Abstract
BACKGROUND
Acute otitis media (AOM) results from a complex interplay between the infectious agents and host immune responses. Cytokines play a major role in the pathogenesis of AOM, but there are limited studies on the systemic cytokine response during AOM.
METHODS
Sera were collected from 145 children (median age = 13.5 months) at the time of diagnosis of AOM. Concentrations of 17 cytokines (IL-1β, -2, -4, -5,-6, -7, -8, -10, -12, -13,-17,GCSF, GM-CSF, IFN-γ, MCP-1, MIP-1β, TNF-α) were determined and correlated with viral etiology and clinical outcome. The statistical analysis was conducted using bioinformatics software.
RESULTS
Cluster patterns of concentrations of cytokines were examined by unsupervised hierarchical clustering algorithms. Four major cluster groups were identified, one the groups was significantly enriched for cases of RSV-induced AOM as compared to other viruses. Specifically, RSV-induced AOM had significantly higher concentrations of G-CSF, MCP-1, IL-10, IL-6, IFN-γ, and IL-8 (P<0.05). Using a decision tree classifier, higher G-CSF concentrations produced 87.6% accuracy to predict RSV-induced AOM. Overall, higher IL-13 concentrations produced 84.2% accuracy to predict early clinical failure of antibiotic treatment.
CONCLUSIONS
Children with AOM have a unique pattern of systemic cytokine response that relates to virus etiology and clinical outcome. Based on G-CSF and IL-13 measurements, it is possible to accurately classify RSV-induced AOM and early treatment failure, respectively; these observations will need to be validated in an independent population.
Keywords: acute otitis media, cytokines, respiratory viruses
INTRODUCTION
Acute Otitis Media (AOM) is one the most common diseases seen in pediatric practice and makes a significant impact on healthcare in the U.S. and worldwide. AOM is a multifactorial disease representing a complex interplay of genetic, environmental and infectious etiologies. Preceding viral upper respiratory infection (URI) is the most important predisposing factor for the bacterial invasion of middle ear resulting in AOM.1 With their immunoregulatory and proinflammatory properties, cytokines may play an important role in the pathogenesis of virus-induced AOM. Numerous cytokines and inflammatory mediators have been detected in the nasopharynx of children with viral URI and in the middle ear fluid of children with AOM.1–2
There are very few published studies with respect to the systemic cytokine responses in children with AOM. Johnston et al reported that children with chronic or recurrent OM have higher serum concentrations of T-helper (Th)-2 cytokines, namely, IL-4 and IL-5, as compared to healthy children.3 We have previously shown that serum IL-6 concentrations are elevated in children with AOM due to Streptococcus pneumoniae.4 However, there are no published studies of systemic concentrations of a large number of cytokines during AOM.
In the present study, we examined the patterns of concentrations of systemic cytokines in children presenting with AOM. The cytokines measured were broadly related to systemic inflammation including those involved in the acute phase response, Th1/Th2 balance and leukocyte chemotaxis (chemokines).
METHODS
Subjects
We studied 145 children, age 3 months to 6 years, who were enrolled in two AOM treatment trials at UTMB, Galveston between 1995 and 2000.5–6 Children were otherwise healthy except for history of otitis media. Children with anatomic and physiologic defect of the ear or nasopharynx, major medical condition, or treatment with other medication were excluded. In addition, none of the subjects had received antibiotics during the preceding week or were treated for AOM in the previous 30 days. The diagnosis of AOM was based on symptoms of fever, irritability or earache, signs of inflammation of the tympanic membrane, and the presence of middle ear fluid (MEF) as documented by otoscopic examination and tympanogram analysis at enrollment.
Of 145 children, 52 were enrolled in one of the treatment trials that included tympanocentesis prior to and 5 days after treatment5; 27 (52%) had had 2 or more AOM episodes. Ninety-three children were enrolled in the second treatment trial for which no tympanocentesis was performed6; therefore, bacteriologic data of the middle ear fluid (MEF) were not available in this group. All children in the second study had at least 2 AOM episodes previously, or had the first AOM episode prior to 6 months of age.
All children were treated with a single dose of intramuscular ceftriaxone and randomized into one of the four adjunctive treatment groups; (chlorpheniramine prednisolone, both or neither). Each child was clinically reassessed at 3–5 days (on-therapy) and 9–12 days (end-of-treatment) after the initiation of antibiotic treatment to document clinical response to antibiotic treatment. Failure of treatment was defined as worsening or recurrent recurrence of AOM signs and symptoms that required a new antibiotic.
