Abstract
The effect of glutathione S-transferase variants on pediatric busulfan metabolism was investigated by noncompartmental and population pharmacokinetic modeling. Twenty-nine children who underwent related or unrelated bone marrow or umbilical cord blood hematopoietic cell transplant were retrospectively studied. GSTA1, GSTP1, and GSTM1 variants were explored for their effects on busulfan exposures. Noncompartmental pharmacokinetic analyses showed that carriers of GSTA1*B had a 2.6-fold higher busulfan area under the curve and concentration at steady state compared with noncarriers (P ≤ .01). Population pharmacokinetic modeling demonstrated that carriers of GSTA1*B reduced busulfan clearance by 30%. Monte Carlo simulations were then performed to assess busulfan dosing regimens based on GSTA1 genotypes. Simulations determined that dosing based on GSTA1 genotype, weight, and age resulted in fewer children exceeding the upper therapeutic limit compared with dosing using age and weight only. Larger, prospective studies are needed to confirm these findings.
Keywords: Glutathione S-transferase, polymorphisms, busulfan, pharmacokinetics
Busulfan is an alkylating agent commonly used in conditioning regimens prior to hematopoietic cell transplantation (HCT) for the treatment of hematologic malignancies and nonmalignant disorders.1–3 Because busulfan exhibits an exposure-response relationship and high pharmacokinetic variability, therapeutic drug monitoring is standard clinical practice for patients receiving full-dose busulfan prior to transplantation, particularly in children.4 Busulfan exposure is commonly measured by either area under the curve concentration (AUC) or steady-state plasma concentration (Css) and is associated with concentration-dependent toxicities, including hepatic veno-occlusive disease.5–7 A busulfan average Css > 900 ng/mL is associated with greater toxicity in busulfan and cyclosphosphamide preparative regimens,8–10 whereas a Css < 600 ng/mL is associated with poorer engraftment and, in some settings, predicts graft rejection.1,11 Hence, the typical busulfan plasma target is a Css of 600 to 900 ng/mL or AUC 221.5 to 369 mcg·mL/min (900–1500 μM·min).3,7,8,10 Consequently, plasma concentration targeting may improve clinical outcomes. Thus, better targeting strategies are needed to improve initial dosing and lessen the time that a subject would be exposed to sub- or supratherapeutic concentrations.
Busulfan is metabolized extensively in the liver through conjugation with glutathione by glutathione S-transferase (GST) enzymes.12–14 In vitro studies using liver and intestinal cytosols have demonstrated that busulfan is metabolized primarily by GSTA1, with minor contributions by GSTM1 and GSTP1.15,16 In vitro studies have shown that variants in GSTP1 result in functional alterations in the activity of the GSTP1 enzymes conferring decreased enzyme activity.17
Four variants in the promoter region of the GSTA1 gene, −631T>G, −567T>G, −69 C>T, and −52G>A, are in linkage disequilibrium and result in decreased enzyme expression, whereas the GSTM1*0 results in no enzyme production. Previous studies in HCT found a significantly higher incidence of hepatic venoocclusive disease in thalassemic patients carrying the GSTM1*0 genotype (GSTM1 null) compared with those who were GSTM1 positive (46.5% vs 18.3%; P = .001), presumably due to higher busulfan exposure.18 Kusama et al19 examined the effects of the GSTA1 variant, GSTA1*B, on oral busulfan pharmacokinetics in a Japanese population (n = 9). They observed that individuals with the GSTA1*B variant had a lower busulfan clearance (0.118 ± 0.013 vs 0.196 ± 0.011 L/h/kg, P = .004) and higher busulfan plasma concentrations (1344 ± 158 vs 854 ± 44 ng/mL, P = .001) after the fifth dose of busulfan compared with subjects without the variant. However, the pharmacogenetic effect was confounded by oral administration and the high variability in busulfan bioavailability. To date, no studies have evaluated the clinical effect of the GSTA1, GSTP1, and GSTM1 variants on intravenous (IV) busulfan exposures in the pediatric population. Therefore, this study investigated the role of the GSTA1, GSTM1, and GSTP1 variants toward busulfan AUC and Css concentrations following the first dose of IV busulfan using a noncompartmental pharmacokinetic analysis. In addition, a nonlinear mixed-effects population pharmacokinetic model was developed to quantify the effects of age, body weight, and genotype on the pharmacokinetics of busulfan. Finally, Monte Carlo simulations of the final population pharmacokinetic model were used to evaluate various dosing strategies in children with GSTA1 variants with respect to literature-based target exposures.
METHODS
Patients
Twenty-nine subjects who underwent related or unrelated bone marrow or umbilical cord blood HCT for malignant (n = 10) or nonmalignant diseases (n = 19) at the University of Minnesota were studied. Patients included were <18 years of age who had undergone HCT at the University of Minnesota and underwent busulfan pharmacokinetic sampling with first busulfan dose as part of routine clinical management. This study protocol was approved by the University of Minnesota Institutional Review Board. All patients or parents signed informed consent or assent, as appropriate. Patient demographics are given in Table I. The median age (range) and weight was 5.58 years (0.08–18.25) and 16.70 kg (5.58–99.10), respectively. All children received intravenous busulfan. Children ≤12 kg received busulfan 1.1 mg/kg IV every 6 hours and, if >12 kg, 0.8 mg/kg IV every 6 hours. The median dose was 0.8 mg/kg (0.8–1.1 mg/kg) every 6 hours. Busulfan was infused over 2 hours at a constant rate. Fosphenytoin was given for seizure prophylaxis from 1 day prior to initiation of the busulfan regimen and continuing through until 24 hours after the final dose of busulfan. All children received one of the following disease-specific conditioning regimens: busulfan 0.8 to 1.1 mg/kg/dose IV every 6 hours for 16 doses given on days −9, −8, −7, and −6 plus cyclophosphamide 50 mg/kg IV daily on days −4, −3, −2, and −1 (n = 21); busulfan 0.8 mg/kg/dose IV every 12 hours for 8 doses on days −7 and −6 and IV fludarabine 35 mg/m2 daily for 4 days on days −5, −4, −3, and −2 (n = 1); busulfan 0.8 to 1.1 mg/kg/dose IV every 6 hours for 16 doses given on days −8, −7, −6, and −5 and fludarabine 25 mg/m2 IV daily for 3 days on days −4, −3, and −2 (n = 3); or busulfan 0.8 to 1.1 mg/kg/dose IV every 6 hours for 16 doses given on days −7, −6, −5, and −4 plus cyclophosphamide 60 mg/kg IV daily on days −3 and −2 (n = 4). No patients received total body irradiation. All patients underwent therapeutic monitoring with first dose of busulfan, and doses were adjusted to achieve a Css range of 600 to 900 ng/mL using dosage recommendations provided by the analytical laboratory at the University of Pennsylvania.
