Abstract
Context
Vernonia amygdalina Del. (VA; Asteraceae or Compositae) is a small tree growing throughout tropical Africa. It is widely used for food and medicinal purposes by local people. It was reported that it had several qualities, including anticancer activity.
Objective
A sesquiterpene lactone, vernodalinol, was isolated from VA leaves. The first reported source of vernodalinol was in 2009 from a different plant, only 1H NMR spectrum and no detailed structural analysis was carried out. No whole spectroscopic data were provided.
Materials and methods
VA dried leaves were extracted with 85% ethanol followed by further separation into four fractions by liquid–liquid extraction technique using various solvents: hexane, chloroform, and n-butanol. Vernodalinol was separated from the n-butanol fraction by column chromatography. The biological activity of vernodalinol was evaluated in estrogen receptor-positive (ER+) human breast carcinoma cells (MCF-7) in vitro.
Results
Results indicated that vernodalinol (25 and 50 μg/mL) inhibited breast cancerous cell growth (DNA synthesis) by 34% (P < 0.025) and 40% (P < 0.025), respectively. It is reasonable to expect an LC50 of 70–75 μg/mL for vernodalinol in MCF-7 cells.
Discussion and conclusion
Vernodalinol structure was confirmed using a battery of spectroscopic methods, 1D and 2D NMR, high-resolution mass spectrometry (HR-MS), UV, IR, and X-ray. These results suggest that vernodalinol, although it has some biological activity, is likely to work in concert with other ingredients responsible for the anticancer activity exhibited of VA.
Keywords: Vernonia amygdalina, vernodalinol, sesquiterpene lactone, breast cancer
Introduction
Vernonia amygdalina Del. (VA), a member of the Asteraceae (or Compositae) family, commonly called “bitter leaf,” is a small tree that grows up to 5 m in height. It grows throughout tropical Africa. VA is widely used for food and medicinal purposes. The water extracts of its leaves have been used as tonic drinks to prevent and/or treat various illnesses (Farombi, 2003). Also, it was reported that VA has antioxidant (Oboh, 2005; Iwalokun et al., 2006; Odukoya et al., 2007), hepatoprotective (Iwalokun et al., 2006), antimicrobial (Jisaka et al., 1992b; Akinpelu, 1999), antiparasitic (Koshimizu et al., 1994), antiplasmodial (Tona et al., 2004), and anticancer (Jisaka et al., 1992b; Izevbigie, 2003; Izevbigie et al., 2004) activities.
In our previous research, we reported that VA aqueous extracts potently inhibited the growth of human estrogen receptor-positive (ER+) cell line (MCF-7) in vitro at low concentrations (12.5 μg/mL) by modulating the extracellular signal-regulated kinases 1 and 2 (ERK-1 and -2) activities on MCF-7 cells (Izevbigie et al., 2004), cytochrome P450 enzymes (Howard et al., 2003), and membrane disruption (Opata & Izevbigie, 2006). Also, we showed that using solvent extraction, spectrophotometric, and chromatographic techniques can fractionate and identify anticancer activity markers in VA extracts (Oyugi et al., 2009). With these previous research results, the active components of VA, with anticancer activities, need to be identified in next step. We used the bioassay method from our previous research to track the active components in VA ethanol extract and found that two fractions of ethanol extract showed anti-cancer activities. One was mid-polar fraction, which was chloroform fraction. The other fraction was high-polar fraction, which was n-butanol fraction.
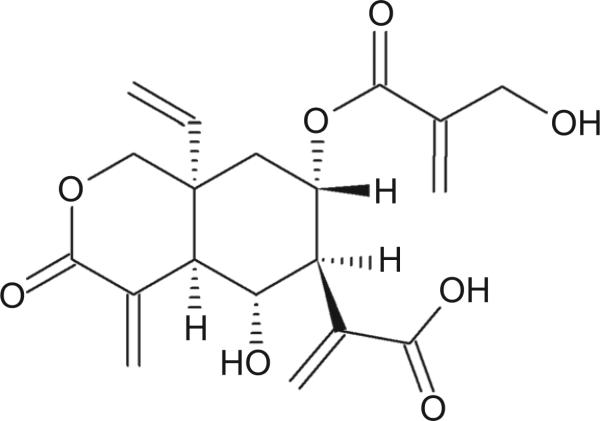
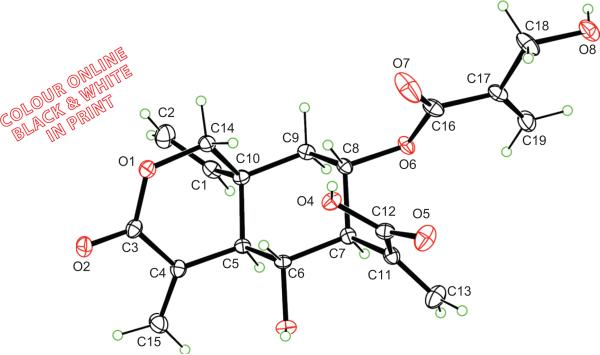
In this study, vernodalinol (Figure 1), (4aR,5R,6S,7S,8aR)-8a-ethenyloctahydro-5-hydroxy-7-[[2-(hydroxymethyl)-1-oxo-2-propen-1-yl]oxy]-α,4-bis(methylene)-3-oxo-1H-2-benzopyran-6-acetic acid (CAS 1158650-97-5), was isolated from n-butanol fraction, which was one of the active fractions of VA ethanol extract. A battery of spectroscopic methods, 1D and 2D NMR, IR, UV, and high-resolution mass spectrometry (HR-MS), was used for unequivocal structural determination. This is the first time obtaining the pure form of vernodalinol and its detailed spectroscopic data. The isolated vernodalinol was also tested for inhibition of DNA synthesis in MCF-7 cells in vitro to see if this compound is the active ingredient for the anticancer activity exhibited by VA.
