5, 30.0) |
| Similarities, total | 18 (2, 26) | 17 (6, 25) | 18 (2, 26) | 17 (5, 25) |
Note: SD=standard deviation; WMH=white matter hyperintensities; for WMH and temporal horn volume, negative values indicate worse pathology;
n=423;
n=88;
n=245;
n=90;
are natural log transformed;
expressed as percentage of total cranial volume
Table 3.
Left ventricular ejection fraction, brain magnetic resonance imaging, and neuropsychological regression data
| LVEF Quintiles n=1114 | |||||||
|---|---|---|---|---|---|---|---|
| LVEF n=1114 |
Quintile 1 (bottom/low) |
Quintile 2–4 (middle) |
Quintile 5 (top/high) | ||||
| beta±SE | P | beta±SE | P | beta±SE | P | ||
| Brain MRI Data | |||||||
| WMH | 0.000±0.03 | 0.999 | 0.13±0.08 | 0.079 | Referent | 0.04±0.08 | 0.584 |
| Total brain volume | 0.003±0.02 | 0.903 | 0.13±0.23 | 0.564 | Referent | 0.13±0.23 | 0.593 |
| Frontal lobar volume | −0.03±0.03 | 0.186 | 0.13±0.21 | 0.551 | Referent | −0.21±0.21 | 0.319 |
| Temporal horn volume | 0.02±0.02 | 0.349 | −0.04±0.06 | 0.542 | Referent | −0.03±0.06 | 0.577 |
| Hippocampal volume† | 0.06±0.05 | 0.208 | −0.002±0.01 | 0.808 | Referent | 0.01±0.01 | 0.430 |
| Neuropsychological Data | |||||||
| WMS Logical Memory Delayed | −0.01±0.03 | 0.821 | −0.12±0.08 | 0.159 | Referent | −0.18±0.08 | 0.031 |
| WMS Visual Reproduction Delayed | 0.05±0.03 | 0.131 | −0.27±0.08 | <0.001 | Referent | −0.17±0.08 | 0.029 |
| Boston Naming Test-30 Item | −0.01±0.03 | 0.780 | −0.05±0.08 | 0.521 | Referent | −0.05±0.08 | 0.519 |
| Trail Making Test Part B-Part A | −0.01±0.04 | 0.750 | −0.13±0.09 | 0.174 | Referent | −0.22±0.09 | 0.022 |
| WAIS-R Similarities Subtest | 0.000±0.03 | 0.997 | −0.12±0.08 | 0.114 | Referent | −0.11±0.08 | 0.178 |
| Hooper Visual Organization Test | −0.006±0.03 | 0.856 | −0.27±0.08 | <0.001 | Referent | −0.20±0.08 | 0.015 |
Note:
based on n=423;
SE=standard error; WMH=white matter hyperintensities; Models adjusted for age, sex, systolic blood pressure, smoking status, diabetes mellitus, hypertension treatment, atrial fibrillation and prevalent CVD; LVEF quintiles are: <62.00 for Q1 (n=225), Q2=62.00 to 65.90 (n=217), Q3=65.90 to 68.80 (n=226), Q4=68.80 to 73.19 (n=226), and >73.19 for Q5 (n=220).
Table 4.