Table I.
Patient Demographics
| Age, y, median (range) | 5.58 (0.08–18.25) |
| Gender, male/female | 17/12 |
| Weight, kg, median (range) | 16.70 (5.58–99.10) |
| Disease | |
| ALD | 6 |
| AML | 4 |
| Hurler syndrome | 4 |
| ALL | 2 |
| I-cell disease (mucolipidosis) | 2 |
| JMML | 2 |
| Krabbe | 2 |
| MLD | 2 |
| Fanconi anemia/AML | 1 |
| T cell ALL | 1 |
| α-Thalassemia | 1 |
| Batten disease | 1 |
| Niemann-Pick disease | 1 |
| Race/ethnicity | |
| Caucasian | 17 |
| African American | 3 |
| Unknown | 3 |
| Asian | 2 |
| Hispanic | 2 |
| Other | 2 |
| Donor type | |
| Unrelated | 22 |
| Related | 7 |
| Stem cell source | |
| Cord blood | 21 |
| Bone marrow | 8 |
| Preparative regimen | |
| Busulfan, cyclophosphamide | 25 |
| Busulfan, fludarabine | 4 |
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; ALD, adrenoleukodystrophy; MDL, metachromatic leukodystrophy; JMML, juvenile myelomonocytic leukemia.
Noncompartmental Pharmacokinetic Analysis
Busulfan concentrations were measured at 120, 135, 240, and 360 minutes after the start of infusion with the first dose of busulfan. At each time point, 2 mL of blood was collected and placed on wet ice. Busulfan samples were converted to the 1,4-diiodobutane derivative and subsequently measured using gas chromatography with electron capture as previously described.20 Briefly, the iodinated derivative of busulfan, 1,5-diiodopentane, was used as the internal standard. The selective ion monitoring was used at m/z of 183 and 197. The busulfan assay was linear from 0.04 to 4.0 mg/L, with the lowest limit of quantification being 0.04 mg/L. Accuracy and precision were within 6% of nominal values for all standards.20
For purposes of this analysis, plasma concentration-time data were reanalyzed by noncompartmental and population pharmacokinetic analyses. First, noncompartmental analyses were conducted using standard software (WinNonLin Professional 5.2, Pharsight Corp, Mountain View, California). Area under curve (AUC0-∞), average steady-state plasma concentration (Css), and Cmax were determined. The AUC was estimated using the trapezoidal rule. Css was the ratio of AUC0-∞ and time between doses in minutes. Cmax was the highest observed concentration. AUC0-∞ and Css were adjusted for dose in mg prior to statistical analysis.
Population Pharmacokinetic Modeling of Busulfan
A nonlinear mixed-effects analysis was undertaken to characterize the population pharmacokinetics of busulfan. NONMEM Version VI with PDxPop Version 2.2a (Icon Development Solutions, Ellicott City, Maryland) was used to define a 1-compartment model parameterized in terms of clearance (CL) and volume of distribution (V). Busulfan clearances were modeled by creating a dichotomous variable of busulfan dosing by age (≤4 years, >4 or <6 years, ≥6 years).21 Six years of age was chosen as a cut point because this was the median age of the population. Modeling based on the 6-year age cut point did not generate a sufficient model. Data were also modeled by creating a dichotomous variable for weight (≤12, >12 kg).21 This model was also not sufficient. Study population was modeled based on GST1A*B carrier status. The only useful model was when data were stratified by GSTA1*B carrier status, whereby subjects who carried 1 or 2 of the GSTA1*B alleles were grouped together and compared with noncarriers of the GSTA1*B allele. Additional models were also constructed to evaluate the effect of GSTM and GSTP polymorphisms on busulfan clearance with and without GST1A*B polymorphism, but these models were not significant.
The final regression model was as follows:
TVCL is the population mean clearance, and θ(1) is the mixed-effects regression parameter of the population that is ≤4 years of age. θ(2) is the mixed-effects regression parameter of the population that is >4 years of age. θ(3) is the regression parameter of the population mean volume. θ(4) is the mixed-effect regression parameter of the population that carries 1 or 2 GSTA1*B alleles. SIZE is the ideal body weight (IBW).
The intersubject clearance variability was described by the exponential error model, where
η1 is an estimated vector of the individual-specific random effects of CL. η2 is an estimated vector of the individual-specific random effects of V. The remainder of the variability was estimated by the proportional error model, whereby
The first-order conditional estimation procedure with interaction was used in the analysis. Forward inclusion/backward elimination of each of the age, weight, and genotype groups was used to develop the population pharmacokinetic model. The likelihood ratio test was used to establish the level of significance of any covariate where alpha was set at 0.05 (χ2, df = 1). A decrease in objective function value of 3.8 was considered significant. Diagnostic plots and visual predictive checks were used as qualification steps in the evaluation of the final regression model. For the visual predictive check, 100 individuals were simulated using Monte Carlo sampling techniques from the parameter distributions defined by the final model. The age, busulfan dose, and weight covariate triads were the same as the original distribution. Intuitively, if a model is reasonable, approximately 80% of the observed concentrations should be contained within the 10th and 90th quantiles of the predictions under the model of these 100 in silico individuals. This is evaluated visually, rather than statistically, and is a useful tool in evaluating the appropriateness of population pharmacokinetic models.