Figure 1.
The structure of vernodalinol.
Methods
General experimental procedures
The melting point of vernodalinol was measured on the X-4 microscopic melting point apparatus, made by Beijing Tech Instrument. Optical rotation was measured on WXG-4 disc polarimeter, made by Shanghai Precision & Scientific Instrument. UV and IR were measured on Unico (China) UV-2102PCS and Nicolet NEXUS470 FT-IR, respectively. 1H, 13C NMR, gradient heteronuclear single quantum coherence (HSQC), gradient heteronuclear multiple-bond correlation (HMBC), and gradient correlation spectroscopy (COSY) and distortionless enhancement by polarization transfer (DEPT) spectra were recorded on a Varian Unity INOVA 500 FT-NMR at 500 MHz for 1H and 125 MHz for 13C, respectively, in CH3OD with TMS as internal standard. Chemical shifts are given in δ (ppm) and coupling constants in Hz. High-resolution electrospray ionization-mass spectrometry (HR ESI-MS) was obtained on a Agilent 6200 series accurate-mass time-of-flight (TOF) LC/MS. Column chromatography was performed over silica gel (300–400 mesh; Haiyang, China) and thin-layer chromatography (TLC) with silica gel HF254. X-Ray crystal structure analysis was carried out with Nonius Kappa CCD diffractometer equipped with an Oxford Cryostream cryostat.
Plant material
Pesticide-free, fresh VA leaves were collected in Benin City, Nigeria, following Good Agricultural Practices (GAP) in January 2006 and shipped to United States. The leaves were rinsed with distilled water and spread out evenly on galvanized-wire screens with the edges bent upward 2 in. on all sides. The galvanized-wire screen were placed in a specially constructed dryer and heated to 55–60°C for complete dryness in 4 h. The VA leaves were authenticated by Plant Quarantine Service, Federal Department of Agriculture, Nigeria (Phyto-sanitary Certificate No. IK2006/075).
Extraction and isolation
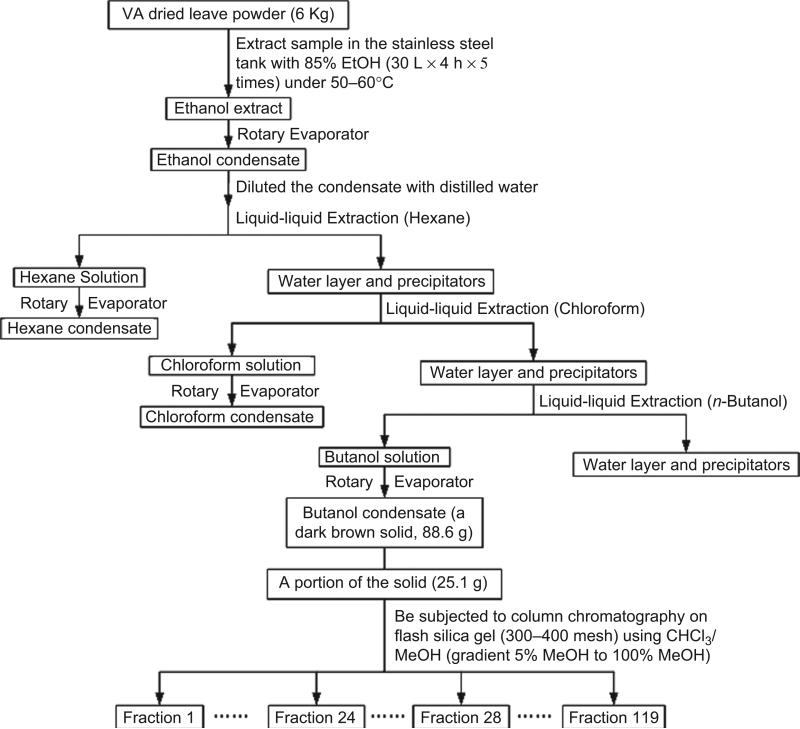
The extraction and isolation processes followed Figure 2. From fractions 24 to 28, brown crystals appeared in the vials. These fractions had the same components shown by TLC analyses (Rf = 0.61 in the 80% chloroform and 20% methanol developing solvents). Combined fractions 24 to 28 (150 mg) were recrystallized five times from anhydrous MeOH in a Teflon crystallizer. After each recrystallization, the crystals were washed with EtOAc and acetone separately. Colorless crystals (120 mg) were obtained.