Left ventricular ejection fraction, brain magnetic resonance imaging, and neuropsychological regression data excluding cardiovascular disease
| LVEF Quintiles n=1037 | |||||||
|---|---|---|---|---|---|---|---|
| LVEF n=1037 | Quintile 1 (lowest) | Quintiles 2–4 (middle) |
Quintile 1 (lowest) | ||||
| beta±SE | P | beta±SE | P | beta±SE | P | ||
| Brain MRI Data | |||||||
| WMH | 0.01±0.03 | 0.768 | 0.10±0.08 | 0.193 | Referent | 0.02±0.08 | 0.805 |
| Total brain volume | 0.01±0.03 | 0.808 | 0.13±0.24 | 0.594 | Referent | 0.20±0.24 | 0.410 |
| Frontal lobar volume | −0.04±0.03 | 0.164 | 0.14±0.22 | 0.514 | Referent | −0.22±0.22 | 0.333 |
| Temporal horn volume | 0.01±0.03 | 0.690 | −0.01±0.06 | 0.930 | Referent | −0.03±0.06 | 0.574 |
| Hippocampal volume† | 0.07±0.05 | 0.151 | −0.002±0.01 | 0.841 | Referent | 0.01±0.01 | 0.425 |
| Neuropsychological Data | |||||||
| WMS Logical Memory Delayed | −0.02±0.03 | 0.495 | −0.11±0.09 | 0.213 | Referent | −0.20±0.09 | 0.020 |
| WMS Visual Reproduction Delayed | 0.05±0.03 | 0.129 | −0.24±0.08 | 0.003 | Referent | −0.14±0.08 | 0.073 |
| Boston Naming Test-30 Item | −0.03±0.03 | 0.377 | −0.003±0.08 | 0.973 | Referent | −0.06±0.09 | 0.465 |
| Trail Making Test Part B-Part A | −0.01±0.04 | 0.806 | −0.12±0.10 | 0.202 | Referent | −0.24±0.10 | 0.017 |
| WAIS-R Similarities Subtest | 0.03±0.03 | 0.425 | −0.15±0.08 | 0.069 | Referent | −0.10±0.08 | 0.203 |
| Hooper Visual Organization Test | 0.01±0.03 | 0.886 | −0.27±0.08 | 0.001 | Referent | −0.15±0.08 | 0.078 |
Note:
based on n=391;
SE=standard error; WMH=white matter hyperintensities; Models adjusted for age, sex, systolic blood pressure, smoking status, diabetes mellitus, hypertension treatment and atrial fibrillation
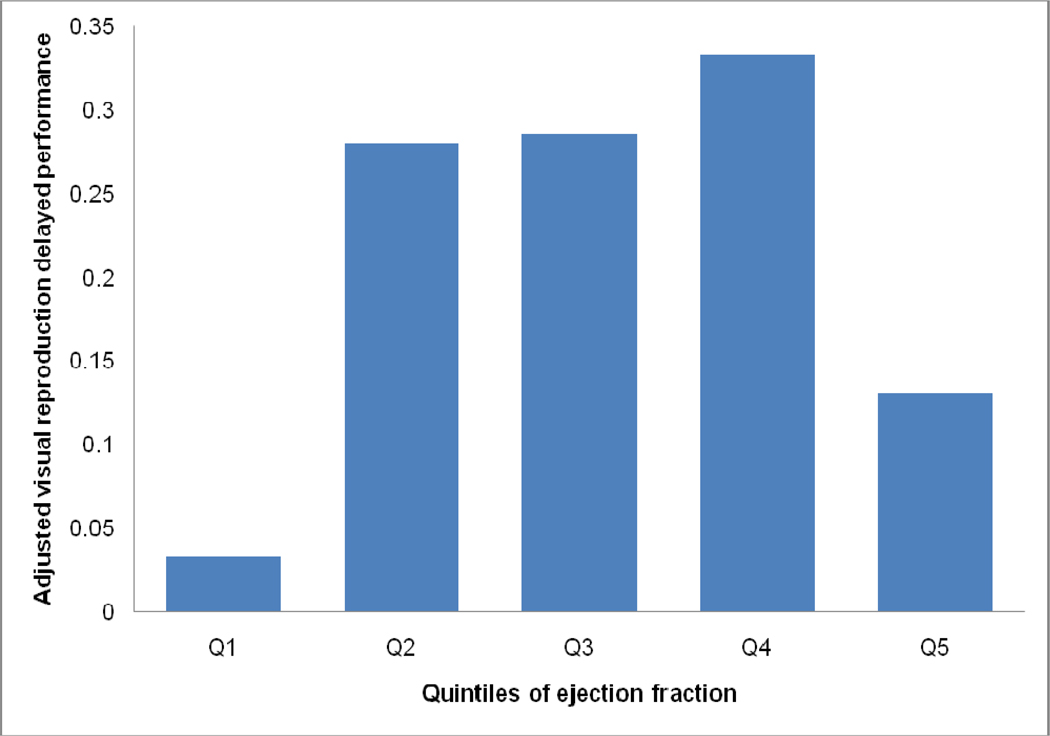
When LVEF quintiles were compared to assess associations with brain aging variables, participants in Q1 did not differ from the referent group (Q2–Q4) for any of the brain MRI variables (Table 3). However, participants in Q1 differed from the referent group for Visual Reproduction Delayed Recall (p<0.001) and Hooper Visual Organization Test (p<0.001; Table 3), such that lower LVEF values were associated with poorer mean cognitive performance. When participants with prevalent CVD were excluded, findings were similar (Table 4). As compared to the referent, participants in Q5 performed more poorly on Logical Memory Delayed Recall (p=0.03), Visual Reproduction Delayed Recall (p=0.03), Trail Making Test Part B-Part A (p=0.02), and the Hooper Visual Organization Test (p=0.02, Table 3). The non-linear association between LVEF quintiles and Visual Reproduction Delayed Recall is illustrated in the Figure 2. When analyses were repeated excluding participants with prevalent CVD, findings were similar (Table 4).