Dosing Regimen Simulations
Using the original 29 subject data set, 1000 bootstrapped subjects were selected at random with replacement. This generated 1000 subjects with paired ages and weights in a distribution similar to the original 29 subjects. GSTA1 genotype classification of a carrier or noncarrier was randomly assigned to each subject at the same fraction (0.52) found in the original subjects. Given each subject’s age, weight, and genotype, a clearance was simulated under the derived population pharmacokinetic model. Age and weight stratifications were selected based on the manufacturer’s dosing recommendations.21 Three dosing schemes were developed and busulfan Css simulated based on clearance (derived from the population pharmacokinetic model), GSTA1 variant status, age, and weight. Model 1 simulated the conventional busulfan dosing strategy based on weight, where genotype was not considered in dose selection. If weight was ≤12 kg, then the simulated busulfan dose was 1.1 mg/kg; otherwise, a dose of 0.8 mg/kg was simulated. Model 2 simulated conventional busulfan dosing based on weight but added a genotype effect. If an individual was a carrier of the GSTA variant, the simulated dose was reduced by 30% (≤12 kg = 0.8 mg/kg; >12 kg = 0.6 mg/kg). The dose reduction was derived from the population pharmacokinetic model, which showed that children heterozygous or homozygous for GSTA1 had a 30% lower clearance compared with noncarriers. Model 3 simulated an age-based dosing strategy along with GSTA1 carrier status. If age ≤4 years, then the simulated busulfan dose was 1.0 mg/kg; otherwise, a dose of 0.8 mg/kg was simulated. If an individual was also a carrier of the GSTA variant, the simulated dose was reduced by 30% (≤4 years = 0.7 mg/kg; >4 years = 0.6 mg/kg). The simulated clearances were then used to compute the expected busulfan steady-state concentration (Css) for the 1000 simulated subjects. The percentages of subjects within, below, and above the target Css range of 600 to 900 ng/mL were calculated for the 3 dosing models.
Diagnosis of Veno-Occlusive Disease, Treatment-Related Mortality, and Engraftment
Veno-occlusive disease (VOD) was diagnosed within 30 days posttransplant using the Baltimore clinical criteria,22 including presence of total bilirubin >2 mg/dL, plus 2 of the following 3 findings: hepatomegaly, ascites, and weight gain >5%. Alternatively, VOD was diagnosed as reversal of portal blood flow on ultrasound plus 1 of the previous signs. Treatment-related mortality was characterized as death from any cause prior to day 100. Engraftment was defined as the first of 3 consecutive days of an absolute neutrophil count >500 cells/mm3.
DNA Isolation and Genotype Determination
Pretransplant genomic DNA was isolated from peripheral blood lymphocytes and extracted using a Qiagen DNA extraction kit. The TaqMan allelic discrimination method was used for the GSTM1 gene deletion and the GSTP1 p.I105V (c.A313G) and GSTP1 p.A114V (c.C341T) single-nucleotide polymorphisms (SNPs). Primer Express v1.5 software (PerkinElmer/Applied Biosystems, Inc, Foster City, California) was used to develop gene-specific primers for polymerase chain reaction (PCR) and a TaqMan fluorogenic probe for allelic discrimination for GSTM1. SNPs for GSTP1 I105V and A114V were analyzed using TaqMan SNP Genotyping Assays (PerkinElmer/Applied Biosystems, Inc). These assays were performed per kit instructions. The GSTA1-69 SNP C-69T was amplified by PCR followed by restriction fragment length polymorphism (RFLP) using the HinfI enzyme and visualized on a 2% agarose gel by electrophoresis. Confirmation of genotyping results was performed on 10% of samples for each SNP by either TaqMan for GSTA1*B or DNA sequencing (Biomedical Genomics Center, University of Minnesota, Minneapolis) for GSTM1 and GSTP1.
Statistical Analyses
Comparisons of busulfan exposure measures derived from noncompartmental modeling (AUC0-∞ and Css) were made between GST wild-type, heterozygous, and homozygous individuals. In addition, heterozygotes and homozygotes for the variant allele were grouped and compared with wild-type subjects. All statistical analyses were performed using the SAS software, Version 9.1 for Windows (SAS Institute, Cary, North Carolina). Statistical comparisons of genotype groups and demographics were made using 1-way analysis of variance (ANOVA). Adjustments for multiple comparisons were done using the Tukey method.
RESULTS
Patient Characteristics and Frequency of GST Variants
Patient demographics are shown in Table I. Fourteen of the 29 (48.2%) children were heterozygous (n = 7) or homozygous (n = 7) for GSTA1*B. The GSTM1*0 (null) homozygous genotype was observed in 16 of 29 patients (55%). Nineteen of the 29 children (65.5%) were heterozygous for GSTP1*2 variant, and 6 of 29 (20.6%) were heterozygous for the GST P1*3. All variants were in Hardy-Weinberg equilibrium. The median number of days to achieve neutrophil engraftment was 17 (range, 11–36). Two (6.8%) individuals had treatment-related mortality by day 100. Overall, 11 (38%) patients were not within the therapeutic range (Css 600–900 ng/mL) with the first IV dose of busulfan. Of the 11 patients, 7 had plasma busulfan Css above 900 ng/mL, 3 (43%) of whom went on to develop VOD. These 3 individuals had a busulfan Css of 1236, 1123, and 928 ng/mL with the first dose.