Cytokine assay
Sera collected at the initial visit of AOM diagnosis were analyzed by Bio-Plex Human Cytokine 17-Plex Panel (BIO-RAD, Hercules, CA) using the xMAP detection technology. Quantitative analysis was performed for interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-17, tumor necrosis factor (TNF)-α, granulocyte-colony stimulating factor (G-CSF), granulocyte-monocyte-colony stimulating factor (GM-CSF), interferon (IFN)-γ, monocyte chemotactic protein (MCP)-1, and macrophage inflammatory protein (MIP)-1β. Cytokine concentrations were determined based on a simultaneously measured standard curve using a logistic curve fitting algorithm (Bio-Plex Manager 3.0 Software). The detection limits of the cytokines were 0.1–32,000 pg/ml, however, mathematical extrapolation allowed the lower limit measurements of 0.02 pg/ml. The samples that were read as below the range of the lowest detection limit were assigned a value of 0.01 pg/ml for statistical comparison.
Virologic Studies
Viral respiratory pathogens were identified by either viral culture or antigen detection tests of either MEF or nasal wash (NW) samples, or by serology as described previously.7 A fourfold or higher rise in viral titers between acute and convalescent sera was also considered as proof of the viral infection. In children with positive viral findings in the MEF, the specimens from the left and right ears had been combined before viral culture or antigen detection test. A positive virus identification from any one of these samples or serology was considered the viral cause of URI associated with the AOM episode.
Statistical Analysis
The data from 17 cytokine measurements were clustered using unsupervised agglomerative (“bottoms up”) hierarchical clustering as previously published by us.8 This analysis was performed on 50 percentile normalized data by using the unweighted paired group mean with correlation as the similarity measure (Spotfire Decision Site 9.0, Somverille, MA).
To conduct Classification and Regression Trees (CART) analysis, the data was Z-score transformed, and a feed-forward filtering strategy was used. The cytokines were rank ordered based on their nonparametric (Kruskall-Wallis) test statistic (comparing RSV vs non-RSV infection) and used to feed into the CART model. The CART tree was built using the “twoing” rule for splitting (Salford Systems, Inc. San Diego). Accuracy was determined by 10-fold cross validation (WEKA 3.5.6 Software, University of Waikato, Hamilton, New Zealand).
For above modeling, ANOVA with multiple comparisons and Kruskall-Wallis tests were performed by using SAS, versoin 9.1 (SAS Inc, Cary, NC) and SPSS, release 11.0.1 (SPSS Inc, Chicago, IL).
RESULTS
Demographic, virologic and bacteriologic data
The study population included 74 (51%) males and 71 females, with median age of 13.5 months. The virus were identified in 65 (45%) of AOM cases: respiratory syncytial virus (RSV) = 16, adenovirus = 13, influenza A or B = 10, parainfluenza types 1, 2 and 3 = 9, rhinoviruses = 9, and cytomegalovirus = 8. In a subset of 52 who were enrolled in the tympanocentesis study, approximately 70% of children yielded bacteria (S. pneumoniae, H. influenzae, and / or M. catarrhalis) in the MEF5; approximately 83% of children in the study without tympanocentesis6 yielded the pathogenic bacteria in the nasopharyngeal swab specimens at the time of AOM diagnosis. Bacteriologic results were similar to those reported in AOM literature and have been previously described 5, 6 In view the small number of MEF samples studied for bacteria, no further analysis of the impact of bacterial pathogens on cytokine concentrations and clinical outcome was performed.
Cytokine concentrations at initial AOM presentation
Table 1 shows the concentrations of all cytokines in descending order of median values among those with values within the detection limit of the assay. Cytokines that were found in at least 50% of samples within the detection range of the assay were MIP-1β, MCP-1, IL-6. IL-5, IL-7, IL10 and IL-13 (in descending order). The chemokines MCP-1 and MIP-1β correlated positively with each other [correlation coefficient (CR) 0.47; P <0.001]. Similarly, the acute phase cytokines IL-1β, IL-6 and TNF-α correlated with each other (IL-1β vs IL-6, CR = 0.26, P = 0.002; IL-1β vs TNF-α, CR = 0.81, P<0.001; TNF-α vs IL-6 P, CR = 0.28, P <0.001).
Table 1.
