Abstract
Studies on familial aggregation of cancer may suggest an overall contribution of inherited genes or a shared environment in the development of malignant disease. We performed a meta-analysis on familial clustering of prostate cancer. Out of 74 studies reporting data on familial aggregation of prostate cancer in unselected populations retrieved by a Pubmed search and browsing references, 33 independent studies meeting the inclusion criteria were used in the analysis performed with the random effects model. The pooled rate ratio (RR) for first-degree family history, i.e. affected father or brother, is 2.48 (95% confidence interval: 2.25–2.74). The incidence rate for men who have a brother who got prostate cancer increases 3.14 times (CI:2.37–4.15), and for those with affected father 2.35 times (CI:2.02–2.72). The pooled estimate of RR for two or more affected first-degree family members relative to no history in father and in brother is 4.39 (CI:2.61–7.39). First-degree family history appears to increase the incidence rate of prostate cancer more in men under 65 (RR:2.87, CI:2.21–3.74), than in men aged 65 and older (RR:1.92, CI:1.49–2.47), p for interaction = 0.002. The attributable fraction among those having an affected first-degree relative equals to 59.7% (CI:55.6–63.5%) for men at all ages, 65.2% (CI:57.7–71.4%) for men younger than 65 and 47.9% (CI:37.1–56.8%) for men aged 65 or older. For those with a family history in 2 or more first-degree family members 77.2% (CI:65.4–85.0%) of prostate cancer incidence can be attributed to the familial clustering. Our combined estimates show strong familial clustering and a significant effect-modification by age meaning that familial aggregation was associated with earlier disease onset (before age 65).
Introduction
Prostate cancer is one of the most common cancers among males in developed countries [1]. A lot of evidence shows that a family history of the disease is an important risk factor [1], [2]. In 2003, three meta-analyses evaluated the increase in the risk of prostate cancer in relatives of affected men [3]–[5]. Since then, familial clustering has been assessed in a number of new populations. Furthermore, more recent data is available from the big cohorts in Sweden [6] and the US [7]. We studied all the data available up to September 2010 to assess the strength of prostate cancer familial aggregation. In order to evaluate the impact of a family history of prostate cancer on the disease incidence, we also estimated the attributable fractions among men with affected relatives.
Methods
Searching
We browsed the PubMed database using the search term ‘(prostate cancer) and (family history)’. The last update was performed September 21, 2010. Out of 801 initially identified articles, 53 reports provided data on the relationship between family history and risk of prostate cancer in an unselected population of men (see Figure S1). Case-control studies using selected populations as cases (example: patients undergoing prostatectomy [8], [9]) and cohort studies with a specific cohort (example: smokers [10]) were excluded to avoid bias or heterogeneity due to these population characteristics. Additional 21 study reports were found through the references of the studies identified via the PubMed database.
Selection
74 relevant articles were coded. As quality control, we considered study design, control for age and the way family history was ascertained. Cohort studies and case-control studies reporting age-adjusted estimates or using age-matched controls were included. Cross-sectional studies [11]–[17] were excluded. Two studies in which the attempt to match for age did not result in a similar age of the cases and the controls [18], [19] and one study in which the controls were not age-matched and age-adjusted estimates were not reported [20] were excluded. Additionally, one case-control study was excluded [21], because none of the participants reported a family history of prostate cancer. The study of McCahy et al. [22] was not used in the main analysis, because it was characterized by clearly outlying results (odds ratio for first-degree family history 17.83). The influence of exclusion of this study on the estimates was assessed in the sensitivity analysis.
Five studies [23]–[27] were excluded, because the investigated type of familial clustering did not correspond to any of the exposures and reference categories considered in this meta-analysis (affected first-degree relatives, i.e. father and/or brother(s), versus not, affected father versus not, affected father versus no affected first-degree relatives, affected brother(s) versus not, affected brother(s) versus no affected first degree-relatives, affected first or second-degree relative(s) versus not, two or more affected first-degree relatives versus no history in first-degree relatives). Two articles [28], [29] were excluded because of the lack of definition of ‘family history’.
Duplications in study populations were avoided so that each pooled estimate was based on independent studies. In case of an overlap between populations from several studies using the same design, a case-control study with the largest number of participants or the most recent cohort study was included. The case-control study reported by Negri et al. [30] was preferred over Gallus et al. [31] and Randi et al. [32], and Krain, 1974 [33] over Krain, 1973 [34]. Out of many reports based on the Swedish cancer register [6], [35]–[45], only the most recent [6] was used. Similarly, the most recent findings were included from the studies using the Utah population database [7], [46], [47] and the US Health Professionals Cohort [48], [49]. When there was a population overlap between studies using a different design, the cohort study [6] was preferred over the case-control studies [50]–[53], and the nested case-control study [7] over the study of West et al. [54]. A summary of the 25 case-control studies [30], [33], [55]–[77] and 8 cohort-based studies [6], [7], [49], [78]–[82] included in the analysis is presented in Table 1 and Table 2.
Table 1. Summary of the case-control studies included in the analysis.
