Endothelium exerts diverse effects within the vessel wall and on nearby target cells (1). Through these effects endothelial cells inhibit vascular tone and vascular growth, protect against thrombosis and progression of atherosclerosis, as well as increases in blood pressure (1,2). Much of the influence of endothelium on other cells is mediated by intercellular signals carried by diffusible factors. Of these endothelium-derived signals, nitric oxide (NO) produced by the endothelial form of NO synthase (eNOS) plays a particularly prominent role in both large and small blood vessels in diverse experimental models and normal humans (1,3–5). Impairment of eNOS-driven signaling underlies vascular dysfunction in a broad spectrum of diseases and is a risk factor for cardiovascular events and stroke (1,2).
The function of eNOS is regulated at many levels including expression of mRNA and protein, post-translational modifications, subcellular trafficking, substrate and cofactor availability, associations with other protein regulators, as well as interactions of NO with superoxide (1,6). A key set of mechanisms that regulate activity of eNOS involves reversible phosphorylation of specific amino acid residues (mainly serine, threonine and tyrosine residues) resulting in increases or decreases in eNOS activity. Kinase-dependent and phosphatase-mediated dephosphorylation of these residues are a common element of cell signaling in general. Within the eNOS molecule, there are multiple phosphorylation sites, including Ser 116 (Ser 114 and 116 in human and bovine eNOS, respectively) in the oxygenase domain of the enzyme. Phosphorylation of Ser 116 inhibits activity of eNOS while dephosphorylation of the same residue increases activity (7)(Figure).
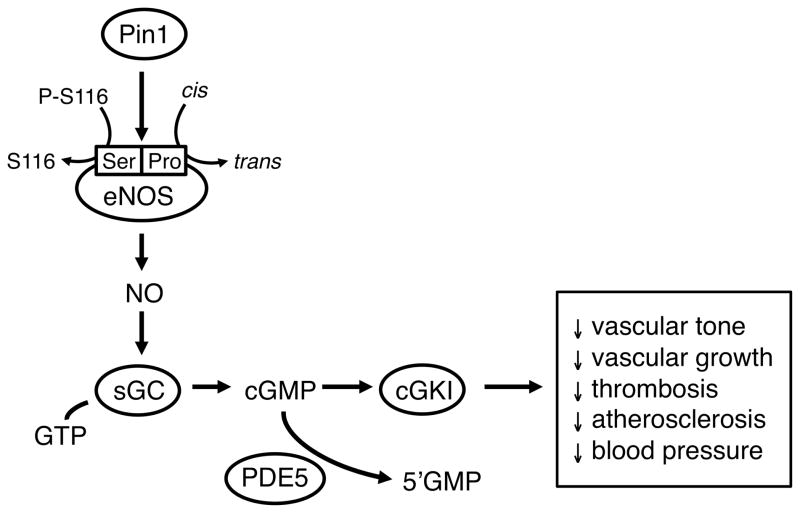
Figure.
Mechanisms that regulate production of nitric oxide (NO) by endothelial NO synthase (eNOS). Activity of eNOS is dependent in part on the phosphorylation status of serine 116 (S116 or Ser116) in the oxidase domain of the molecule (P-S116 = phosphorylated S116). Pin1 (a prolyl-peptidyl cis/trans isomerase) recognizes the P-Serine-Proline motif and promotes the removal of the inhibitory phosphate group from P-Ser116 leading to eNOS activation. Once produced, the primary molecular target for NO is soluble guanylate cyclase (sGC), which produces cGMP from GTP. A key target for cGMP is cGMP-dependent protein kinase I (cGKI). Steady-state levels of cGMP are also affected by activity of type 5 phosphodiesterase. (PDE5). Major effects of eNOS- and cGMP-mediated signaling are also listed.
A major post-translational modification of eNOS involves proline directed protein kinases that recognize and phosphorylate a Ser/Thr-Pro motif (8)(Figure). Proline has unique stereochemistry where it can exist in cis or trans conformations. The conversion between these two conformations is catalyzed by peptidyl-prolyl cis/trans isomerases, which may act as a “molecular switch” where enzymatic activity is conformation-specific (ie, trans = on, cis = off). While in the “on” state, local structural changes may occur and allow other post-translational modifications to further affect enzyme activity. Pin1 is a prolyl-peptidyl cis/trans isomerase that regulates the dephosphorylation of proteins through isomerization of peptide bonds that link phosphoserine or phosphothreonine to proline (8). The result of this interaction is phosphorylation-dependent conformation changes. In relation to eNOS specifically, a very recent study provided evidence that Pin1 binds with and regulates activity of eNOS through site-specific phosphorylation of Ser 116 (7) resulting in changes to both basal and agonist-induced NO production (Figure). These conclusions were based on pharmacological and molecular approaches using cultured endothelium and isolated aorta.
In this issue of Hypertension, Chiasson et al (9) interrogate this concept further while adding new approaches. Using a series of complementary methods, they studied endothelium and aorta in culture as well as performing experiments designed to evaluate the impact of Pin1 in vivo. There were several major findings. First, they confirmed the interaction between Pin1 and eNOS and then found that knockdown (using RNAi) or pharmacological inhibition [using juglone (5-hydroxyl-1,4-napthoquinone)] of Pin1 increased eNOS Ser 116 phophorylation, reduced NO production, and decreased endothelium-dependent relaxation to acetylcholine. These findings are consistent with those of Ruan et al (7) that highlight the complexity of eNOS regulation and support the concept that Pin1 affects basal and agonist induced eNOS activity (Figure).