Figure 2.
Mean Visual Reproduction Delayed performance (with standard error bars) adjusted for age, sex, systolic blood pressure, cigarette smoking status, diabetes mellitus, hypertension treatment, atrial fibrillation, and prevalent CVD is depicted by quintile of LVEF. The referent (Quintiles 2–4) is significantly different from Q1 (p<0.001) and Q5 (p=0.03)
To determine if clinically low LVEF accounted for the association between Q1 and cognition, participants in Q1 were dichotomized: <55% LVEF (n=41) and ≥55% LVEF (n=184). Compared to the referent (Q2–Q4), the lowest (<55%) LVEF subgroup had worse Visual Reproduction Delayed Recall (β=−0.42, p=0.01) but not Hooper Visual Organization Test performance (β=−0.16, p=0.34). However, the low normal (≥55%) LVEF subgroup had worse Visual Reproduction Delayed Recall (β=−0.24, p=0.004) and Hooper Visual Organization Test performances (β=−0.29, p<0.001) as compared to the referent. In post-hoc analyses, the Q1 subgroups did not significantly differ for Hooper Visual Organization Test (p=0.49) or Visual Reproduction Delayed Recall performances (p=0.30). Findings were similar when excluding individuals with prevalent CVD.
To better understand the observed U-shaped association (and the relation between Q5 LVEF and worse cognitive performances), the multivariable adjusted three-category models were repeated, excluding participants with prevalent CVD and adding heart rate, C-reactive protein, body mass index (BMI), cardiac index,18 and height-indexed LV mass as covariates, which resulted in strengthened statistical significance of the primary findings (see Supplemental Table). The frequency of both mitral and aortic regurgitation was not disproportionately higher or lower in the highest LVEF quintile.
There was an interaction between sex and the categorical LVEF variable (Q1, Q2–Q4, Q5) in their association with Boston Naming Test-30 item (p=0.03); however, there was no effect in stratified analyses (all p-values >0.09). No interactions were observed between LVEF category and age or APOE-ε4 status in relation to the brain aging variables.
Comment
Our epidemiological findings suggest a U-shaped association, rather than a linear relation, between LVEF and markers of abnormal brain aging. Participants in both the lowest and highest LVEF quintiles had cross-sectional evidence of abnormal cognitive changes as compared to the middle referent group. The observation that lower LVEF is associated with abnormal brain changes extends prior literature examining patients with severe cardiomyopathies, which reported reduced LVEF was associated with memory,4,21 reasoning,22 and sequencing impairments.22 In the absence of clinical heart failure and prevalent CVD, our findings suggest that lower levels of LVEF are also related to abnormal brain aging. It is noteworthy that the lowest quintile of LVEF (which had significant associations with visuospatial memory and object recognition) included a majority of participants with clinically normal values (i.e., 55–62%). The observation that even low normal values of systolic function can be associated with cross-sectional markers of abnormal brain aging is consistent with our recent work reporting low normal values of cardiac index are associated with smaller brain volumes.18
The mechanism underlying associations between lower resting LVEF and abnormal brain aging is unknown. Despite auto-regulatory mechanisms, cerebral blood flow values are low in heart transplant candidates but return to normal following heart transplantation.23 Disruption of cerebral perfusion may contribute to clinical or subclinical brain injury by propagating or exacerbating cerebrovascular disease, including alterations in microvessel structure, expression of vascular cell receptors, microvessel permeability changes, and vascular remodeling.24,25 Chronic cerebral hypoperfusion in animals leads to the development8,9 and progression8 of white matter changes. Another pathological mechanism could be AD, as rats develop AD-related neuropathology, including diffuse beta-amyloid peptide and amyloid precursor protein expression in the hippocampus, entorhinal cortex, and neocortex, following acute cessation of blood flow.26 Chronic cerebral hypoperfusion places the brain at risk for amyloid deposition, resulting in neuronal death in transgenic AD mice.27 More research is needed to understand the mechanism accounting for the associations reported here.