| First author and reference | Date | Place | Race | Mean age at diagnosis | No. cases | No. controls | ControlsA |
| Beebe-Dimmer55 | 1996–2002 | USA: Michigan | African | 65 | 121 | 179 | P C |
| Fincham56 | 1981–1983 | Canada: Alberta | Caucasian | NA, age 45 or older | 382 | 625 | P C M |
| Ghadirian57 | 1989–1993 | Canada: Montreal, Toronto, Vancouver | Caucasian | NA, median 70 | 640 | 639 | P D M |
| Glover58 | 1998 B | Jamaica: Kingston | African | 73,3 | 263 | 263 | H C M |
| Hayes59 | 1986–1989 | USA: Atlanta, Detroit, New Jersey | Mixed | 61,5C | 905 | 1264 | P C M |
| Honda60 | 1979–1982 | USA: Los Angeles County | Caucasian | NA, 60 or younger | 216 | 216 | P C M |
| Justine61 | 2001–2002 | Australia: Perth | Caucasian | 63,8 C | 560 | 450 | P C M |
| Kolonel62 | 1977–1983 | USA: Hawaii | Mixed | NA | 452 | 899 | P C M |
| Krain33 | 1971–1972 | USA: Los Angeles | Mixed | median 69 | 210 | 215 | H C M |
| Lesko63 | 1992–1994 | USA: Massachusetts | Caucasian | NA, median 65, age 70 or less | 563 | 703 | P D M |
| Magura64 | 2004–2006 | USA: North Dakota | Caucasian | 64,2 C | 312 | 319 | H C M |
| Mettlin65 | 1995 B | USA: Buffalo | Caucasian | 67,6 | 1271 | 1909 | H C M |
| Negri30 | 1991–2002 | Italy | Italian | 65,7 C | 1294 | 1451 | H C |
| Rovito66 | 1998–2001 | USA: New York | Caucasian | 63,3 C | 152 | 161 | H C M |
| Rybicki67 | 2001–2004 | USA: Detroit | Mixed | NA, median 63 | 637 | 244 | P C M |
| Salinas68 | 1993–1996, 2002–2005 | USA: King County, Washington | Caucasian | 59,9 | 1211 | 1208 | P C M |
| Schuman69 | 1977 B | USA: Minnesota | Caucasian | NA, median 64 | 36 | 41 | H D M |
| Spitz70 | 1985–1989 | USA: Texas | Caucasian | 66,2 | 378 | 383 | H C M |
| Staples71 | 1994–1998 | Australia: Melbourne, Sydney, Perth | Caucasian | 60 | 1475 | 1405 | P D M |
| Steele72 | 1968–1969 | Canada: Ontario | Caucasian | 69 | 39 | 39 | H C M |
| Stone73 | 1994–1995 | USA: New Mexico | Mixed | 66,1 | 244 | 526 | P C M |
| Strom74 | 1998–2005 | USA: Texas | Hispanic | 62,2 | 176 | 174 | P C M |
| Suzuki75 | 1988–2004 | Japan | Japanese | NA | 257 in total | H C M | |
| Whittemore76 | 1987–1991 | USA, Canada | Mixed | NA, mean age at interview: 71 | 1500 | 1581 | P C M |
| Zhu77 | 1989–1991 | USA: Washington State | Caucasian | 64 | 175 | 258 | P D M |
P–population based controls, H-hospital based controls, C–cumulative sampling, D–density sampling, M–age-matched controls.
The year of publishing. The period of collecting the data not reported.
Calculated from the reported distribution of age of the cases at diagnosis.
NA–not available.
Table 2. Summary of the cohort-based studies included in the analysis.
| Author | Date | Place | Race | Mean age at diagnosis | Design | Cohort |
| Brandt6 | 1961–2006 | Sweden | Caucasian | NA, aged younger than 75 | Cohort study | 3,900,000 men from the Swedish cancer registry |
| Cerhan78 | 1987–1995 | USA, Iowa | Caucasian | 73,6 A | Prospective cohort study | 1557 population-based controls from case-control study in Iowa from 1987–1989 |
| Chen49 | 1990–2004 | USA | Caucasian | NA | Prospective cohort study B | 43494 men from the Health Professionals Follow-Up Study cohort |
| Kalish79 | 1987–1997 | USA, Boston area | Caucasian | 65,2 A | Retrospective cohort study | 1149 men from the Massachusetts Male Aging Cohort |
| Kerber7 | 1966–2000 | USA, Utah | Caucasian | NA | Nested case-control, density samping | 11572 cases and 11572 controlsfrom the Utah Population Database |
| Park80 | 1993–2006 | USA, California | Mixed | NA | Nested case-control, cumulative sampling | 729 cases and 729 controls from the Multiethnic Cohort |
| Schuurman81 | 1986–1992 | The Netherlands | Caucasian | 64,2 A | Prospective cohort study | 52879 men from the Municipal population registries |
| Sun82 | 1992–2006 | USA | Caucasian | NA | Nested case-control, density sampling | 1157 cases and 1157 controls from the Prostate Lung and Ovarian Cancer Screening Trial cohort |
Calculated from the reported distribution of age of the cases at diagnosis.
Data on exposure to family history was available at baseline, however the updated data from 1996 was used in the analysis.
Data analysis
The combined estimates were expressed with the ratio of incidence rates (RR) among those exposed and those not exposed. The hazard ratios, the odds ratios from the logistic regression models, and the odds ratios calculated from the contingency tables were assumed to estimate the rate ratios. This measure of association was considered appropriate to express the combined estimates for several reasons. Firstly, the hazard ratios, which can be estimated in cohort studies and density sampling case-control studies [83], are valid estimates of the rate ratios [84]. Also the odds ratios from the logistic regression models from density sampling case-control studies can be used to estimate the rate ratios without any adjustments [85]. Moreover, the bias introduced by estimating the rate ratios with the odds ratios is in general smaller than when the risk ratios are estimated from the odds ratios [85].
When only raw data in a case-control study with age-matched controls was available, the odds ratio and the confidence interval were calculated from the contingency table. In case several measures were available, the one adjusted for more variables was preferred. If the results were reported in strata, the fixed effects model was used to obtain the pooled estimate from the study. Similarly to the previous meta-analyses on familial clustering of prostate cancer [3]–[5], the estimates for men with affected father relative to men without affected father were pooled with those based on the reference category of men without family history in first-degree family members. The same analysis strategy was applied for men with affected brother(s). The estimates based on the reference categories ‘men without affected brothers’ and ‘men without affected first-degree relatives’ were pooled in one analysis.
In order to assess the public health implications of our findings, we estimated the attributable fractions among those with a family history of prostate cancer [86]. The measure was defined as the proportion of the disease incidence attributable to the exposure. The attributable fraction among those exposed can be expressed as: AFE = (RR-1)/RR [84]. We estimated AFE by plugging in our estimates of the rate ratios in the equation, which is often referred to as the Mantel-Haenszel approach [87]. The confidence bounds were obtained using the Wald method [88], with the standard deviations estimated by the Monte Carlo simulation [89].