Figure 2.
The flow chart of vernodalinol isolation.
Inhibition of DNA synthesis assay
DNA synthesis was determined by the incorporation of [3H]-thymidine into cellular nucleic acids as we previously described (Izevbigie et al., 2000, 2002). Cells were grown to about 60% confluence in 35-mm diameter tissue culture plates with RPMI-1640 medium containing 1% antibiotic–antimycotic solution containing 10,000 U/mL penicillin, 10,000 μg/mL streptomycin, and 25 μg/ mL amphotericin B (fungizone), and 10% fetal bovine serum. Stock solution (5 mg/mL) was prepared by dissolving 5 mg of vernodalinol into 1 mL EtOH, and the cells were treated with either 25 or 50 μg/mL final concentrations of vernodalinol. Untreated cells served as negative controls, whereas vehicle-treated cells served as positive controls. The cells were then incubated for 18 h in a 37°C humidified 5% CO2 incubator before the addition of 1 μCi/mL of [3H]-thymidine per 35-mm well, following by an additional 4 h. The incubation was stopped by media aspirated, then followed by three sequential washes with cold phosphate-buffered saline at pH 7.4. Two times (2 mL/35 mm) of ice-cold 10% trichloroacetic acid (TCA) were added and the cells were incubated for 10 min at 4°C. TCA solution was aspirated and the cells were washed three times with ice-cold distilled water, and then solubilized with 2 mL of 0.5 M NaOH solution at 37°C for 30 min. Upon solubilization, 1 mL per well aliquot samples was transferred to scintillation vials into which 5 mL of scintillation cocktail was added. DNA synthesis in treated and controlled cells was quantified by a Liquid Scintillation Analyzer (Try-Carb 2700TR).
Statistical analysis
Data collected were analyzed using one-way analysis of variance (ANOVA) with post test (GraphPad Prism, version 4). Means were further classified using Tukey–Kramer honestly significant difference (HSD) test. P-values of <0.05 were considered significant.
Spectroscopic analyses
The colorless crystals from the above recrystallization has a melting point 217–218°C; <math/> +178.70 (c 0.253, dry MeOH); UV (dry MeOH) λmax (log ε): 235 (2.76) nm; IR (KBr) νmax 3600–2600 (–COOH), 3443 (s, O–H), 3108 (w, =C–H), 3083 (w, =C–H), 1707 (vs C=O), 1626 (m, C=C) cm−1; for 1H and 13C NMR spectroscopic data (CH3OD), see Table 1. HR ESI-MS m/z 379.1383 [M + H]+ (calcd 379.1387 for C19H23O8).
Table 1.
1H and 13C NMR (500 and 125 MHz, methanol-d) data for vernodalinol.
| Position | δH (mult., J, Hz) | δ C | gHMBC |
|---|---|---|---|
| 1 | 5.76 (dd, 11.0, 17.5) | 139.7 | 5, 9, 10, 14 |
| 2 | a: 5.21 (d, 11.0)a | 112.9 | 1, 5, 10 |
| b: 5.25 (d, 17.5)a | 1, 5, 10 | ||
| 3 | — | 163.7 | — |
| 4 | — | 131.4 | — |
| 5 | 2.55 (dd, 10.5, 2.0) | 49.2 | 1, 3, 4, 6, 7, 9, 10, 14, 15 |
| 6 | 4.08 (dd, 11.0, 10.5)b | 67.0 | 4, 5, 7, 11, 15 |
| 7 | 2.74 (dd, 11.5, 11.0)b | 52.7 | 5, 6, 8, 9, 11, 12, 13 |
| 8 | 5.38 (dt, 11.5, 11.5, 4.5) | 68.0 | 7, 9, 11, 16 |
| 9 | a: 1.64 (dd, 13.5, 11.5) | 35.9 | 1, 5, 7, 8, 10 |
| b: 2.01 (dd, 13.5, 4.5) | 1, 5, 7, 8, 10 | ||
| 10 | — | 38.1 | — |
| 11 | — | 136.2 | — |
| 12 | — | 166.5 | — |
| 13 | a: 5.79 (d, 1.0) | 127.0 | 7, 8, 11, 12 |
| b: 6.34 (d, 1.0) | 7, 8, 11, 12 | ||
| 14 | a: 4.42 (dd, 12.0, 2.0) | 69.0 | 1, 3, 5, 9, 10 |
| b: 4.76 (d, 12.0) | 1, 3, 5, 9, 10 | ||
| 15 | a: 5.77 (d, 1.0) | 131.2 | 3, 4, 5, 6, 10 |
| b: 6.54 (d, 1.0) | 3, 4, 5, 6, 10 | ||
| 16 | — | 163.3 | — |
| 17 | — | 138.6 | — |
| 18 | 4.19 (dd, 4.0, 2.0) | 58.5 | 16, 17, 19 |
| 19 | a: 5.85 (dd, 4.0, 3.0) | 122.3 | 16, 17, 18 |
| b: 6.19 (dd, 2.0, 3.0) | 16, 17, 18 |
The gem-coupled resonance between these two hydrogens is invisible in the spectrum.