Effect of Variants on Busulfan Noncompartmental Pharmacokinetics
Median busulfan concentrations at 120, 135, 240, and 360 minutes after the start of IV busulfan infusion were 1034 (range, 720–1564), 938 (range, 635–1225), 595 (range, 271–990), and 362 (range, 113–627) ng/mL, respectively. The median AUC0-∞ and Css were 302.6 (range, 155.3–518.9) mcg min/mL and 868.0 (range, 425.7–1753.2) ng/mL, respectively. Children heterozygous or homozygous (n = 14) for the GSTA1*B variant had a 2.6-fold higher dose-corrected busulfan AUC0-∞ (P < .01) and and Css (P = .01)a 28% higher median Cmax (P = .02) compared with patients with the wild-type GSTA1 gene (n = 15) (Table II). This relationship was independent of the number of variant GSTA1*B alleles, as there was no difference in busulfan exposure between children heterozygous or homozygous for this mutation. There were also no significant differences in AUC0-∞, Css, or Cmax in children with or without the GSTM1*0, GSTP1*2, or GSTP1*3 variants (Table III). Children with the GSTA1 variants (*A/*B, *B/*B) were significantly younger (P < .01) and weighed less (P = .02), confounding the genotype effect; therefore, a population pharmacokinetic analysis was undertaken to control for the effect of these covariates on exposure.
Table II.
Effect of GSTA1*B on Busulfan Noncompartmental Pharmacokinetics
| GSTA1 | ||||
|---|---|---|---|---|
| *A/*A | *A/*B | *B/*B | P Valuea | |
| n (%) | 15 (52) | 7 (24) | 7 (24) | |
| Age, y | 10.25 (2.08–18.25) | 1.50 (0.58–10.08) | 1.33 (0.08–13.75) | <.01 |
| Weight, kg | 33.30 (9.30–99.10) | 8.17 (6.26–33.80) | 12.00 (5.58–53.20) | .02 |
| AUC0-∞b | 11.22 (4.62–23.67) | 33.42 (10.02–55.22) | 24.02 (8.28–67.25) | <.01 |
| Css c | 30.00 (13.13–64.84) | 92.83 (27.46–152.59) | 64.94 (24.37–184.10) | .01 |
| Cmax, ng/mL | 922 (720–1274) | 1129 (882–1307) | 1246 (881–1564) | .02 |
Data are in median (range).
P is comparison of *A/*A (wild type) and *A/*B and *B/*B (heterozygous and homozygous mutant, respectively).
AUC, area under the curve adjusted for dose in mg; units are mcg·min/mL/mg.
Css, steady-state plasma concentration adjusted for dose in mg; units are ng/mL/mg.
Table III.
Effect of GSTM1*0, GSTP1*2, and GSTP*3 on Busulfan Noncompartmental Pharmacokinetics
| GSTM1
|
GSTP1*2
|
GSTP1*3
|
||||
|---|---|---|---|---|---|---|
| *0/*0 (Null) | *A/*A or *A/*0 | *1/*1 | *1/*2 | *1/*1 | *1/*3 | |
| n (%) | 16 (55) | 13 (45) | 10 (34) | 19 (66) | 23 (79) | 6 (21) |
| Age, y | 6.08 (0.67–18.25) | 4.58 (0.08–18.25) | 3.38 (0.08–18.25) | 8.17 (0.58–18.25) | 5.25 (0.08–18.25) | 6.88 (0.67–16.33) |
| P value | .85 | .24 | .90 | |||
| Weight, kg | 17.31 (6.26–99.10) | 16.70 (5.58–94.60) | 15.05 (5.58–94.60) | 15.50 (5.58–99.10) | 26.05 (6.26–61.50) | 27.00 (6.26–99.10) |
| P value | .35 | .36 | .95 | |||
| AUC0-∞ a,b | 16.41 (4.62–55.22) | 12.19 (4.73–67.25) | 21.28 (5.63–67.25) | 12.19 (4.62–55.22) | 14.25 (4.62–67.25) | 17.72 (8.28–55.22) |
| P value | .85 | .39 | .61 | |||
| Css,0-∞ a,c | 46.72 (13.79–152.59) | 33.87 (13.13–184.10) | 57.92 (15.42–184.10) | 33.87 (13.13–152.59) | 39.60 (13.13–184.10) | 47.47 (24.37–152.59) |
| P value | .85 | .40 | .62 | |||
| Cmax, ng/mL | 979 (827–1307) | 1059 (720–1564) | 1048 (881–1417) | 922 (720–1564) | 1033 (720–1564) | 1107 (893–1285) |
| P value | .27 | .28 | .60 | |||
Adjusted for dose in mg; data are in median (range).
AUC, area under the concentration curve adjusted for dose in mg; units are mcg·min/mL/mg.
Css, steady-state plasma concentration adjusted for dose in mg; units are ng/mL/mg.