The estimates from the individual studies were combined on the log scale. The Cochran's Q statistic showed evidence of heterogeneity greater than expected by the sampling variance alone. Therefore, we used the random effects model to obtain the combined estimates. For every combined estimate the funnel plot, i.e. a plot of effects estimates versus their standard error, was visually examined and the Egger's asymmetry test [90] was used in order to assess the presence of a publication bias. A meta-regression was carried out to investigate the effect of study design (cumulative sampling case-control versus other), ethnicity (Caucasian versus other), country (US versus other) and publication year on the rate ratio for first-degree family history. The effect of ethnicity on the combined estimate was estimated using the mixed effects model. To evaluate the effect of using the odds ratios from the cumulative-sampling case-control studies as estimates of the rate ratio, we also estimated the rate ratio for first-degree family history using only the cumulative-sampling case-control studies and compared it with the rate ratio based only on the density-sampling case-control studies. Sensitivity of the findings was examined by recalculation of the pooled association sizes after exclusion of studies one by one. As all studies reporting familial aggregation for men under 65 years old also provided data for the age group 65 and older, the paired t-test was used to assess the significance of the difference. The analysis was conducted in SAS (version 9.2; Cary, NC, USA). The rmeta package of the R software (version 2.11.1) was used for the plots.
Results
Pooled estimates
The pooled rate ratio for men with first-degree family history, i.e. affected father or/and brother(s), was based on 19 case-control [30], [57]–[60], [62]–[68], [70]–[72], [74]–[77], 3 nested case-control [7], [80], [82] and 4 cohort studies [6], [49], [78], [81]. 18 independent studies [6], [30], [49], [55]–[57], [60], [61], [63]–[66], [70], [71], [73], [75], [78], [81] provided data for men with history of prostate cancer in father and 16 [6], [30], [49], [57], [61], [63]–[66], [70], [71], [73], [75], [78], [79], [81] for men with history of prostate cancer in a brother(s). The combined estimate for history of prostate cancer in a second-degree relative was based on 5 studies [7], [58], [64], [66], [70]. Seven studies [6], [57], [62], [63], [66], [71], [76] were used to estimate the rate ratio for 2 or more affected first-degree family members. Five studies [6], [49], [63], [65], [71] provided data for the different age groups.
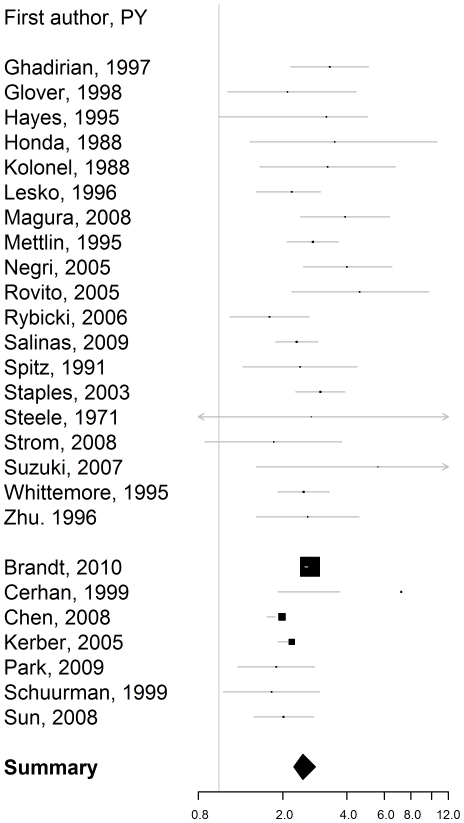
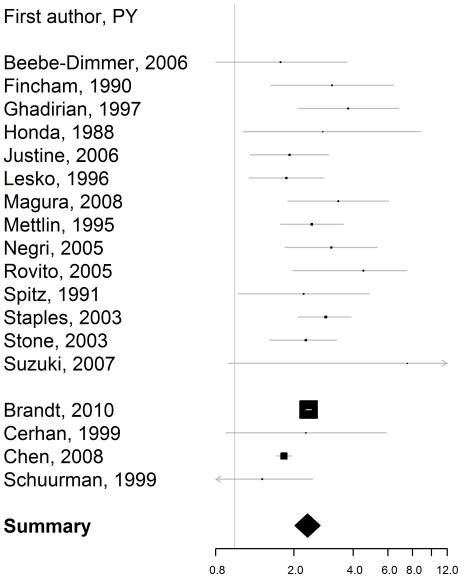
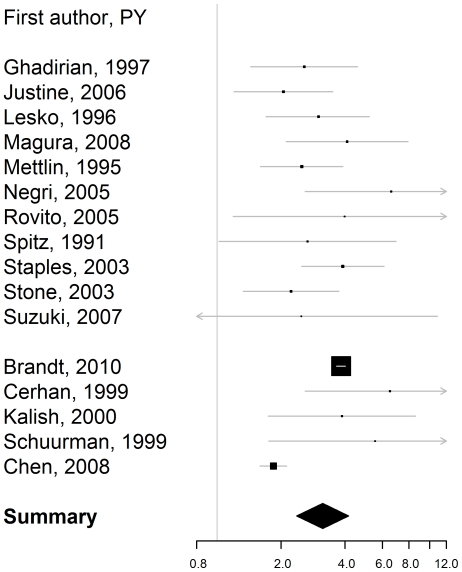
The combined rate ratios and the corresponding attributable fractions among those exposed are reported in Table 3. The rate ratios for firstdegree family history, i.e. affected father or/and brother(s) (Figure 1), affected father (Figure 2), and affected brother(s) (Figure 3) were bigger than 2, with the confidence level 95%. The estimated rate ratio for two or more affected first-degree relatives equaled 4.39 (95% confidence interval: 2.61–7.39). The rate ratio for first-degree family history was significantly higher for men younger than 65, than for men aged 65 or older, p = 0.002.
Table 3. Estimates of the rate ratios and the attributable fractions among men with different types of family history.
| Type of clustering | RR (95% CI) | AFE (95% CI) | N |
| 1st degree relatives | |||
| For all men | 2.48 (2.25–2.74) | 59.7% (55.6–63.5%) | 26 |
| For men before the age of 65 | 2.87 (2.21–3.74) | 65.2% (57.7–71.4%) | 5 |
| For men aged 65 or older | 1.92 (1.49–2.47) | 47.9% (37.1–56.8%) | 5 |
| Affected father | 2.35 (2.02–2.72) | 57.4% (50.7–63.1%) | 18 |
| Affected brother(s) | 3.14 (2.37–4.15) | 68.1% (58.1–75.7%) | 16 |
| 2+ 1st degree relatives | 4.39 (2.61–7.39) | 77.2% (65.4–85.0%) | 7 |
| 2nd degree relatives | 2.52 (0.99–6.46) | 60.4% (19.8–80.4%) | 5 |
RR: Rate Ratio, AFE: attributable fraction among those exposed, N: number of studies the estimates are based on.