The coupling constants of H-6 and H-7 are very close and their multiplicities in the spectrum look like triplet, not doublet–doublet.
X-ray structure analysis of vernodalinol
A suitable colorless crystal, grown by slow evaporation of dry MeOH solution in a Teflon crystallizer, was mounted on a Nonius Kappa CCD diffractometer equipped with MoKα radiation (λ = 0.71073 Å) and an Oxford Cryostream low-T device. Crystal data: C19H22O8, Mr = 378.37 g/ mol, monoclinic, P21, a = 6.1981(8) Å, b = 9.7736(10) Å, c = 15.545(2) Å, β = 91.317(7)o, V = 941.4(2) Å3, Z = 2, Dcalc = 1.335 mg/m3, μ (MoKα) = 0.11 mm−1, T = 90 K, crystal size = 0.25 × 0.18 × 0.10 mm3. Total 21,053 reflections were collected in the range 3.2° < θ < 30.9° (Rint = 0.066). The total number of independent reflections measured was 5800, of which 5036 were observed [I > 2σ(I)]. Hydroxyl hydrogen atoms were refined individually. Those on carbons were visible on difference maps, and were placed in idealized positions. The final indices were R1 = 0.040, wR2 = 0.092 with goodness-of-fit = 1.03. The absolute configuration was chosen to be that accepted for sesquiterpene lactones from higher plants (Fischer et al., 1979). Analysis of 2498 Bijvoet pairs by the method of Hooft et al. (2008) based on resonant scattering of the light atoms yielded the parameter y = −0.1(3). This corresponds to a probability of 0.998 that this configuration is correct. Data have been deposited with the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication No. CCDC 748346. Copies of the deposited crystal data can be obtained, free of charge, at via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-(0)-1223 336033 or deposit@ccdc.cam.ac.uk).
Results
Structure elucidation
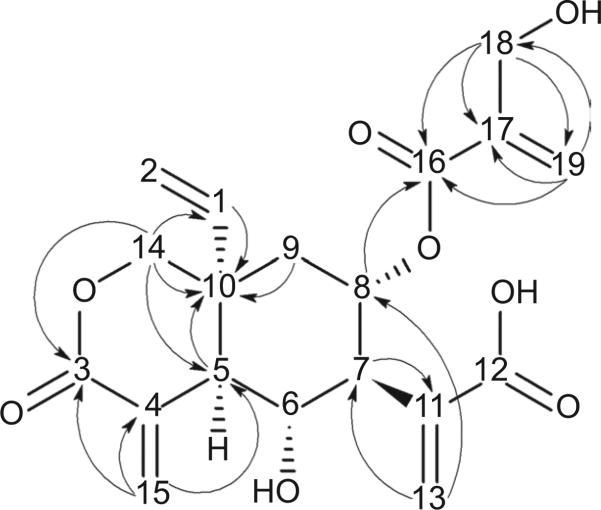
Vernodalinol was isolated and purified from VA as colorless crystals. The molecular formula was established as C19H22O8 from the positive HR ESI-MS at m/z 379.1383 [M + H]+. Its IR spectrum displays carboxyl (3600–2600 cm−1), hydroxyl (3443 cm−1), carbonyl (1707 cm−1), and alkene (1626 cm−1) functional groups. The absorption spectrum at λmax = 235 nm shows the presence of enones. The assignments of 1H and 13C NMR spectroscopic data of vernodalinol are summarized in Table 1, based on HSQC, HMBC (Figure 3), and COSY spectra. The 13C NMR spectrum shows 19 resonances. A DEPT NMR experiment classifies the 19 13C NMR resonances into seven methylene, five methine, and seven quaternary carbons.
Figure 3.