Effect of GST Variants on Busulfan Clearance Using Population Pharmacokinetics
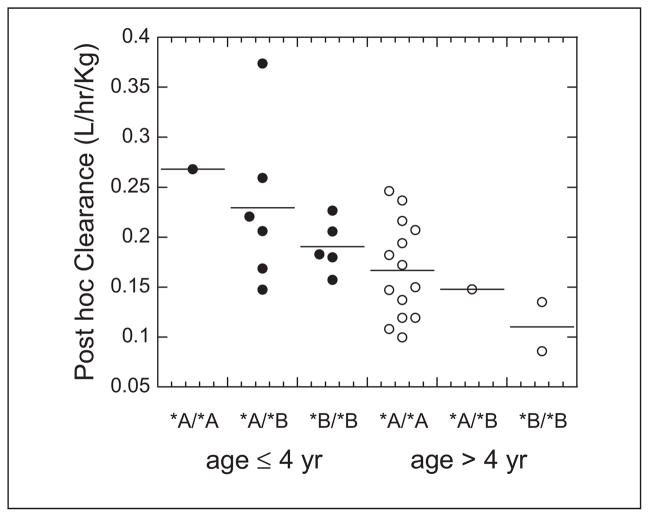
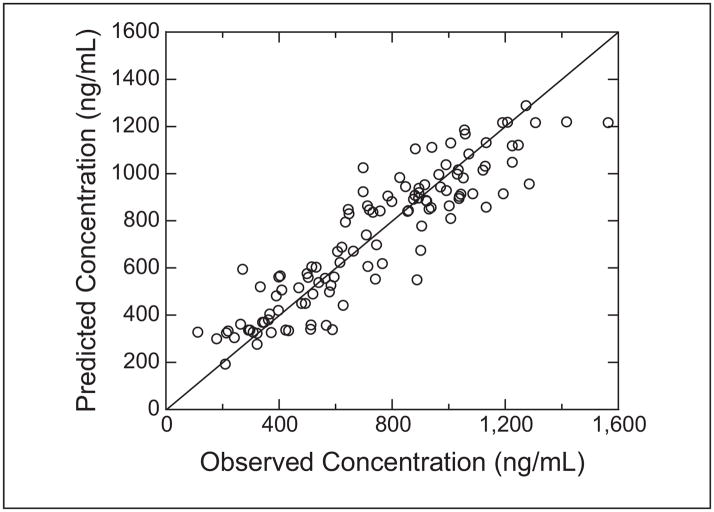
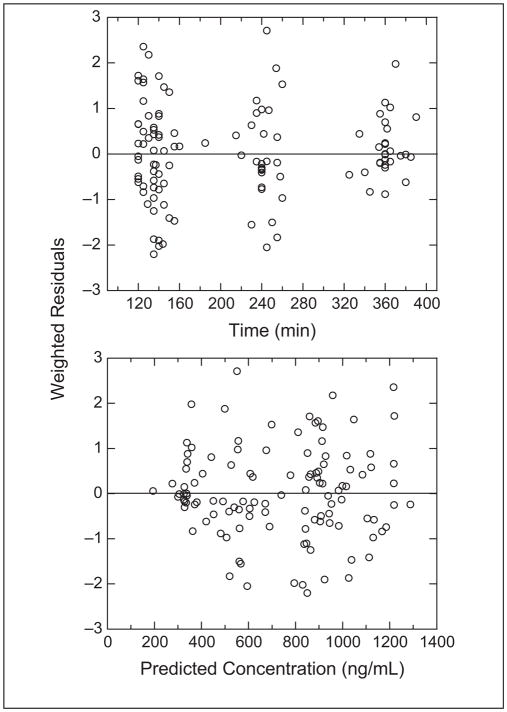
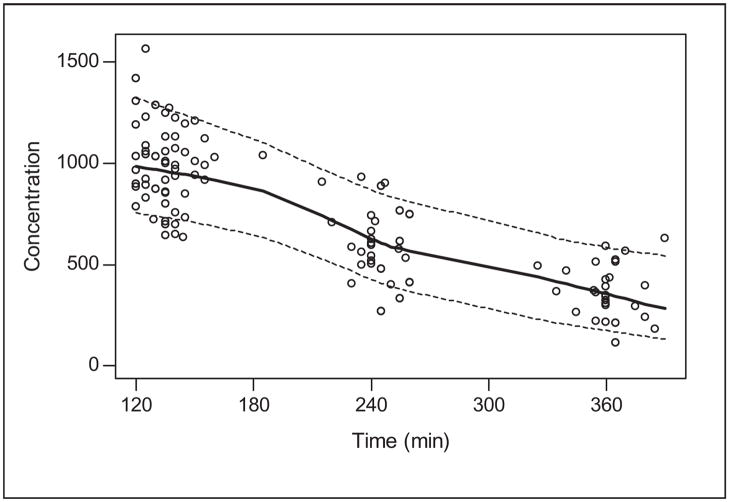
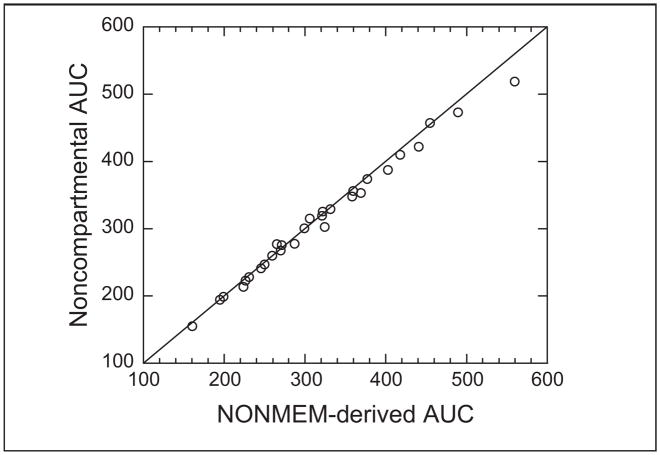
The busulfan population pharmacokinetic estimates are given in Table IV. Age was converted to a dichotomous variable for analysis. Children ≤4 years of age had a significantly higher weight-normalized busulfan clearance than those >4 years (P < .0005). Regardless of age, clearance was reduced by ~30% if the child was heterozygous or homozygous for the GSTA1*B variant (Figure 1). There was no effect of GSTM1 or P1 variants on busulfan clearance. There was a strong relationship between observed busulfan concentrations and those predicted by the population pharmacokinetic model (Figure 2). Figure 3 shows the weighted residual for time after dose and predicted busulfan concentrations. The randomness of these diagnostic plots indicates that the population pharmacokinetic model is sufficient. In addition, the results from the visual predictive check of the model also indicate the suitability of the final model and the estimated parameters (Figure 4). The observed concentrations are centered about the simulated 50th percentile, and approximately 12% are outside the simulated 10th and 90th quantiles. Finally, the relationship between the standard noncompartmental trapezoidal estimate of AUC and population-predicted AUC was excellent and also supported model sufficiency (Figure 5). All these findings support the appropriateness of the final population model and parameter estimates.
Table IV.