Figure 1. Rate ratio of prostate cancer for first-degree family history, i.e. affected father or brother relative to no first-degree family history.
Estimates from the case-control studies are presented at the top. They are separated from the estimates from the cohort-based studies with a line break.
Figure 2. Rate ratio of prostate cancer for a history of prostate cancer in father.
Estimates from the case-control studies are presented at the top. They are separated from the estimates from the cohort studies with a line break.
Figure 3. Rate ratio of prostate cancer for a history of prostate cancer in brother(s).
Estimates from the case-control studies are presented at the top. They are separated from the estimates from the cohort studies with a line break.
59.7% of the incidence of prostate cancer in men with an affected first-degree relative could be attributed to this risk factor (CI: 55.6–63.5%). When two or more first-degree family members were affected, the attributable fraction equaled 77.2% (CI: 65.4–85.0%). For men younger than 65, the estimated attributable fraction equaled 65.2% (CI: 57.7%–71.4%) and for men 65 or older 47.9% (CI: 37.1–56.8%).
Publication bias and sensitivity analysis
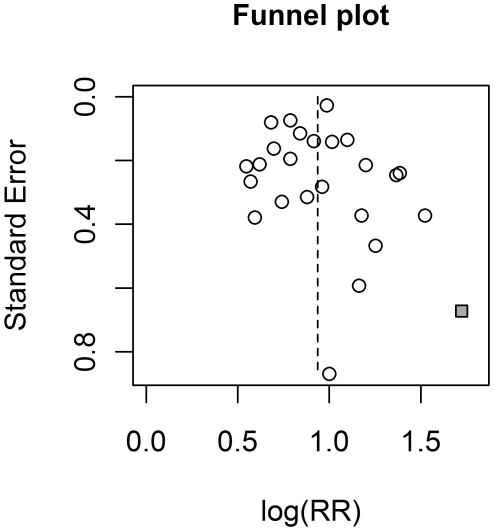
The results of the Egger's test for first-degree family history (p = 0.99), affected father (p = 0.86), affected brother(s) (p = 0.33), and affected second-degree relative (p = 0.06) did not indicate a publication bias. The funnel plot for first-degree family history suggests a potential publication bias (see Figure 4). In particular, the rate ratio for the study by Suzuki et al. [75], a relatively small study, is by far the largest from all the studies included in the analysis. The sensitivity analysis showed that neither a decision to include this study, nor any other shifted the estimate of the rate ratio for first-degree family history or affected father more than by 0.1. Analysis conducted without the study by Chen et al. [49] gave the rate ratio for affected brother(s) 0.18 bigger than the estimate based on all the studies. Excluding none of the other studies led to a change by more than 0.12.
Figure 4. Funnel plot for affected first-degree relatives.
The study of Suzuki et al., which may be subject to publication bias, is indicated with a square.
The combined estimate for men aged 65 or older did not change by more than 0.1, when one of the studies it was based on was excluded. For the age category of men younger than 65, it increased from 2.87 to 3.38 when the study by Chen et al. [49] was not included, which was the largest change of the estimate when one of the studies it was based on was excluded. The rest of the estimates appeared to be more sensitive to changes in the sample of studies. The studies by Magura et al. [64] and Kerber et al. [7] had the largest effect on the estimate of the rate ratio for affected second-degree family members. Excluding them changed the estimate from 2.52 to 2.08 and 3.29 respectively. Not including the study by Staples et al. [71] in the sample used to estimate the rate ratio for two or more affected first-degree relatives led to a decrease of the estimate from 4.39 to 3.89, and not using the study by Lesko et al. [63] to an increase to 4.96, which were the largest changes of the estimate when one of the studies it was based on was excluded. Including the study by McCahy et al. [22] would not change any of the combined estimates by more than 0.1.
The meta-regressions showed that neither the study design, country, nor year of publication had a significant influence on the combined estimates for first-degree relatives (p = 0.40 for study design, p = 0.08 for country, and p = 0.29 for publication year). The estimated multiplicative effect of ethnicity on the rate ratio equaled 1.04 (CI:0.83–1.30). The estimated rate ratio equaled 2.50 for Caucasian populations and 2.41 for other populations. This difference was not significant, p = .75. The estimated rate ratio based only on the cumulative-sampling case-control studies and only on the density-sampling case control studies equaled 2.61 (CI:2.25–3.02) and 2.44 (CI:2.08–2.87) respectively.
Discussion
We identified 74 articles reporting information on the association between family history and prostate cancer from studies conducted in 16 countries in North America, Europe, Asia and Australia. The identified studies differed regarding the study design, the analysis, the way family history was ascertained, the investigated type of clustering, and the reference category that they used. 25 case-control studies and 8 cohort-based studies with non-overlapping populations from 8 countries and 4 continents met the inclusion criteria and reported data on the considered types of clustering. According to our estimates, almost 60% of the prostate cancer incidence among men with first-degree family history is attributable to this risk factor.
The sensitivity analysis showed that the individual study results had a small influence on the pooled estimates of the rate ratio for first-degree family history, affected brother(s), and affected father. The results for affected second-degree relatives and to 2 or more affected first-degree family members, which are based on a small number of studies, are more sensitive to changes in the sample of studies used in the analysis and, therefore, should be treated with caution. The meta-regression and the similarity between the combined estimates based only on the cumulative-sampling case-control studies and only on the density-sampling case-control studies suggested that using the odds ratios from the cumulative-sampling case-control studies as estimates of the rate ratios did not substantially bias our estimates.
We did not attempt to identify unpublished studies. However, neither the visual examination nor the statistical procedures suggested that publication bias could have an important effect on the estimates. With the exception of the study by Glover et al. [58], the studies included in the analysis were conducted in developed countries, most of them in the USA. Our analysis did not suggest that a difference in the strength of familial clustering between the USA and other countries exists. However, generalizing the results to males not living in developed countries may be inappropriate. The population in most of the considered studies was Caucasian. However, the meta-regression showed that the effect of ethnicity on the rate ratio for first-degree family history was small and not significant. This finding suggests that the strength of the association between family history and prostate cancer for Caucasian males is similar as in other populations.