Concentrations of 17 serum cytokines in 145 children at the initial visit for AOM
| Serum samples with > 0.02 pg/ml |
||||
|---|---|---|---|---|
| Cytokine | No. Samples (%)* |
Mean ± S.D. | Median (pg/ml) |
Range (min – max) |
| MIP-1β | 145 (100) | 76.8 ± 53.7 | 62.26 | 10.1 – 290.6 |
| MCP-1 | 144 (99) | 48.3 ± 41.3 | 40.40 | 0.18 – 338.2 |
| IL-6 | 129 (89) | 173.4 ± 413.2 | 63.91 | 0.34 – 4035.4 |
| IL-5 | 96 (66) | 5.8 ± 47 | 0.78 | 0.03 – 461.1 |
| IL-7 | 96 (66) | 6.8 ± 34.3 | 3.05 | 0.16 – 338.7 |
| IL-10 | 81 (56) | 56.4 ± 426.2 | 2.70 | 0.07 – 3841 |
| IL-13 | 75 (52) | 7.9 ± 42.4 | 1.90 | 0.04 – 368.7 |
| IL-8 | 62 (43) | 5.6 ± 18.9 | 2.63 | 0.03 – 150.5 |
| IL-1β | 50 (34) | 5.6 ± 13.9 | 1.25 | 0.24 – 88.6 |
| TNF-α | 48 (33) | 13 ± 26.9 | 2.28 | 0.42 – 123.1 |
| IFN-γ | 30 (21) | 83.2 ± 122.9 | 21.46 | 0.36 – 410.6 |
| GM-CSF | 27 (19) | 473.5 ± 1059.5 | 161.60 | 7.5 – 5472.3 |
| IL-4 | 25 (17) | 111.8 ± 198.8 | 24.16 | 1.3 – 760.4 |
| G-CSF | 19 (13) | 73.4 ± 87.2 | 37.01 | 0.56 – 278.7 |
| IL-2 | 16 (11) | 71 ± 98.4 | 30.17 | 0.23 – 390 |
| IL-17 | 3 (2) | 34 ± 56.6 | 1.84 | 0.95 – 99.4 |
| IL-12 | 2 (1) | 194 ± 259.5 | 194.03 | 10.6 – 377.5 |
Figures in parenthesis are percent samples in detectable range out the total 145 samples;
The lowest limit of detection was 0.02 pg/ml, samples with results below this level are not shown above;
S.D. = standard deviation
Hierarchical clustering
To reveal the presence of any common grouping of cytokine profile with respect to viral infection at AOM diagnosis or success or failure of AOM treatment, we analyzed the 17 cytokine measurements by unsupervised agglomerative hierarchical clustering (Figure 1). Four groups (G) were identified, with G2 and G4 containing the majority of subjects. From inspection of the clustering data, the G2 group contained high values for IL-1β, TNF-α, IL-8 and IL-13. Similarly, the G4 group contained high values of IL-2, IL-4, IFN-γ, GM-CSF and G-CSF.
Figure 1. Unsupervised hierarchical clustering of serum cytokine values.
Each row is an individual serum sample from 145 children. The intensity of the color (from light green, red to black) indicates the relative concentration of each cytokine. Dendogram shows similarity of the closely related samples. Four groups are identified, with Groups (G) 2 and - 4 containing the majority of individuals.
Features of virus-induced AOM
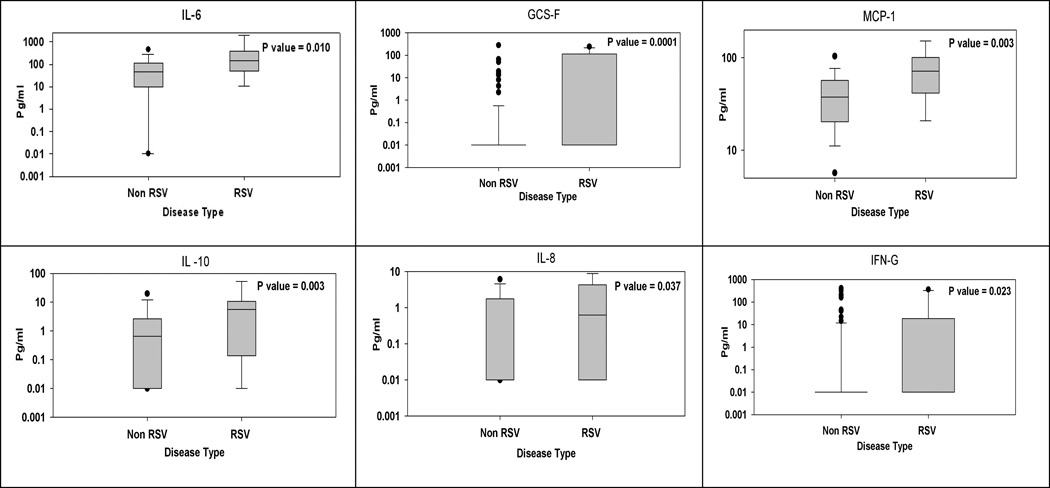
Comparison of the above hierarchical cluster groups was found to be significantly different for RSV as compared to non-RSV group, with 18.8% of RSV versus 51.2% of non-RSV cases in G2, and 68.8% of RSV versus 45.7% of non-RSV cases in G4 (Table 2). The cytokine measurements in the RSV group versus non-RSV group were further compared using a non-parametric t-test. Six cytokines, namely, G-CSF, MCP-1, IL-10, IL-6, IFN-γ, and IL-8, were significantly higher in the RSV group than in the non-RSV group (Figure 2).