Final Population Pharmacokinetic Model Parameter Estimates
| Parameter | Estimate | Lower 95% Confidence Interval | Upper 95% Confidence Interval |
|---|---|---|---|
| CL,a age ≤4 years | 0.288 | 0.213 | 0.363 |
| CL,a age >4 years | 0.173 | 0.148 | 0.197 |
| GSTA1*B CL multiplierb | 0.704 | 0.519 | 0.889 |
| Vd | 0.682 | 0.650 | 0.714 |
| BSV on CL, % | 24.7 | 14.3 | 31.9 |
| BSV on Vd, % | 11.1 | 0 | 15.9 |
| RUV, proportional, % | 6.87 | 4.79 | 8.46 |
Vd, volume of distribution; BSV, between-subject variability; RUV, residual unexplained variability.
Clearance (CL) = L/h/kg.
Reduction in CL in children heterozygous or homozygous for the GSTA1*B variant.
Figure 1.
Post hoc intravenous busulfan clearance estimates in patients with GSTA1*A/*A, GSTA1*A/*B, and GSTA1*B/*B genotypes. Filled circles are the individual data for children age ≤ 4 years. Open circles are the individual data for children age > 4 years. The solid lines represent the means for each genotype.
Figure 2.
Observed versus population-predicted intravenous busulfan concentrations. Solid line represents the line of identity. The open circles are the population-predicted/observed concentration pair.
Figure 3.
The weighted residuals versus time (top panel) and predicted concentrations (lower panel).
Figure 4.
Visual predictive check of the final busulfan population model. Open circles are observed data. Solid line is a smooth of the medians of 100 simulated concentrations at each time point. Dashed lines are smoothes of the upper and lower 90% confidence intervals of the simulated data. Busulfan concentration is in ng/mL.
Figure 5.
Relationship between noncompartmental busulfan AUC and NONMEM-derived AUC. AUC is in mcg·min/mL.
Simulation of Busulfan Exposures Using GST Genotypes, Weight, and Age
Simulation modeling was performed to explore 3 dosing regimens. Using the demographics data from the original 29 subjects, 1000 bootstrap subjects were selected. Table V displays the percentage of patients within the targeted therapeutic range for busulfan after simulating busulfan dosing regimens based on weight alone, weight and GSTA1 genotype, and age and GSTA1 genotype. In the simulated data set, using weight alone to determine dose (model 1), approximately 42% of individuals were in the desired therapeutic range. With dosing regimens using weight and GSTA1 genotype (model 2) or age and GSTA1 genotype (model 3), 48% and 54% of simulated subjects achieved the targeted concentration. Using weight or age plus GSTA1 genotype reduced the number of subjects with a Css over the therapeutic limit from 37% to 19% and 21%, respectively; however, consequently, more subjects were below the therapeutic target.
Table V.
Percentage of Patients Within Targeted Busulfan Range After Population Simulation of 1000 Subjects
| Model | Weight, kg | GSTA1a | Dosage Regimen, mg/kgb | % Subjects
|
||
|---|---|---|---|---|---|---|
| 600–900 ng/mL | <600 ng/mL | >900 ng/mL | ||||
| 1 | ≤12 | — | 1.1 | 42 | 20 | 37 |
| >12 | — | 0.8 | ||||
| 2 | ≤12 | WT | 1.1 | 48 | 33 | 18 |
| MUT | 0.8 | |||||
| >12 | WT | 0.8 | ||||
| MUT | 0.6 | |||||
|
| ||||||
| Model | Age, y | GSTA1a | Dosage Regimen, mg/kgb | 600–900 ng/mL | <600 ng/mL | >900 ng/mL |
|
| ||||||
| 3 | ≤4 | WT | 1.0 | 54 | 29 | 16 |
| MUT | 0.7 | |||||
| >4 | WT | 0.8 | ||||
| MUT | 0.6 | |||||
WT, wild type; MUT, mutant.
GSTA genotype was categorized as WT for GSTA1*A/*A genotype and MUT for individuals with either GSTA1*A/*B or GSTA1*B/*B genotype.
Alternative busulfan dosing was selected if the individual was a carrier of the GSTA1*B genotype. Carriers of the GSTA1*B genotype received a 30% reduction in dose, which was determined by the population pharmacokinetic model clearance estimates.
DISCUSSION
Therapeutic monitoring of busulfan concentrations and dose adjustments in the pediatric population is important for avoiding serious hepatic toxicity and possibly for enhancing stem cell engraftment. This study is the first study to describe the clinical importance of GST variants on intravenous busulfan exposure in pediatric patients. Variability in busulfan concentrations is well described, and 30% to 60% of children do not achieve the therapeutic target with the initial dose and require dose adjustments.23 Previous simulation studies in children demonstrated that only 56% to 60% of pediatric patients achieved the targeted busulfan concentration (AUC of 900–1350 μM·min) with the first dose using multiple weight cutoffs and dosing regimens.24 The authors concluded that an initial busulfan dose of 0.8 mg/kg for children ≤12 kg and 1.1 mg/kg if >12 kg was clinically most optimal. These data are now the dosing recommendations in the IV Busulfex product package insert and the dosing adopted by most US transplant centers. Recent European studies found that 76.4% of children achieved an AUC of 900 to 1350 μM·min, and 91% achieved the broader AUC target of 900 to 1500 μM·min using doses based on 5 different strata of body weights.25,26 As of yet, this dosing strategy has not been adopted in the United States or approved by the Food and Drug Administration (FDA). In the United States, the use of weight cutoffs for dosing of busulfan in pediatric patients has reduced the variability in busulfan exposure and provides safer therapeutics. However, 29% to 40% of individual patients, despite weight-or age-based dosing, as recommended by the package insert, do not achieve the therapeutic target.21
Genetic variants in GST promoter or genes result in reduced enzyme expression (GSTA1), enzyme deletion (GSTM1), or reduced enzyme activity (GSTP1) and may explain a significant portion of pharmacokinetic variability. We found that 48% of our primarily Caucasian population were carriers of the GSTA1*B variant, resulting in a minor allele frequency of 36%. This is similar to the frequencies reported by Robert et al27 (40%). Similarly, allele frequencies observed for the GSTM1*0, GSTP1*2, and GSTP1*3 variant were comparable to those reported in the literature.28
The pharmacokinetics of busulfan have been studied extensively in children.23,24,29–34 It is well characterized that age and body weight are important covariates toward busulfan exposures in children,23,24,30–33 but they only partially explain the observed variation in pharmacokinetics. Therefore, we postulated that recipient genetic status may provide additional explanation for the pharmacokinetic variability. In our noncomparmental pharmacokinetic analysis, we found that the presence of 1 or more GSTA1*B variant alleles was associated with a 2.6-fold increase in busulfan AUC and Css (Table II). However, children heterozygous or homozygous for GSTA1*B were significantly younger and weighed less, thereby confounding the interpretation. There was no effect of GSTM1, GSTP1*2, or GSTP1*3 on busulfan exposures, with age and weight being evenly distributed between genotype groups (Table III).