The amount of evidence on the relationship between family history and prostate cancer that was available in our study was much larger, than in the previous meta-analysis. Since 2003, the strength of the associations has been investigated in a number of new populations and a more recent data has been reported from the Swedish cancer register and the Utah population database. This allowed using stringent inclusion criteria and at the same time retaining a substantial number of studies. In contrast to the previous works, several measures were taken in order to improve the quality of the analysis. To avoid a possible bias caused by confounders the studies among specific populations such as smokers [10] were excluded. As referring to prostate examination may occur more frequently among men with prostate cancer family history, the cross-sectional data gathered among males referred to examination by a doctor [11], [12], [14] was not used. Homogeneity of the studies was assured by including only the studies in which family history was defined and corresponding to one of the investigated types of clustering. Finally, as pooling of the studies required the assumption of independence, overlaps in study populations were avoided.
The results confirm the conclusions made in the previous meta-analyses [3]–[5] and support the American Cancer Society guidelines. We observed more than a 2-fold increase in the incidence rate of the disease for all of the investigated types of familial clustering, meaning that over 50% of prostate cancer cases among men with a certain type of family history are attributable to familial clustering of the disease. Having a brother with prostate cancer appears to be associated with a larger increase in the incidence rate than being a son of a father with prostate cancer. The incidence rate increases with an increasing number of affected family members. Family history appears to increase the incidence rate of prostate cancer for males younger than 65 more, than for males aged 65 and older, which suggests the relative importance of genetic factors and/or shared environment and/or food factors in an early onset of prostate cancer. In line with our conclusions, the American Cancer Society (ACS) recommends that men at average risk should be offered testing beginning at age 50, and that men at increased risk for prostate cancer, such as those with a history of the disease in a father or brother at a young age, should begin testing with both the prostate specific antigen blood test and the digital rectal examination at age 45, or even younger if they have multiple relatives with the disease.
Supporting Information
Flow of Included Studies.
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The study was supported by Scientific Fund FWO (URL: www.fwo.be, krediet aan navorsors; 1.5.158.09.N.00), and Micha$ Kiciński has a Ph.D. fellowship of the Research Foundation – Flanders (FWO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Damber JE, Aus G. Prostate cancer. Lancet. 2008;371:1710–21. doi: 10.1016/S0140-6736(08)60729-1. [DOI] [PubMed] [Google Scholar]
- 2.Noe M, Schroy P, Marie-France D, Babayan R, Geller A. Increased cancer risk for individuals with a family history of prostate cancer, colorectal cancer, and melanoma and their associated screening recommendations and practices. Cancer Causes and Control. 2007;19:1–12. doi: 10.1007/s10552-007-9064-y. [DOI] [PubMed] [Google Scholar]
- 3.Bruner DB, Moore D, Parlanti A, Dorgan J, Engstrom P. Relative risk of prostate cancer for men with affected relatives: systematic review and meta-analysis. Int J Cancer. 2003;107:797–803. doi: 10.1002/ijc.11466. [DOI] [PubMed] [Google Scholar]
- 4.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU International. 2003;91:789–794. doi: 10.1046/j.1464-410x.2003.04232.x. [DOI] [PubMed] [Google Scholar]
- 5.Zeegers MPA, Jellema A, Ostrer H. Empiric risk of prostate carcinoma for relatives of patients with prostate carcinoma. A meta-analysis. Cancer. 2003;97:1894–1903. doi: 10.1002/cncr.11262. [DOI] [PubMed] [Google Scholar]
- 6.Brandt A, Bermejo JL, Sundquist J, Hemminki K. Age-specific risk of incident prostate cancer and risk of death from prostate cancer defined by the number of affected family members. European Urology. 2010;58:275–280. doi: 10.1016/j.eururo.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Kerber RA, O'Brien EO. A cohort study of cancer risk in relation to family histories of cancer in the Utah population database. Cancer. 2005;103:1906–1915. doi: 10.1002/cncr.20989. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs SD, Kiemeney L, Baffoe-Bonnie A, Beaty TH, Walsh PC. Risk of cancer in relatives of prostate cancer probands. J Natl Cancer Inst. 2005;87:991–996. doi: 10.1093/jnci/87.13.991. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC. Family history and the risk of prostate cancer. The prostate. 1990;17:337–347. doi: 10.1002/pros.2990170409. [DOI] [PubMed] [Google Scholar]
- 10.Ahn J, Moslehi R, Weinstein J, Snyder K, Virtamo J, et al. Family history of prostate cancer and prostate cancer risk in the alpha-tocopherol, beta-carotene caner preventions (ATBC) study. Int. J Cancer. 2008;123:1154–1159. doi: 10.1002/ijc.23591. [DOI] [PubMed] [Google Scholar]
- 11.Aprikian AG, Bazinet M, Plante M, Meshref A, Trudel C, et al. Family history and the risk of prostatatic carcinoma in a high risk group of urological patients. The journal of urology. 1995;154:404–406. doi: 10.1097/00005392-199508000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez DJ, Han M, Humphreys EB, Mangold LA, Taneja SS, et al. Predicting the outcome of prostate biopsy: comparison of a novel logistic regression-based model, the prostate cancer risk calculator and prostate-speciic antigen level alone. BJU International. 2008;103:609–614. doi: 10.1111/j.1464-410X.2008.08127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mäkinen T, Tammela TL, Stenman U, Määttänen L, Rannikko S, et al. Family history and prostate cancer screening with prostate-specific antigen. J Clin Oncol. 2002;20:2658–2663. doi: 10.1200/JCO.2002.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Nam RK, Toi A, Laurence HK, Trachtenberg J, Jewett MA, et al. Assessing individual risk for prostate cancer. J Clin Oncol. 2007;25:3582–3588. doi: 10.1200/JCO.2007.10.6450. [DOI] [PubMed] [Google Scholar]