Selected heteronuclear multiple-bond correlations (HMBC) for vernodalinol.
Three carbonyl carbons are noted at δC 163.3 (C-16), 163.7 (C-3), and 166.5 (C-12). Eight double-bond carbons are found at 139.7 (C-1), 112.9 (C-2), 131.4 (C-4), 136.2 (C-11), 127.0 (C-13), 131.2 (C-15), 138.6 (C-17), and 122.3 (C-19). Four oxygenated carbon resonances are shown at 67.0 (C-6), 68.0 (C-8), 69.0 (C-14), and 58.5 (C-18). On the basis of HSQC data, five protonated double-bond carbons are present: four gem-coupled resonances at δH/δH 5.21/5.25, 5.79/6.34, 5.77/6.54, and 5.85/6.19 are assigned to H-2a/b, H-13a/b, H-15a/b, and H-19a/b, which are attached to C-2, C-13, C-15, and C-19, respectively. One methylene group shows its resonance at δH/δC 5.76/139.7, which is assigned to H-1/C-15. Also, according to COSY data with coupling constants and multiplicities in Table 1, C-1 and H-2 are part of a vinyl group and H-1 and H-2b are trans to each other.
The COSY experiment shows the sequence: H-5–H-6–H-7–H-8–H-9. Resonances at δH/δC 2.55/49.2 (H-5/C-5) and 1.64 and 2.01/35.9 (H-9a and b/C-9) display in the HMBC correlations with the carbon at δC 38.1 (C-10). From these data, a cyclohexane structure is assigned. Because of the relatively large coupling constants ranging from 10 to 11.5 Hz, H-5, H-6, H-7, H-8, and H-9a must be in the axial positions of the cyclohexane ring. According to HMBC data in Table 1 and Figure 3, C-16, C-17, C-18, and C-19 compose an independent system, a [2-(hydroxymethyl) acryloyl]oxy substituent. This substituent should link the C-8 through an ether bond because of the resonance at 5.38/68.0 (H-8/C-8) that displays the HMBC correlation at δC 163.3 (C-16). The HMBC data also indicate that C-7 in the cyclohexane ring connects with a prop-2-enoic acid moiety through its 2 position. The propenoic acid is composed of C-11, C-12, C-13, and H-13a/b. The vinyl group connects with the C-10 due to the resonance at δH/δC 5.76/139.7 (H-1/C-1) with C-10 at δC 38.1. Because of HMBC, the carbons (C-3, C-4, and C-14) and hydrogens (H-14a/b) are in a δ-lactone ring, which is fused with the cyclohexane ring with C-5 and C-10. C-15 and H-15a/b form an exocyclic double bond with C-4 in the lactone ring.
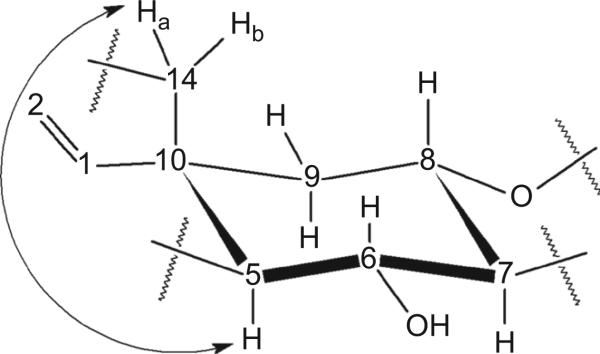
For the bond between C-10 and C-14, there are two options, axial or equatorial. In COSY spectrum, H-14a has correlation with H-5 (Figure 4), the coupling constant J = 2.0 Hz. This long range four-bond coupling is only possible if all four bonds linking the two coupled nuclei are arranged in a sterically fixed “W” configuration (Friebolin, 2005). Therefore, the bond between C-10 and C-14 must be in the axial position to produce a “W” pattern between H-14a and H-5 (Figure 4), which matches the X-ray crystal structure in Figure 5.
Figure 4.
The conformation of cyclohexane ring for vernodalinol and the key correlation spectroscopy (COSY) correlation.
Figure 5.
Crystal structure of vernodalinol. (See colour version of this figure online at www.informahealthcare.com/phb).