In the population pharmacokinetic analysis, children ≤4 years of age had a significantly higher weight-normalized busulfan clearance than those >4 years. These clearance values are in agreement with other studies evaluating IV busulfan pharmacokinetics in children.23,24,30–33 In addition, children who were heterozygous or homozygous for the GSTA1*B polymorphism (regardless of age) exhibited a 30% decrease in busulfan clearance (Table IV). Our data are consistent with a previous in vitro study in which human liver samples, genotyped for the GSTA1*B variant, were associated with a reduced expression of the GSTA1 enzyme.35 GST variants and busulfan pharmacokinetics also were evaluated recently in a small number of adult HCT recipients (n = 12) with the first dose of oral busulfan.19 Kusama et al19 demonstrated that subjects who were heterozygous for the GSTA1*B variant (n = 3) had lower busulfan CL/F (~0.099 L/h/kg) compared with noncarriers (~0.225 L/h/kg; n = 9; P ≤ .01). GSTP is a minor contributor in vitro to busulfan metabolism, and we found that neither GSTP1*2 nor GSTP1*3 variants were important covariates, although in vitro studies have demonstrated that variants in the promoter region of GSTP were 1.7-fold less efficient in conjugating antidiol epoxides of methylchrysenes.17
Veno-occlusive disease is one of the major dose-dependent toxicities occurring primarily when busulfan Css is greater than 900 ng/mL.1,6,8,10 Busulfan-induced VOD occurs in approximately 20% (oral) and 5% (IV) of individuals, with an associated mortality rate between 20% (oral) and 3% (IV).36 In our population, 3 of 29 (10%) developed VOD. We observed that 2 of the 3 patients with VOD were carriers of the GSTA1*B variant. However, overall, 14 children were shown to have the GSTA1*B variant, and only 2 went on to develop VOD. A greater number of children ultimately may have developed VOD, but given that dose adjustments were done on all children with out-of-range Css, this cannot be determined. Further studies are needed to confirm the relationship between the GSTA1 genotype and VOD, but because most centers today perform therapeutic drug monitoring in children, this is unlikely confirmable. Conversely, correlative studies by Srivastava et al18 reported that thalassemic patients undergoing hematopoietic cell transplantation with the GSTM1*0 (null) genotype had a significant increase in the incidence of hepatic VOD compared with those who were GSTM1 positive (wildtype; 46.5% vs 18.3%; P = .001). Patients with the GSTM1*0 genotype had a higher busulfan clearance and lower plasma concentrations. This effect was hypothesized to be caused by the increased expression of GSTA due to the absence of GSTM1, but the association between GSTA1 and VOD was not investigated.18 This latter study demonstrates that compensatory mechanisms might increase the expression of GSTA1 when GSTM1 is absent. However, studies by Bredschneider et al37 demonstrated that neither GSTA1 protein expression nor conjugation activity was affected by GSTM1 status in human liver tissue. The combined effects of being a carrier of multiple GST variants were investigated in our study, but future studies should examine these effects in a larger population.
We found that the GSTA1 variant was associated with a 30% reduction in busulfan clearance and a 2.6-fold higher busulfan Css. GSTM and GSTP do not appear to contribute substantially. Simulations to determine the potential effect of using genotype in addition to weight or age on busulfan exposure showed that fewer children would be expected to exceed the upper therapeutic limit compared with dosing using age or weight only. As a consequence, more children would fall below the lower limit. Our findings do not suggest elimination of therapeutic drug monitoring but that fewer children might have high busulfan exposures if genotype is used in the dosing decisions. Future studies in larger populations are needed to validate these finding and to prospectively test busulfan dosing based on genotype and weight. Such models have the potential to improve the safety of busulfan therapy.
Footnotes
Financial disclosure: This work was supported by T32 CA099936 and the Children’s Cancer Research Fund, Minneapolis, Minnesota (LJ).