- 15.Monroe KR, Mimi CY, Kolonel LN, Coetzee GA, Wilkens LR, et al. Evidence of an X-linked recessive genetic component to prostate cancer risk. Nature Medicine. 1995;1:827–829. doi: 10.1038/nm0895-827. [DOI] [PubMed] [Google Scholar]
- 16.Narod SA, Dupont A, Cusan L, Diamond P, Gomez J, et al. The impact of family history on early detection of prostate cancer. Nature medicine. 1995;1:99–101. doi: 10.1038/nm0295-99. [DOI] [PubMed] [Google Scholar]
- 17.Roemeling S, Monique JR, Vries SH, Gosselaar C, Van der Kwast TH, et al. Prevalence, treatment modalities and prognosis of familial prostate cancer in a screened population. The Journal of Urology. 2006;175:1332–1336. doi: 10.1016/S0022-5347(05)00698-1. [DOI] [PubMed] [Google Scholar]
- 18.Ghadirian P, Cadotte M, Lacroix A, Perret C. Family aggregation of cancer of the prostate in Quebec: The tip of the Iceberg. The prostate. 1991;19:43–52. doi: 10.1002/pros.2990190105. [DOI] [PubMed] [Google Scholar]
- 19.Multigner L, Ndong JR, Giusti A, Romana M, Delacroix-Maillard H. Exposure and Risk of Prostate Cancer. J Clin Oncol. 2010;28:3457–3462. doi: 10.1200/JCO.2009.27.2153. [DOI] [PubMed] [Google Scholar]
- 20.Rennert H, Zeigler-Johnson CM, Addya K, Finley MJ, Walker AH, et al. Association of susceptibility alleles in ELAC2/HPC2, RNASEL/HPC1, and MSR1 with prostate cancer severity in European American and African men. Cancer Epidemiol Biomarkers Prev. 2005;14:949–957. doi: 10.1158/1055-9965.EPI-04-0637. [DOI] [PubMed] [Google Scholar]
- 21.Bai Y, Gao Y, Deng J, Sesterhenn IA, Fraumeni JF, et al. Risk of prostate cancer and family history of cancer: a population based study in China. Prostate Cancer and Prostatic Diseases. 2005;8:60–65. doi: 10.1038/sj.pcan.4500775. [DOI] [PubMed] [Google Scholar]
- 22.McCahy PJ, Harris PJ, Neal DE. Breast and prostate cancer in the relatives of men with prostate cancer. Br J Urol. 1996;78:552–556. doi: 10.1046/j.1464-410x.1996.17111.x. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham GR, Ashton CM, Annegers JF, Souchek J, Klima M, et al. Familial aggregation of prostate cancer in African-Americans and white Americans. The Prostate. 2003;56:256–262. doi: 10.1002/pros.10252. [DOI] [PubMed] [Google Scholar]
- 24.Eldon BJ, Jonsson E, Tomasson J, Tryggvadottir L, Tulinius H. Familial risk of prostate cancer in Iceland. BJU International. 2003;92:915–919. doi: 10.1111/j.1464-410x.2003.04536.x. [DOI] [PubMed] [Google Scholar]
- 25.Matikainen MP, Pukkala E, Schleutker J, Tammela TL, Kovisto P, et al. Relatives of prostate cancer patients have an increased risk of prostate and stomach cancers: a population-based, cancer registry study in Finland. Cancer Causes and Control. 2001;12:223–230. doi: 10.1023/a:1011283123610. [DOI] [PubMed] [Google Scholar]
- 26.Keetch DW, Rice JP, Suarez BK, Catalona WJ. Familial aspects of prostate cancer: a case control study. The Journal of Urology. 1995;154:2100–2102. [PubMed] [Google Scholar]
- 27.Verhage BA, Aben KK, Witjes JA, Straatman H, Schalken JA, et al. Site-specific familial aggregation of prostatic cancer. Int. J Cancer. 2004;109:611–617. doi: 10.1002/ijc.20015. [DOI] [PubMed] [Google Scholar]
- 28.Lightfoor N, Conlon M, Kreiger N, Sass-Kortsak A, Purdham J, et al. Medical history, sexual, and maturational factors and prostate cancer risk. Ann Epidemiol. 2004;14:655–662. doi: 10.1016/j.annepidem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Pu YS, Wu H, Wu T, Lai MK, et al. Cadmium burden and the risk and phenotype of prostate cancer. BMC Cancer. 2009;9:429. doi: 10.1186/1471-2407-9-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Negri E, Pelucchi C, Talamini R, Montella M, Gallus S, et al. Family history of cancer and the risk of prostate cancer and benign prostatic hyperplasia. Int. J Cancer. 2005;114:648–652. doi: 10.1002/ijc.20755. [DOI] [PubMed] [Google Scholar]
- 31.Gallus S, Roberto F, Foschi R, Talamini R, Altieri A, et al. Risk factors for prostate cancer in men aged less than 60 years: a case-control study from Italy. Urology. 2007;70:1121–1126. doi: 10.1016/j.urology.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 32.Randi G, Pelucchi C, Negri E, Talamini R, Galeone C, et al. Family history of urogenital cancers in patients with bladder renal cell and prostate cancers. Int. J Cancer. 2007;121:2748–2752. doi: 10.1002/ijc.23037. [DOI] [PubMed] [Google Scholar]
- 33.Krain LS. Some epidemiologic variables in prostatic carcinoma in California. Preventive Medicine. 1974;3:154–159. doi: 10.1016/0091-7435(74)90070-x. [DOI] [PubMed] [Google Scholar]
- 34.Krain LS. Epidemiologic variables in prostatic cancer. Incidence rates on prostatic cancer relate to veneral disease, coital frequency, number of sexual partners, and use of contraceptives. Geriatrics. 1973:93–98. [PubMed] [Google Scholar]
- 35.Kristoffersson U, Lundgren R, Olsson H. The risk of malignant tumors in first-degree relatives of men with early onset prostate cancer: a population-based cohort study. European Journal of Cancer. 1997;33:2237–2240. doi: 10.1016/s0959-8049(97)00320-1. [DOI] [PubMed] [Google Scholar]