Table 2.
Comparison of hierarchical cluster groups of cytokine concentrations in RSV and non-RSV induced AOM
| Group | Non-RSV AOM* (N = 129) |
RSV AOM** (N = 16) |
|---|---|---|
| 1 | 2 (1.6%) | 2 (12.5%) |
| 2 | 66 (51.2%) | 3 (18.8%) |
| 3 | 2 (1.6%) | 0 (0%) |
| 4 | 59 (45.7%) | 11 (68.8%) |
The groupings are based on the cluster pattern identified in Figure 1;
Figures in parenthesis indicate percent of the column total;
Non-RSV group included 49 cases of other viruses, and 80 cases with no virus identified;
RSV versus Non-RSV groups, p = 0.011 (Chi square analysis)
Figure 2. Group-wise comparison of serum cytokines in RSV (n = 16) versus non-RSV (n = 129) induced AOM.
Shown are box and whisker plots of 6 cytokines that showed between-group differences.
We next sought to classify RSV-from non-RSV viral infections by decision tree analysis using CART. Using this strategy, we obtained a classification model that grouped subjects into RSV-induced AOM and non-RSV-induced AOM on the basis of a single split based on the G-CSF measurement alone (Figure 3). With higher G-CSF concentrations, 53.8% of the AOM cases were due to RSV, while in those with lower G-CSF concentrations, only 6.8% of the cases were due to RSV. The overall accuracy of the classifier (based on the accuracy of predicting RSV and that of predicting non-RSV classes in 10-fold cross-validation) was 87.6%.
Figure 3. CART Classification of G-CSF.
Decision performed on Z score normalized cytokine data. G-CSF is the sole contributor to the model and accurately classifies RSV from non-RSV. Each terminal node shows the percent of RSV and Non RSV identified by the split criteria.
Relationship with clinical outcome
CART decision tree analysis was also employed to obtain a classification model that grouped subjects into clinical improvement or failure (ongoing or worsening AOM) at the on-treatment visit (3–5 days). Of 139 subjects available for assessment at this visit, 14 (10%) subjects had clinical failure of treatment, but this was not related to any specific assignment to a treatment category (data not shown). A best single split for AOM improvement versus failure was achieved by the measurement of IL-13 alone (Figure 4). With higher IL-13 concentrations at the initial visit, 38.1% subjects had failure of treatment, while with lower IL-13 concentrations, 5.1% of subjects had failure. The overall accuracy of the classifier based on the accuracy of predicting early, on-treatment improvement or failure of AOM treatment was 84.2%.
Figure 4. CART Classification of IL-13.
Decision performed on Z score normalized cytokine data. IL-13 is the sole contributor to the model and accurately classifies AOM treatment failure from success. Each terminal node shows the percent of failure and success identified by the split criteria. ‘Sym’ = symptomatic at the on-treatment visit at 3–5 days (early treatment failure).
The pre-treatment IL-6 concentrations had the highest accuracy (78%) of predicting clinical improvement at the end-of-treatment visit (9–12 days), however, as the CART model was excessively trained, only limited conclusions can be drawn. Additional analysis showed that the levels of serum cytokines at the pretreatment visit were not associated with the end-of-treatment clinical outcome.
DISCUSSION
In the present study, we report results of the largest panel of cytokines studied to-date in the sera of children with AOM. Among 17 cytokines analyzed, MIP-1β, MCP-1, IL-6. IL-5, IL-7, IL10 and IL-13 were found in at least 50% of the samples. G-CSF was the predominant cytokine that predicted RSV-associated AOM, while IL-13 predicted early treatment response. These are important findings as there have been no prior studies of systemic cytokine responses in relation to viral causes associated with or treatment response for AOM.