Crystal structure of vernodalinol shows a cis-fusion between cyclohexane and δ-lactone rings, which is a common structural feature in sesquiterpene from Vernonia plants. The structure of α-methylene-δ-lactone in the vernodalinol is very similar to what was reported previously (Erasto et al., 2006). The bond angles around the quaternary carbon, C-10 are in the range of 108.1°– 112.5° in vernodalinol, in comparison the methyl ester, vernodalol, was reported to be in the range of 107°–117° (McPhail & Sim, 1971). The cyclohexane ring adopts a slightly distorted chair conformation, with the endocylic torsion angle magnitudes in the range of 50.60(14)– 58.53(14)°. The δ-lactone ring adopts a twisted envelope conformation, with C-5 at the flat position. While C-5 is 0.534(1) Å out of plane, the other five carbons of the ring are approximately coplanar, with mean deviation of 0.110 Å from planarity. The twist axis bisects the C-3–O-1 and C-5–C-10 bonds, such that O-1, C-3, C-4, and C-14 exhibit a mean deviation of 0.019 Å from planarity, whereas C-5 and C-10 lie 0.197(1) and 0.494(1) Å, respectively, on opposite sides of the plane. This is a typical conformation of cis-fused α-methylene-δ-lactone ring.
Inhibition of DNA synthesis by vernodalinol
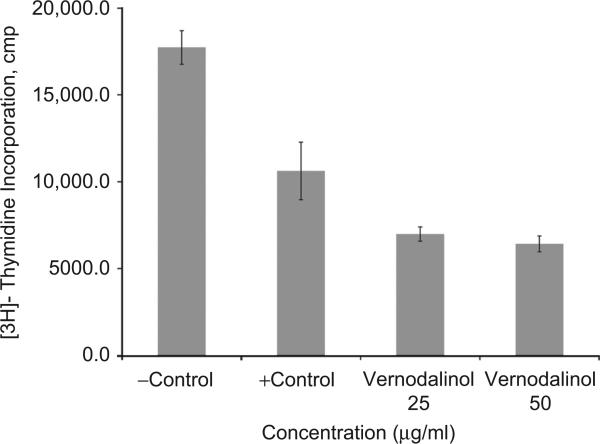
Exposure of MCF-7 cells to 25 and 50 μg/mL vernodalinol inhibited DNA synthesis by 34% and 40% (P < 0.025), respectively, compared with solvent control (second bar in Figure 6). While the inhibition activity of vernodalinol is relatively modest, it is comparable with epigallocatechin-3-gallate (EGCG) LC50 value of 84 μM in human diploid fibroblasts (Meng et al., 2008). Due to quantity limitation, we were unable to determine the LC50 for vernodalinol in the present study. Based on the 34% and 40% inhibition by 25 and 50 μg/mL, it is reasonable to expect an LC50 of 70–75 μg/mL for vernodalinol in these cells.
Figure 6.
Inhibition of DNA synthesis by vernodalinol. Cells at the logarithmic growth phase were treated with either 25 or 50 μg/mL for 18 h before addition of 1 μCi/mL [3H]-thymidine for 4 h. Each data point represents the mean of three independent experiments done in duplicates (n = 3). Exposure of vernodalinol inhibited DNA synthesis in both concentration- and solvent-dependent fashion. Vernodalinol at 25 μg/mL inhibited DNA synthesis by ~34% (P-value <0.025), while at 50 μg/mL vernodalinol inhibited DNA synthesis by 40% (P-value <0.025).
Discussion
Thus far, 32 compounds were isolated and identified from VA. They are classified into three flavonoids (Igile et al., 1994): one steroidal alcohol (Arene, 1972), 10 steroid glucosides (Ohigashi et al., 1991; Jisaka et al., 1992a, 1993; Kamperdick et al., 1992; Igile et al., 1994, 1995), 12 fatty acids (Erast et al., 2007b), and six sesquiterpene lactones that were vernolide (Erasto et al., 2006, 2007a); vernodalol (Erasto et al., 2006); 11,13-dihydrovernodalin (Ganjian et al., 1983); vernodalin (Kupchan et al., 1969); vernomygdin (Kupchan et al., 1969); and hydroxyvernolide (Jisaka et al., 1992a). These sesquiterpene lactones show a range of activities from antifeedant (Ganjian et al., 1983), antioxidant (Erasto et al., 2006, 2007a), antimicrobial (Kupchan et al., 1969), antiparasitic (Jisaka et al., 1992b) to anticancer activities (Koshimizu et al., 1994).
In this article, we report the isolation and structural elucidation of another sesquiterpene lactone, vernodalinol. This lactone, the free acid form has not been isolated, but the methyl ester was isolated by several reports. McPhail and Sim (1971) isolated the methyl ester of vernodalinol, which was vernodalol, from Vernonia hymenolepis, a different species of VA, and obtained X-ray crystal structure in 1971. Koul et al. (2005) synthesized the diacetate of vernodalol in 2005. In 2006, Erasto et al. (2006) isolated vernodalol from VA. However, their focus was on bactericidal, antifungal, and antioxidant activities of vernodalol (Erasto et al., 2006, 2007a), not structural elucidation. The free acid form was reported by Pedersen et al. (2009), who obtained a 1H NMR spectrum of vernodalinol from HPLC-PDA-MS-SPE-NMR. The compound itself was not isolated in pure form from a different plant, Distephanus angulifolius (DC.) H. Rob. & B. Kahn (Asteraceae).