An unexpected observation from the current study was that participants with the highest (top quintile) LVEF values also had poorer cognitive performances in verbal and visuospatial memory, executive functioning, and visuoperceptual abilities as compared to the referent. These findings persisted despite adjusting for multiple covariates, excluding participants with prevalent CVD, and post-hoc consideration of additional possible confounders (e.g., enhanced inflammatory process, greater BMI, lower cardiac index, or LV hypertrophy). The mechanism underlying this observation is unknown. Whereas healthy LVEF values may be good for brain health, very high LVEF values may correspond to subtle cognitive impairment. Alternatively, our observation may reflect an epiphenomenon or another pathological process that was not analytically considered in our models, such as anemia or thyroid disease.28 The observed U-shaped association between LVEF and cognitive aging requires further study, including the clinical significance of cognitive impairment, such as early functional loss.29
Our study has several strengths, including the large community-based cohort free of clinical dementia and stroke, comprehensive ascertainment of possible confounders, innovative cardiac imaging, rigorous quality control procedures, and core reading laboratory for processing measurements, blinded to the participants’ cognitive status. However, there are methodological limitations. The cohort is predominantly white, of European descent, and middle-aged to elderly, so the generalizability to other races, ethnicities, and age groups is unknown. The ambulatory nature of the cohort, exclusion of participants with clinical stroke or dementia, and inclusion of individuals willing to undergo MRI yielded a healthier sample, reducing the likelihood of detecting relations that may be present in individuals with more comorbities. The smaller dataset available for analyses relating LVEF to hippocampal volume may have been insufficiently powered. Analyses were cross-sectional and observational; hence, we are unable to establish a causal connection between cardiac function and brain measures. The potential for false positive findings given the multiple statistical tests is also a concern. By accounting for multiple potential confounders, we may have ‘over-adjusted’ our models, as LVEF may predispose to cognitive impairment through intermediate mechanisms, such as hypertension or diabetes. Finally, the cardiac MRI data were acquired on average 2.5 years prior to the brain MRI and neuropsychological data.
Supplementary Material
Acknowledgments
Grant support: NHLBI’s Framingham Heart Study N01-HC25195; AG027480, AG030962 (Paul B. Beeson Career Development Award in Aging Program), Alzheimer’s Association IIRG-08-88733 (ALJ); P30-AG013846 (Boston University Alzheimer’s Disease Core Center (ADCC)); AG08122, NS017950, AG033040, AG16495 (PAW); AG028321, HL092577 (EJB); AG033193, AG031287 (SS); AG021028 (CD), AG010129 (University of California at Davis ADCC); HL070279 (WJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zuccala G, Cattel C, Manes-Gravina E, Di Niro MG, Cocchi A, Bernabei R. Left ventricular dysfunction: a clue to cognitive impairment in older patients with heart failure. J Neurol Neurosurg Psychiatry. 1997;63:509–512. doi: 10.1136/jnnp.63.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogels RL, van der Flier WM, van Harten B, Gouw AA, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Brain magnetic resonance imaging abnormalities in patients with heart failure. Eur J Heart Fail. 2007;9:1003–1009. doi: 10.1016/j.ejheart.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 3.Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- 4.Deshields TL, McDonough EM, Mannen RK, Miller LW. Psychological and cognitive status before and after heart transplantation. Gen Hosp Psychiatry. 1996;18:62S–69S. doi: 10.1016/s0163-8343(96)00078-3. [DOI] [PubMed] [Google Scholar]
- 5.Massaro AR, Dutra AP, Almeida DR, Diniz RV, Malheiros SM. Transcranial Doppler assessment of cerebral blood flow: effect of cardiac transplantation. Neurology. 2006;66:124–126. doi: 10.1212/01.wnl.0000191397.57244.91. [DOI] [PubMed] [Google Scholar]