There are very few prior studies of systemic concentrations of a limited number of cytokines during AOM episodes. Chkhaidze et al studied sera of 14 adolescents and adults with AOM for IL-6 and TNF-α; only TNF-α levels were significantly elevated when compared with controls.9 We have previously shown in a study of 184 children with AOM that serum IL-6 levels are significantly higher in children with S. pneumoniae when compared with other bacterial pathogens in MEF.3 Scharer et al studied sera of 10 children with AOM due to S. pneumoniae for IL-1, IL-6, IL-8 and TNF-α; only IL-6 concentrations were significantly elevated at presentation.10 Our present study did not take into account the bacterial cause of AOM as tympanocentesis was not peformed in all subjects. Additional studies are needed to analyze the specific interactions between bacteria and viruses in relation to the systemic cytokine pattern.
Our study suggests that there is a role for G-CSF in RSV-induced AOM. Elliott et al have shown that G-CSF is present in the nasopharyngeal secretions during RSV and parainflueza virus infection and its quantity correlates negatively with the severity of hypoxic bronchiolitis.11 In vitro, G-CSF is produced by RSV-infected airway epithelial cells and monocytes isolated from peripheral blood.12 G-CSF is known to promote inflammatory cell survival and differentiation, however, what specific role it plays in the pathogenesis of RSV infection and development of AOM is not clear. Furthermore, it is not clear why the G-CSF response for RSV is different form other respiratory viruses associated with AOM.
In the present study, AOM due to RSV was associated with significantly higher concentrations of IL-6, MCP-1, IL-10, IFN-γ and IL-8, when compared with other viruses. This is in contrast to the observations of Oda et al- sera of hospitalized children with lower airway infection due to adenovirus contained significantly higher quantities of IL-6 as compared to RSV infection.13 A possible explanation is that systemic cytokine responses involved in the middle ear and lower airway inflammation are different.
The role of IL-13 in AOM treatment failure shown in our study is intriguing. Indeed, the role of IL-13 in AOM pathogenesis has never been investigated. IL-13 may play an important role in AOM because of its ability to mediate an allergic type Th2 response leading to IgE synthesis. It is possible that children producing higher quantities of IL-13 have an underlying allergic diathesis; these children may produce an allergic type inflammatory response to acute infection in the middle ear that is not altered by the use of antibiotics, hence leading to clinical failure. The role of other Th2 cytokines in chronic middle ear disease has been reported previously: Johnston et al have shown that children with chronic or recurrent otitis media have higher serum concentrations of Il-4 and IL-5.3 In a similar population, Bernstein et al have shown that adenoid and blood lymphocytes produce larger quantities of IL-4 and IL-5.14
Systemic as well as local cytokine responses in the nasopharynx and middle ear probably act in concert in the pathogenesis of AOM. However, the quantitative and temporal responses in various sites may not correlate with each other. Hayden et al reported that in adult volunteers experimentally infected with influenza virus, the different cytokines peak at different time points.15 In the nasopharynx, IL-6 and IFN-α peaked earlier than TNF-α and IL-8. In serum, IL-6 and TNF-α peaks occurred at the same time point as nasopharynx, but the levels were lower than in the nasopharynx; and IFN-α and IL-8 were undetectable. These observations suggest that different cell types may be involved in systemic and local cytokine responses at different time points of infection.
In summary, our study shows that cytokines that mediate acute phase response, Th1/Th2 balance and leukocyte chemotaxis (chemokines) are present in the sera during the acute phase of AOM. Association between G-CSF and RSV infection suggests that the cytokine response varies according to the virus type. Furthermore, Th2 cytokines such as IL-13 may play a role in AOM treatment failure. Additional studies are needed to compare the local and systemic cytokine responses during AOM as well as virus-bacteria interactions, and to further delineate the risk factors that predict the development of AOM and subsequent recovery.
ACKNOWLEDGEMENT
The authors thank the children and their families, nurses and technical staff involved in the conduct of this study, and to Shauna Reid for assistance with data management.
Financial Support :
This work was supported by the National Institutes of Health grant R01 DC 2620 (to T.C.). The study was conducted at the General Clinical Research Center at the University of Texas Medical Branch at Galveston, funded by grant M01 RR 00073 from the National Center for Research Resources, NIH. Informatics support was partially provided by the Integrated Health Science Facility Core (P30 ES06676, to J. Halpert, UTMB).