References
- 1.McCune JS, Gooley T, Gibbs JP, et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;30:167–173. doi: 10.1038/sj.bmt.1703612. [DOI] [PubMed] [Google Scholar]
- 2.Vassal G, Koscielny S, Challine D, et al. Busulfan disposition and hepatic veno-occlusive disease in children undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1996;37:247–253. doi: 10.1007/BF00688324. [DOI] [PubMed] [Google Scholar]
- 3.Andersson BS, Thall PF, Madden T, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8:477–485. doi: 10.1053/bbmt.2002.v8.pm12374452. [DOI] [PubMed] [Google Scholar]
- 4.McCune JS, Gibbs JP, Slattery JT. Plasma concentration monitoring of busulfan: does it improve clinical outcome? Clin Pharmacokinet. 2000;39:155–165. doi: 10.2165/00003088-200039020-00005. [DOI] [PubMed] [Google Scholar]
- 5.Copelan EA, Bechtel TP, Avalos BR, et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complications following marrow transplantation. Bone Marrow Transplant. 2001;27:1121–1124. doi: 10.1038/sj.bmt.1703047. [DOI] [PubMed] [Google Scholar]
- 6.Slattery JT, Risler LJ. Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit. 1998;20:543–549. doi: 10.1097/00007691-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Slattery JT, Clift RA, Buckner CD, et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89:3055–3060. [PubMed] [Google Scholar]
- 8.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 9.Slattery JT, Sanders JE, Buckner CD, et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 1995;16:31–42. [PubMed] [Google Scholar]
- 10.Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993;20:18–25. quiz 26. [PubMed] [Google Scholar]
- 11.Bolinger AM, Zangwill AB, Slattery JT, et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant. 2000;25:925–930. doi: 10.1038/sj.bmt.1702371. [DOI] [PubMed] [Google Scholar]
- 12.Gibbs JP, Liacouras CA, Baldassano RN, Slattery JT. Up-regulation of glutathione S-transferase activity in enterocytes of young children. Drug Metab Dispos. 1999;27:1466–1469. [PubMed] [Google Scholar]
- 13.Gibbs JP, Murray G, Risler L, Chien JY, Dev R, Slattery JT. Age-dependent tetrahydrothiophenium ion formation in young children and adults receiving high-dose busulfan. Cancer Res. 1997;57:5509–5516. [PubMed] [Google Scholar]
- 14.Hassan M, Ljungman P, Bolme P, et al. Busulfan bioavailability. Blood. 1994;84:2144–2150. [PubMed] [Google Scholar]
- 15.Czerwinski M, Gibbs JP, Slattery JT. Busulfan conjugation by glutathione S-transferases alpha, mu, and pi. Drug Metab Dispos. 1996;24:1015–1019. [PubMed] [Google Scholar]
- 16.Gibbs JP, Czerwinski M, Slattery JT. Busulfan-glutathione conjugation catalyzed by human liver cytosolic glutathione S-transferases. Cancer Res. 1996;56:3678–3681. [PubMed] [Google Scholar]
- 17.Hu X, Pal A, Krzeminski J, et al. Specificities of human glutathione S-transferase isozymes toward anti-diol epoxides of methylchrysenes. Carcinogenesis. 1998;19:1685–1689. doi: 10.1093/carcin/19.9.1685. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava A, Poonkuzhali B, Shaji RV, et al. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- 19.Kusama M, Kubota T, Matsukura Y, et al. Influence of glutathione S-transferase A1 polymorphism on the pharmacokinetics of busulfan. Clin Chim Acta. 2006;368:93–98. doi: 10.1016/j.cca.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Lai WK, Pang CP, Law LK, Wong R, Li CK, Yuen PM. Routine analysis of plasma busulfan by gas chromatography–mass fragmentography. Clin Chem. 1998;44:2506–2510. [PubMed] [Google Scholar]
- 21.Busulfex [package insert] Redwood City, CA: PDL Bio Pharma, Inc; 2007. [Google Scholar]
- 22.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Dalle JH, Wall D, Theoret Y, et al. Intravenous busulfan for allogeneic hematopoietic stem cell transplantation in infants: clinical and pharmacokinetic results. Bone Marrow Transplant. 2003;32:647–651. doi: 10.1038/sj.bmt.1704209. [DOI] [PubMed] [Google Scholar]
- 24.Booth BP, Rahman A, Dagher R, et al. Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol. 2007;47:101–111. doi: 10.1177/0091270006295789. [DOI] [PubMed] [Google Scholar]
- 25.Vassal G, Michel G, Esperou H, et al. Prospective validation of a novel IV busulfan fixed dosing for paediatric patients to improve therapeutic AUC targeting without drug monitoring. Cancer Chemother Pharmacol. 2008;61:113–123. doi: 10.1007/s00280-007-0455-2. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C. I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant. 2004;33:979–987. doi: 10.1038/sj.bmt.1704446. [DOI] [PubMed] [Google Scholar]
- 27.Robert J, Morvan VL, Smith D, Pourquier P, Bonnet J. Predicting drug response and toxicity based on gene polymorphisms. Crit Rev Oncol Hematol. 2005;54:171–196. doi: 10.1016/j.critrevonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10:1239–1248. [PubMed] [Google Scholar]
- 29.Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006;37:345–351. doi: 10.1038/sj.bmt.1705252. [DOI] [PubMed] [Google Scholar]
- 30.Cremers S, Schoemaker R, Bredius R, et al. Pharmacokinetics of intravenous busulfan in children prior to stem cell transplantation. Br J Clin Pharmacol. 2002;53:386–389. doi: 10.1046/j.1365-2125.2002.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schechter T, Finkelstein Y, Doyle J, et al. Pharmacokinetic disposition and clinical outcomes in infants and children receiving intravenous busulfan for allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13:307–314. doi: 10.1016/j.bbmt.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 32.Zwaveling J, Bredius RG, Cremers SC, et al. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005;35:17–23. doi: 10.1038/sj.bmt.1704707. [DOI] [PubMed] [Google Scholar]
- 33.Shaw PJ, Scharping CE, Brian RJ, Earl JW. Busulfan pharmacokinetics using a single daily high-dose regimen in children with acute leukemia. Blood. 1994;84:2357–2362. [PubMed] [Google Scholar]
- 34.Poonkuzhali B, Chandy M, Srivastava A, Dennison D, Krishnamoorthy R. Glutathione S-transferase activity influences busulfan pharmacokinetics in patients with beta thalassemia major undergoing bone marrow transplantation. Drug Metab Dispos. 2001;29:264–267. [PubMed] [Google Scholar]
- 35.Coles BF, Morel F, Rauch C, et al. Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and GSTA2 expression. Pharmacogenetics. 2001;11:663–669. doi: 10.1097/00008571-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 37.Bredschneider M, Klein K, Murdter TE, et al. Genetic polymorphisms of glutathione S-transferase A1, the major glutathione S-transferase in human liver: consequences for enzyme expression and busulfan conjugation. Clin Pharmacol Ther. 2002;71:479–487. doi: 10.1067/mcp.2002.124518. [DOI] [PubMed] [Google Scholar]