Second, Chiasson et al (9) found that systemic treatment of normal mice with juglone for two weeks had effects on eNOS activity in aorta that were similar to those seen in analogous culture experiments. Importantly, juglone treatment also caused a progressively developing hypertension. To examine this concept further, blood vessels from mice with genetic deletion of Pin1 were studied. Aorta from Pin1 knockout mice (homozygous knockouts) had increased Ser 116 phosphorylation, reduced NO production, and endothelial dysfunction. Pin1-deficient mice were also hypertensive, exhibiting increases in arterial pressure what were similar in magnitude to those seen following systemic treatment with juglone. Because juglone may exert effects unrelated to Pin1, the observations that genetic knockout of Pin1 and systemic treatment with juglone produced similar effects on aortic function and blood pressure suggest effects of the pharmacological inhibitor were relatively selective in this model. Overall, these new and complementary findings are consistent with the concept that Pin1 normally promotes eNOS function by enabling Ser 116 dephosphorylation (Figure).
What are the implications of this study? These latest findings support the larger concept that while functioning as a key signaling molecule with the vessel wall, activity of eNOS is a major determinant of arterial pressure. Production of NO by eNOS is inversely related to the development of hypertension. Although conclusions related to Pin1 in the vasculature to date have come from work on aorta and cultured endothelium (7), the findings that Pin1 has significant effects on blood pressure strongly suggest that similar mechanisms may be active in smaller resistance vessels.
While many previous studies have focused on the interaction between NO and reactive oxygen species, there has been less attention to the role of alterations in eNOS activity per se in vascular disease. The present study provides another example of the complexity of eNOS regulation and implies that reductions in activity of Pin1, as a consequence of disease or genetic influences (genetic deficiency or polymorphisms), may have important effects on eNOS activity and blood pressure. Oxidative stress is a common feature of vascular disease (1) that may also affect Pin1 function, as oxidation of Pin1 reduces its activity (10).
Lastly, peptidyl-prolyl isomerases play a key role in the immunosuppressive effects of agents like cyclosporine, tacrolimus, and sirolimus (11–13). Elevated blood pressure is a common side effect of using such agents for solid-organ transplantation (11–13). As our understanding of mechanisms of innate immunity and autoimmune disease increase, the use of these pharmacologic agents may increase as well. Thus, understanding mechanisms that underlie hypertension induced by immunosuppressive drugs is clinically relevant. Work in this area also highlights the need for more selective and perhaps cell-specific immunotherapy to achieve clinical goals while avoiding effects on eNOS that may promote vascular dysfunction and hypertension.
Acknowledgments
SOURCES OF FUNDING
The authors work has been supported by National Institutes of Health grants HL-38901, NS-24621, and HL-62984, a Bugher Foundation Award in Stroke from the American Heart Association (0575092N), and an American Heart Association Pre-Doctoral Fellowship (0910131G).
Footnotes
DISCLOSURES
None
References
- 1.Faraci FM. Protecting against vascular disease in brain. The Robert M. Berne distinguished lecture. Am J Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
- 3.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci. 2001;98:12814–12819. doi: 10.1073/pnas.221381098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faraci FM, Sigmund CD, Shesely EG, Maeda N, Heistad DD. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol. 1998;274:H564–H570. doi: 10.1152/ajpheart.1998.274.2.H564. [DOI] [PubMed] [Google Scholar]
- 5.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Janssens SP, Wingler K, Schmidt HHHW, Moens AL. Modulating endothelial nitric oxide synthase: A new cardiovascular therapeutic strategy. Am J Physiol. doi: 10.1152/ajpheart.01315.201. [DOI] [PubMed] [Google Scholar]
- 7.Ruan L, Torres CM, Qian J, Chen F, Mintz JD, Stepp DW, Fulton D, Venema RC. Pin1 prolyl isomerase regulates endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:392–398. doi: 10.1161/ATVBAHA.110.213181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu K, Zhou Z. The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nature Rev Molec Cell Biol. 2007;8:904–916. doi: 10.1038/nrm2261. [DOI] [PubMed] [Google Scholar]
- 9.Chiasson VL, Munshi N, Chatterjee P, Young KJ, Mitchell BM. Pin1 deficiency causes endothelial dysfunction and hypertension. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.111.172338. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA. Oxidative modification and down-regulation of PIN1 in Alzheimer’s disease hippocampus: A redox proteomics analysis. Neurobiol Aging. 2006;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Mange KC, Cizman B, Joffe M, Feldman HI. Arterial hypertension and renal allograft survival. JAMA. 2000;283:633–638. doi: 10.1001/jama.283.5.633. [DOI] [PubMed] [Google Scholar]
- 12.Kasiske BL, Anjum S, Shah R, Skogen J, Kandaswamy C, Danielson B, O’Shaughnessy EA, Dahl DC, Dilkensen JR, Sahadevan M, Sunder JJ. Hypertension after kidney transplantation. Am J Kidney Dis. 2004;43:1071–1081. doi: 10.1053/j.ajkd.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Robert N, Wong GWK, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database of Systematic Reviews. 2010;1:CD007893. doi: 10.1002/14651858.CD007893.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]