- 6.Cahn DA, Sullivan EV, Shear PK, Marsh L, Fama R, Lim KO, Yesavage JA, Tinklenberg JR, Pfefferbaum A. Structural MRI correlates of recognition memory in Alzheimer's disease. J Int Neuropsychol Soc. 1998;4:106–114. doi: 10.1017/s1355617798001064. [DOI] [PubMed] [Google Scholar]
- 7.White KG, Ruske AC. Memory deficits in Alzheimer's disease: the encoding hypothesis and cholinergic function. Psychon Bull Rev. 2002;9:426–437. doi: 10.3758/bf03196301. [DOI] [PubMed] [Google Scholar]
- 8.Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizaki K, Adachi K, Kataoka S, Watanabe A, Tabira T, Takahashi K, Wakita H. Chronic cerebral hypoperfusion induced by right unilateral common carotid artery occlusion causes delayed white matter lesions and cognitive impairment in adult mice. Exp Neurol. 2008;210:585–591. doi: 10.1016/j.expneurol.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 11.DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274–284. doi: 10.1097/00004728-199203000-00018. [DOI] [PubMed] [Google Scholar]
- 12.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 13.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D'Agostino R, Wolf PA. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, Yoshita M, Rosenberg IH, D'Agostino RB, DeCarli C. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65:642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeCarli C, Murphy DG, Gillette JA, Haxby JV, Teichberg D, Schapiro MB, Horwitz B. Lack of age-related differences in temporal lobe volume of very healthy adults. AJNR Am J Neuroradiol. 1994;15:689–696. [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 17.Chuang ML, Hibberd MG, Salton CJ, Beaudin RA, Riley MF, Parker RA, Douglas PS, Manning WJ. Importance of imaging method over imaging modality in noninvasive determination of left ventricular volumes and ejection fraction: assessment by two- and three-dimensional echocardiography and magnetic resonance imaging. J Am Coll Cardiol. 2000;35:477–484. doi: 10.1016/s0735-1097(99)00551-3. [DOI] [PubMed] [Google Scholar]
- 18.Jefferson AL, Himali J, Beiser A, Au R, Massaro J, Seshadri S, DeCarli C, O'Donnell C, Benjamin E, Wolf P, Manning W. Cardiac index is associated with brain aging: The Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease, Section 34. (NIH) 1987:87–2703.
- 20.Hixon JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 21.Roman DD, Kubo SH, Ormaza S, Francis GS, Bank AJ, Shumway SJ. Memory improvement following cardiac transplantation. J Clin Exp Neuropsychol. 1997;19:692–697. doi: 10.1080/01688639708403754. [DOI] [PubMed] [Google Scholar]
- 22.Putzke JD, Williams MA, Daniel JF, Foley BA, Kirklin JK, Boll TJ. Neuropsychological functioning among heart transplant candidates: a case control study. J Clin Exp Neuropsychol. 2000;22:95–103. doi: 10.1076/1380-3395(200002)22:1;1-8;FT095. [DOI] [PubMed] [Google Scholar]
- 23.Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 24.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi T, Deguchi K, Nagotani S, Zhang H, Sehara Y, Tsuchiya A, Abe K. Cerebral ischemia and angiogenesis. Curr Neurovasc Res. 2006;3:119–129. doi: 10.2174/156720206776875902. [DOI] [PubMed] [Google Scholar]
- 26.Pluta R. The role of apolipoprotein E in the deposition of beta-amyloid peptide during ischemia-reperfusion brain injury. A model of early Alzheimer's disease. Ann N Y Acad Sci. 2000;903:324–334. doi: 10.1111/j.1749-6632.2000.tb06383.x. [DOI] [PubMed] [Google Scholar]
- 27.Aliev G, Smith MA, de la Torre JC, Perry G. Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer's disease. Mitochondrion. 2004;4:649–663. doi: 10.1016/j.mito.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, Seshadri S. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med. 2008;168:1514–1520. doi: 10.1001/archinte.168.14.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson AL, Byerly LK, Vanderhill S, et al. Characterization of activities of daily living in individuals with mild cognitive impairment. American Journal of Geriatric Psychiatry May. 2008;16(5):375–383. doi: 10.1097/JGP.0b013e318162f197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.