REFERENCES
- 1.Heikkinen T, Chonmaitree T. Importance of respiratory viruses in acute otitis media. Clin Microbiol Rev. 2003;16:230–241. doi: 10.1128/CMR.16.2.230-241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noah TL, Henderson FW, Wortman IA, et al. Nasal cytokine production in viral acute upper Respiratory infection of childhood. J Infect Dis. 1995;171:584–592. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 3.Johnston BN, Preciado DA, Ondrey FG, Daly KA. Presence of otitis media with effusion and its risk factors affect serum cytokine profile in children. International Journal of Pediatric Otorhinolaryngology. 2008;72:209–214. doi: 10.1016/j.ijporl.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Heikkinen T, Ghaffar F, Okorodudu AO, Chonmaitree T. Serum Interleukin-6 in Bacterial and Nonbacterial Acute Otitis Media. Pediatrics. 1998;102:296–299. doi: 10.1542/peds.102.2.296. [DOI] [PubMed] [Google Scholar]
- 5.Chonmaitree T, Saeed K, Uchida T, Heikkinen T, Baldwin CD, Freeman DH, Jr, McCormick DP. A randomized, placebo-controlled trial of the effect of antihistamine or corticosteroid treatment in acute otitis media. J Pediatr. 2003;143:377–385. doi: 10.1067/S0022-3476(03)00293-2. [DOI] [PubMed] [Google Scholar]
- 6.McCormick DP, Saeed K, Uchida T, Baldwin CD, Deskin R, Lett-Brown MA, Heikkinen T, Chonmaitree T. Middle ear fluid histamine and leukotriene B4 in acute otitis media: effect of antihistamine or corticosteroid treatment. Int J Pediatr Otorhinolaryngol. 2003;67:221–230. doi: 10.1016/s0165-5876(02)00372-5. [DOI] [PubMed] [Google Scholar]
- 7.Patel JA, Nguyen DT, Revai K, Chonmaitree T. Role of respiratory syncytial virus in acute otitis media: implications for vaccine development. Vaccine. 2007;25:1683–1689. doi: 10.1016/j.vaccine.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brasier AR, Victor S, Boetticher G, Ju H, Lee C, Bleecker ER, Castro M, Busse WW, Calhoun WJ. Molecular phenotyping of severe asthma using pattern recognition of bronchoalveolar lavage-derived cytokines. J Allergy Clin Immunol. 2008;121:30–37. doi: 10.1016/j.jaci.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chkhaidze I, Nemsadze K, Gotsadze K, Nikoleishvili E, Gordeladze G. Systemic inflammatory responses in patients with acute otitis media and the impact of treatment with sinupret. Georgian Med News. 2007;151:40–44. [PubMed] [Google Scholar]
- 10.Scharer G, Zaldivar F, Gonzalez G, Vargas-Shiraishi O, Singh J, Arrieta A. Systemic inflammatory responses in children with acute otitis media due to Streptococcus pneumoniae and the impact of treatment with clarithromycin. Clin Diagn Lab Immunol. 2003;10:721–724. doi: 10.1128/CDLI.10.4.721-724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott MB, Welliver RC, Sr, Laughlin TS, Pryharski KS, LaPierre NA, Chen T, Souza V, Terio NB, Hancock GE. Matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 in the respiratory tracts of human infants following paramyxovirus infection. J Med Virol. 2007;79:447–456. doi: 10.1002/jmv.20830. [DOI] [PubMed] [Google Scholar]
- 12.Soukup JM, Becker S. Role of monocytes and eosinophils in human respiratory syncytial virus infection in vitro. Clin Immunol. 2003;107:178–185. doi: 10.1016/s1521-6616(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 13.Oda K, Yamamoto Y. Serum interferon-gamma, interleukin-4, and interleukin-6 in infants with adenovirus and respiratory syncytial virus infection. Pediatr Int. 2008;50:92–94. doi: 10.1111/j.1442-200X.2007.02522.x. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein JM, Ballow M, Xiang S, O'Neil K. Th1/Th2 cytokine profiles in the nasopharyngeal lymphoid tissues of children with recurrent otitis media. Ann Otol Rhinol Laryngol. 1998;107:22–27. doi: 10.1177/000348949810700105. [DOI] [PubMed] [Google Scholar]
- 15.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]