- 36.Damber L, Grönberg H, Damber J. Familial prostate cancer and possible associated malignancies: nation-wide register cohort study in Sweden. Int. J Cancer. 1998;78:293–297. doi: 10.1002/(SICI)1097-0215(19981029)78:3<293::AID-IJC5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Grönberg H, Damber L, Damber JE. Familial prostate cancer in Sweden. A nationwide register cohort study. Cancer. 1996;77:138–143. doi: 10.1002/(SICI)1097-0142(19960101)77:1<138::AID-CNCR23>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Grönberg H, Wiklund F, Damber JE. Age specific risks of familial prostate carcinoma. A basis for screening recommendations in high risk populations. Cancer. 1999;86:477–483. [PubMed] [Google Scholar]
- 39.Hemminki K, Dong C. Familial prostate cancer from the family-cancer database. European Journal of Cancer. 2000;36:229–234. doi: 10.1016/s0959-8049(99)00263-4. [DOI] [PubMed] [Google Scholar]
- 40.Hemminki K, Li X, Plna K, Granström C, Vaittinen P. The nation-wide Swedish family-cancer database. Acta Oncologica. 2001;40:772–777. doi: 10.1080/02841860152619214. [DOI] [PubMed] [Google Scholar]
- 41.Hemminki K, Czene K. Attributable risks of familial cancer from the family-cancer database. Cancer Epidemiol Biomarkers Prev. 2002;11:1638–1644. [PubMed] [Google Scholar]
- 42.Hemminki K, Li X, Czene K. Age specific and attributable risks of familial prostate carcinoma from the maily-cancer database. Cancer. 2002;15:346–353. doi: 10.1002/cncr.10819. [DOI] [PubMed] [Google Scholar]
- 43.Hemminki K, Li X, Czene K. Familial risk of cancer: data for clinical counseling and cancer genetics. Int. J Cancer. 2003;108:109–114. doi: 10.1002/ijc.11478. [DOI] [PubMed] [Google Scholar]
- 44.Hemminki K, Sundquist J, Bermejo JL. How common is familial cancer? Annals of Oncology. 2008;19:163–167. doi: 10.1093/annonc/mdm414. [DOI] [PubMed] [Google Scholar]
- 45.Ji J, Eng C, Hemminki K. Familial risk for soft tissue tumors: a nation-wide epidemiological study from Sweden. J Cancer Res Clin Oncol. 2008;134:617–624. doi: 10.1007/s00432-007-0327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon L, Bishop DT, Skolnick M, Hunt S, Lyon JL, et al. Genetic epidemiology of prostate cancer in the Utah Mormon genealogy. Cancer Surveys. 1982;1:47–69. [Google Scholar]
- 47.Goldgar DE, Douglas FE, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. Journal of the National Cancer Institute. 1994;86:1600–1608. doi: 10.1093/jnci/86.21.1600. [DOI] [PubMed] [Google Scholar]
- 48.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int. J Cancer. 2007;121:1571–1578. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Page JH, Chen R, Giovannucci E. Family history of prostate and breast cancer and the risk of prostate cancer in the PSA Era. The Prostate. 2008;68:1582–1591. doi: 10.1002/pros.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersson S, Baron J, Bergström R, Lindgren C, Wolk A, et al. Lifestyle factors and prostate cancer risk: a case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 1996;5:509–513. [PubMed] [Google Scholar]
- 51.Bratt O, Kristoffersson U, Lundgren R, Olsson H. Familial and hereditary prostate cancer in southern Sweden. A population-based case-control study. European Journal of Cancer. 1999;35:271–277. doi: 10.1016/s0959-8049(98)00358-x. [DOI] [PubMed] [Google Scholar]
- 52.Zheng SL, Sun J, Wiklund F, Smith S, Stattin P, et al. Cumulative association of five genetic variants with prostate cancer. N Engl J Med. 2008;358:910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 53.Zheng SL, Sun J, Wiklund F, Gao Z, Stattin P, et al. Genetic variants and family history predict prostate cancer similar to prostate-specific antigen. Clin Cancer Res. 2009;15:1105–1111. doi: 10.1158/1078-0432.CCR-08-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer causes and control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 55.Beebe-Dimmer JL, Drake EA, Dunn RL, Bock CH, Montie JE, et al. Association between family history of prostate and breast cancer among African-American men with prostate cancer. Urology. 2006;68:1072–1076. doi: 10.1016/j.urology.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 56.Fincham SM, Hill GB, Hanson J, Wijayasinghe C. Epidemiology of prostate cancer. The Prostate. 1990;17:189–206. doi: 10.1002/pros.2990170303. [DOI] [PubMed] [Google Scholar]
- 57.Ghadirian P, Howe GR, Hislop TG, Maisonneuve P. Family history of prostate cancer: a multi-center case-control study in Canada. Int. J Cancer. 1997;70:679–681. doi: 10.1002/(sici)1097-0215(19970317)70:6<679::aid-ijc9>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 58.Glover FE, Coffey DS, Douglas LL, Russel H, Cadigan M, et al. Familial study of prostate cancer in Jamaica. Urology. 1998;52:441–443. doi: 10.1016/s0090-4295(98)00200-3. [DOI] [PubMed] [Google Scholar]
- 59.Hayes RB, Lifi JM, Pottern LM, Greenberg RS, Schoenberg JB, et al. Prostate cancer risk in U.S. blacks and whites with a family history of cancer. Int. J Cancer. 1995;60:361–364. doi: 10.1002/ijc.2910600315. [DOI] [PubMed] [Google Scholar]
- 60.Honda GD, Bernstein L, Ross RK, Greenland S, Gerkins V, et al. Vasectomy, cigarette smoking, and age at first sexual intercourse as risk factors for prostate cancer in middle-aged men. Br. J Cancer. 1988;57:326–331. doi: 10.1038/bjc.1988.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Justine L, Gina A, Lin F. Vietnam military service history and prostate cancer. BMC Public Health. 2006;6:75. doi: 10.1186/1471-2458-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol. 1988;127:999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- 63.Lesko SM, Rosenberg L, Shapiro S. Family history and prostace cancer risk. Am J Epidemiol. 1996;144:1041–1047. doi: 10.1093/oxfordjournals.aje.a008876. [DOI] [PubMed] [Google Scholar]