Though our results show that vernodalinol just has a modest activity, previous researches (Kupchan et al., 1969; Ganjian et al., 1983; Jisaka et al., 1992a; Erasto et al., 2006, 2007a) have proved that sesquiterpene lactones isolated from VA are the key active ingredients responsible for most of the activities exhibited of VA, including the anticancer activity. This information suggests that in the future research we can focus on separating other sesquiterpene lactones in VA or modifying sesquiterpene lactones found in VA by organic synthesis.
Conclusion
In this study, another sesquiterpene lactone, vernodalinol, was isolated from VA and its unequivocal structure was determined by a battery of spectroscopic methods and confirmed by a detailed X-ray crystallographic analysis. Its biological activity was evaluated by DNA synthesis assay. Vernodalinol showed a modest inhibition. At the concentrations of 25 and 50 μg/mL, it inhibited 34% (P < 0.025) and 40% (P < 0.025) MCF-7 cell growth, respectively.
Acknowledgement
We thank Dr. Gregorio B. Begonia, Department of Biology, Jackson State University, USA, for his help to verify the VA from Nigeria, and Dr. Azeem Hasan, Mass Spectrometry Facility of Department Chemistry, Louisiana State University, USA, for collecting HR ESI-MS data.
Declaration of interest
This work was supported by National Center for Research Resources (NCRR) (G12RR013459) and National Science Foundation (NSF CHE-0821357) for the 500 MHz NMR. The purchase of the diffractometer was made possible by grant LEQSF (1999–2000)-ENH-TR-13, administered by the Louisiana Board of Regents.
References
- Akinpelu DA. Antimicrobial activity of Vernonia amygdalina leaves. Fitoterapia. 1999;70:432–434. [Google Scholar]
- Arene EO. 7,24(28)-Stimastadien-3β-ol from Vernonia amygdalina. Phytochemistry. 1972;11:2886–2887. [Google Scholar]
- Erasto P, Grierson DS, Afolayan AJ. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacol. 2006;106:117–120. doi: 10.1016/j.jep.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Erasto P, Grierson DS, Afolayan AJ. Evaluation of antioxidant activity and the fatty acid profile of the leaves of Vernonia amygdalina growing in South Africa. Food Chem. 2007a;104:636–642. [Google Scholar]
- Erasto P, Grierson DS, Afolayan AJ. Antioxidant constituents in Vernonia amygdalina leaves. Pharm Biol. 2007b;45:195–199. [Google Scholar]
- Farombi EO. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. Afr J Biotech. 2003;2:662–671. [Google Scholar]
- Fischer NH, Oliver EJ, Fischer HD. Progress in the Chemistry of Organic Natural Products. Vol. 38. Springer; Vienna, Austria: 1979. [Google Scholar]
- Friebolin H. Basic One- and Two-Dimensional NMR Spectroscopy, Fourth Completely Revised and Updated Edition. Wiley-VCH; Weinheim, Germany: 2005. Indirect Spin–Spin Coupling; p. 96. [Google Scholar]
- Ganjian R, Kubo I, Fludzinski P. Insect antifeedant elemanolide lactones from Vernonia amygdalina. Phytochemistry. 1983;22:2525–2526. [Google Scholar]
- Hooft RW, Straver LH, Spek AL. Determination of absolute structure using Bayesian statistics on Bijvoet differences. J Appl Crystallogr. 2008;41:96–103. doi: 10.1107/S0021889807059870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CB, Stevens J, Izevbigie EB, Walker A, McDaniel O. Time and dose-dependent modulation of phase 1 and phase 2 gene expression in response to treatment of MCF-7 cells with a natural anti-cancer agent. Cell Mol Biol (Noisy-le-grand) 2003;49:1057–1065. [PubMed] [Google Scholar]
- Igile GO, Oleszek W, Jurzysta M. Vernoniosides D and E, two novel saponins from Vernonia amygdalina. J Nat Prod. 1995;58:1438–1443. [Google Scholar]
- Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso M, Fasanmade AA. Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem. 1994;42:2445–2448. [Google Scholar]
- Iwalokun BA, Efedede BU, Alabi-Sofunde JA, Oduala T, Magbagbeola OA, Akinwande AI. Hepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage in mice. J Med Food. 2006;9:524–530. doi: 10.1089/jmf.2006.9.524. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB. Discovery of water-soluble anticancer agents (edotides) from a vegetable found in Benin City, Nigeria. Exp Biol Med (Maywood) 2003;228:293–298. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB, Bryant JL, Walker A. A novel natural inhibitor of extracellular signal-regulated kinases and human breast cancer cell growth. Exp Biol Med (Maywood) 2004;229:163–169. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB, Ekunwe SI, Jordan J, Howard CB. Ethanol modulates the growth of human breast cancer cells in vitro. Exp Biol Med (Maywood) 2002;227:260–265. doi: 10.1177/153537020222700406. [DOI] [PubMed] [Google Scholar]