- 64.Magura L, Blanchard R, Hope B. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes Control. 2008;19:1259–1266. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 65.Mettlin C, Natarajan N, Huben R, Raghavan D. Reported family history of cancer in 1,271 prostate cancer cases and 1,909 controls. Urol Oncol. 1995;1:240–245. doi: 10.1016/1078-1439(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 66.Rovito PM, Jr, Morse PD, Spinek K, Newman N, Jones RF, et al. Heterocyclic amines and genotype of N-acetyltransferases as risk factors for prostate cancer. Prostate cancer and prostatic diseases. 2005;8:69–74. doi: 10.1038/sj.pcan.4500780. [DOI] [PubMed] [Google Scholar]
- 67.Rybicki BA, Neslund-Dudas C, Nock NL, Schultz LR, Eklund L, et al. Prostate cancer risk from occupational exposure to polycyclic aromatic hydrocarbons interacting with GSTP1 Ile105Val polymorphism. Cancer detection and prevention. 2006;30:412–422. doi: 10.1016/j.cdp.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salinas CA, Koopmeiners JS, Kwon EM, Fitzgerald L, Lin DW, et al. Clinical utility of five genetic variants for predicting prostate cancer risk and mortality. The prostate. 2009;69:363–372. doi: 10.1002/pros.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuman LM, Mandel J, Blackard C, Bauer H, Scarlett J, et al. Epidemiologic study of prostatic cancer: preliminary report. Cancer Treat Rep. 1977;61:181–186. [PubMed] [Google Scholar]
- 70.Spitz MR, Currier RD, Fueger JJ, Babaian RJ, Newell GR. Familial patterns of prostate cancer: a case-control analysis. The Journal of Urology. 1991;146:1305–1307. doi: 10.1016/s0022-5347(17)38074-6. [DOI] [PubMed] [Google Scholar]
- 71.Staples MP, Giles GG, English DR, McCredie MR, Severi G, et al. Risk of prostate cancer associated with a family history in an era of rapid increase in prostate cancer diagnosis (Australia). Cancer Causes and Control. 2003;14:161–166. doi: 10.1023/a:1023073203467. [DOI] [PubMed] [Google Scholar]
- 72.Steele R, Lees RE, Kfarus AS, Rao C. Sexual factors in the epidemiology of cancer of the prostate. J Chron Dis. 1971;24:29–37. doi: 10.1016/0021-9681(71)90056-7. [DOI] [PubMed] [Google Scholar]
- 73.Stone NS, Hoffman RM, Tollestrup K, Stidley AC, Witter JL, et al. Family history, Hispanic ethnicity, and prostate cancer risk. Ethnicity and disease. 2003;13:233–239. [PubMed] [Google Scholar]
- 74.Strom SS, Yamamura Y, Flores-Sandoval FN, Petteway CA, Lopez DS. Prostate cancer in Mexican-Americans: identification of risk factors. The prostate. 2008;68:563–570. doi: 10.1002/pros.20713. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki T, Matsuo K, Kenji W, Hiraki A, Hiroso K, et al. Effect of familial history and smoking on common cancer risks in Japan. Cancer. 2007;109:2116–2123. doi: 10.1002/cncr.22685. [DOI] [PubMed] [Google Scholar]
- 76.Whittemore AS, Wu AH, Kolonel LN, John EM, Gallagher RP, et al. Family history and prostate cancer risk in black, white, and Asian men in the United States and Canada. Am J Epidemiol. 1995;141:732–740. doi: 10.1093/oxfordjournals.aje.a117495. [DOI] [PubMed] [Google Scholar]
- 77.Zhu K, Stanford JL, Daling JR, MCKnight B, Stergachis A, et al. Vasectomy and prostate cancer: a case-control study in a health maintenance organization. Am J Epidemiol. 1996;144:717–722. doi: 10.1093/oxfordjournals.aje.a008994. [DOI] [PubMed] [Google Scholar]
- 78.Cerhan JR, Parker AS, Putnam SD, Chiu BC, Lynch CF, et al. Family history and prostate cancer risk in a population-based cohort of Iowa men. Cancer Epidemiol Biomarkers Prev. 1999;8:53–60. [PubMed] [Google Scholar]
- 79.Kalish LA, McDougal WS, McKinlay JB. Family history and the risk of prostate cancer. Urology. 2000;56:803–806. doi: 10.1016/s0090-4295(00)00780-9. [DOI] [PubMed] [Google Scholar]
- 80.Park SY, Wilkens LR, Henning SM, Marchand LL, Gao K, et al. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20:211–223. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schuurman AG, Zeegers MP, Goldbohm A, Van Den Brandt PA. A case-cohort study on prostate cancer risk in relation to family history of prostate cancer. Epidemiology. 1999;10:192–195. [PubMed] [Google Scholar]
- 82.Sun J, Chang B, Isaacs SD, Wiley KE, Wiklund F, et al. Cumulative effect of five genetic variants on prostate cancer risk in multiple study populations. The Prostate. 2008;68:1257–1262. doi: 10.1002/pros.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.King G, Zeng L. Estimating risk and rate levels, ratios and differences in case-control studies. Statistics in medicine. 2002;21:1409–1427. doi: 10.1002/sim.1032. [DOI] [PubMed] [Google Scholar]
- 84.Benichou J. Biostatistics and epidemiology: measuring the risk attributable to an environmental or genetic factor. C.R. Biologies. 2007;330:281–298. doi: 10.1016/j.crvi.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 85.Rothman KJ, Greenland S, editors. Philadelphia, PA: Lippincott-Raven; 1998. Modern epidemiology. 2nd ed. [Google Scholar]
- 86.Nawrot TS, Perez L, Künzli N, Munters E, Nemery B. Public health importance of triggers of myocardial infarction: a comparative risk assessment. Lancet. 2011;377:732–740. doi: 10.1016/S0140-6736(10)62296-9. [DOI] [PubMed] [Google Scholar]
- 87.Benichou J. A review of adjusted estimators of attributable risk. Statistical Methods in Medical Research. 2001;10:195–216. doi: 10.1177/096228020101000303. [DOI] [PubMed] [Google Scholar]
- 88.Greenland S. Estimation of population attributable fractions from fitted incidence ratios and exposure survey data, with an application to electromagnetic fields and childhood leukemia. Biometrics. 2001;57:182–188. doi: 10.1111/j.0006-341x.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 89.Greenland S. Interval estimation by simulation as an alternative to and extension of confidence intervals. International Journal of Epidemiology. 2004;33:1389–1397. doi: 10.1093/ije/dyh276. [DOI] [PubMed] [Google Scholar]
- 90.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow of Included Studies.
(DOC)