- Izevbigie EB, Gutkind JS, Ray PE. Angiotensin II and basic fibroblast growth factor mitogenic pathways in human fetal mesangial cells. Pediatr Res. 2000;47:614–621. doi: 10.1203/00006450-200005000-00010. [DOI] [PubMed] [Google Scholar]
- Jisaka M, Ohigashi H, Takagaki T, Nozaki H, Tada T, Hirota M, Irie R, Huffman MA, Nishida T, Kaji M, Koshimizu K. Bitter steroid glucosides, vernoniosides A1, A2, and A3, and related B1 from a possible medicinal plant, Vernonia amygdalina, used by wild chimpanzees. Tetrahedron. 1992a;48:625–632. [Google Scholar]
- Jisaka M, Ohigashi H, Takegawa K, Huffman MA, Koshimizu K. Antischistosomal activities of sesquiterpene lactones and steroid glucosides from Vernonia amygdalina, possibly used by wild chimpanzees against parasite-related diseases. Biosci Biotech Biochem. 1992b;57:833–834. doi: 10.1271/bbb.56.845. [DOI] [PubMed] [Google Scholar]
- Jisaka M, Ohigashi H, Takegawa K, Hirota M, Irie R, Huffman MA, Koshimizu K. Steroid gulcosides from Vernonia amygdalina, a possible chimpanzee medicinal plant. Phytochemistry. 1993;34:409–413. [Google Scholar]
- Kamperdick C, Breitmaier E, von Radloff MA. A new steroid saponin from Vernonia amygdalina (Compositae). J Prakt Chem. 1992;334:425–428. [Google Scholar]
- Koshimizu K, Ohigashi H, Huffman MA. Use of Vernonia amygdalina by wild chimpanzee: possible roles of its bitter and related constituents. Physiol Behav. 1994;56:1209–1216. doi: 10.1016/0031-9384(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Koul JL, Koul S, Taneja SC, Schürmann M, Preut H, Spiteller M. Epivernadol diacetate. Acta Cryst E. 2005;61:3349–3350. [Google Scholar]
- Kupchan SM, Hemingway RJ, Karim A, Werner D. Tumor inhibitors. XLVII. Vernodalin and vernomygdin, two new cytotoxic sesquiterpene lactones from Vernonia amygdalina. J Org Chem. 1969;34:3908–3911. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- McPhail AT, Sim GA. Sesquiterpenoids. Part XI. X-ray determination of the structure of vernolepin p-bromobenzenesulphonate. J Chem Soc (B) 1971:198–202. [Google Scholar]
- Meng Q, Velalar CN, Ruan R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radical Bio Med. 2008;44:1032–1041. doi: 10.1016/j.freeradbiomed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Oboh G. Effect of blanching on the antioxidant properties of some tropical green leafy vegetables. LWT-Food Sci Technol. 2005;38:513–517. [Google Scholar]
- Odukoya OA, Inya-Agha SI, Segun FI, Sofidiya MO, Ilori OO. Antioxidant activity of selected Nigerian green leafy vegetables. Am J Food Technol. 2007;2:169–175. [Google Scholar]
- Ohigashi H, Jisaka M, Takagaki T, Nozaki H, Tada T, Huffman MA, Nishida T, Kaji M, Koshimizu K. Bitter principle and a related steroid glucoside from Vernonia amygdalina, a possible medicinal plant for wild chimpanzees. Agric Biol Chem. 1991;55:1201–1203. [Google Scholar]
- Opata MM, Izevbigie EB. Aqueous Vernonia amygdalina extracts alter MCF-7 cell membrane permeability and efflux. Int J Environ Res Public Health. 2006;3:174–179. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyugi DA, Luo X, Lee KS, Hill B, Izevbigie EB. Activity markers of the anti-breast carcinoma cell growth fractions of Vernonia amygdalina extracts. Exp Biol Med. 2009;234:410–417. doi: 10.3181/0811-RM-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MM, Chukwujekwu JC, Lategan CA, van Staden J, Smith PJ, Staerk D. Antimalarial sesquiterpene lactones from Distephanus angulifolius. Phytochemistry. 2009;70:601–607. doi: 10.1016/j.phytochem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Tona L, Cimanga RK, Mesia K, Musuamba CT, Bruyne TDe, Apers S, Hernans N, van Miert S, Pieters L, Totté J, Vlietinck AJ. In vitro antiplasmodial activity of extracts and fractions from seven medicinal plants used in the Democratic Republic of Congo. J Ethnopharmacol. 2004;93:27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]