Abstract
Background
Adefovir Dipivoxil (ADV) is an important agent to suppress hepatitis B virus (HBV) replication with suboptimal effect on virological and serological response. To optimize Adefovir therapy in chronic hepatitis B (CHB) patients with hepatitis B e antigen (HBeAg) positive, we studied the baseline parameters and on-treatment HBV DNA for favorable outcomes.
Methods
48 patients were enrolled in the study and followed up for 5 years prospectively. Baseline characteristics, virological, serological and biochemical parameters as well as on treatment HBV DNA were assessed in prediction of favorable outcomes.
Results
1. The patients with baseline alanine aminotransferase (ALT) ≥5 × the upper limit of normal (ULN, 40 IU/L) had higher rates of viral response (VR), HBeAg loss and HBeAg seroconversion at year 5 compared to the patients with ALT < 5 × ULN (VR: 75% vs 43.8%, p = 0.035; HBeAg loss: 43.9% vs 13.8%, p = 0.017; HBeAg seroconversion: 37.9% vs 13.8%, p = 0.035); Patients with baseline HBV DNA < 109 copies/ml and ALT ≥3 × ULN had more chance of HBeAg seroconversion (40.9% vs 8.7%, p = 0.012), while in patients with HBeAg < 800 s/co or HBsAg < 5000 IU/ml higher rates of HBeAg loss were achieved. 2. HBV DNA level < 104 copies/ml at week 24 was predictive for VR (96.0% vs 40.9%, P < 0.001), HBeAg loss (84.0% vs 36.3%, P = 0.001) and HBeAg seroconversion (36.0% vs 9.1%, P = 0.030).
Conclusions
ADV treatment should be started for patients with baseline ALT≥5 × ULN or patients with ALT≥3 × ULN and HBV DNA < 109 copies/ml. Lower level of HBeAg(< 800 s/co) and HBsAg(< 5000 IU/ml) may be regarded as referenced factors. In patients with serum HBV DNA < 104 copies/ml at week 24 the therapy should continue, and a favorable outcome may be achieved in 5 years or longer.
Keywords: hepatitis B, chronic, Adefovir Dipivoxil, therapy, hepatitis B virus, hepatitis Be antigen positive, predictor
Background
Adefovir Dipivoxil (ADV) is a nucleotide analogue for treatment of hepatitis B. Long term treatment with ADV is well tolerated and may produce long-term virological, biochemical, serological and histological improvement [1]. Compared with lamivudine (LAM) and telbivudine, ADV correlated resistance develops less frequently but ADV has less potent activity against hepatitis B virus (HBV). It has been reported that the rates of HBV DNA negative, hepatitis B e antigen (HBeAg) loss and HBeAg seroconversion were 39%, 58% and 48% respectively in the subjects with HBeAg positive chronic hepatitis B (CHB) treated with ADV 10 mg once daily for 5 years, and 66% of them achieved alanine aminotransferase (ALT) normalization and 20% developped HBV mutant [2]. These data highlighted an urgent need to identify the predictors that will help to find candidates who may likely benefit from ADV or, alternatively, determine whether other extensive treatment is needed. Recently, It has been reported that baseline and on-treatment serum HBV DNA were significant predictors for sustained viral response in CHB patients with pegylated interferon(Peg-IFN) and nucleos(t)ide analogues(NAs) treatment [3-8]. In the GLOBE study of HBeAg-positive patients treated with telbivudine, HBV DNA negative at week 24 was more predictive for responses than other parameters [9]. Similar results were reported in LAM and ADV therapies [10,11]. However, the association of these predictors with effect of long term ADV treatment has rarely been reported.
The aim of this study is to assess baseline and on-treatment serum markers in prediction of long term response in the early phase of treatment with ADV among HBeAg-positive CHB patients so as to identify the most beneficial patients from ADV treatment and optimize the therapy.
Methods
Subject population
48 HBeAg-positive CHB patients who enrolled in the study were treated with ADV (provided by GlaxoSmithKline) and followed up for 5 years. All patients were seropositive for hepatitis B surface antigen (HBsAg) and HBeAg for more than 6 months and with baseline ALT ≥1.2 × the upper limit of normal (ULN, 40 IU/L) and HBV DNA ≥106 copies/ml before treatment. Criteria for exclusion included serum creatinine greater than 1.5 mg/dl (130μmol/L); seropositivity for hepatitis C or D virus or human immunodeficiency virus. No patients received LAM or any other anti-HBV therapy within 6 months prior to treatment. Other treatments were not used during the therapy. Written informed consent was obtained from all patients and the study was approved by the Ethical Committee of Jinan Infectious Disease Hospital.
Study Design
All patients were followed up at weeks 4, 12, 24, 52 and every 12-16 weeks after week 52 for detection of serum biochemical parameters, serum viral load (Roche COBAS Amplicore HBV Monitor PCR assay, lower limit of detection 300 copies/ml) and HBV markers (micro particle enzyme immunoassay with ABBOTT reagents). DNA sequencing and phylogenetic analysis of HBV genome were evaluated at baseline. Serum samples at first time point from subjects who experienced a breakthrough of HBV DNA (HBV DNA level increased by 1 log10 copies/ml or more than the treatment nadir) were assessed for the development of ADV-associated mutations (rtA181V and rtN236T) in the HBV polymerase.
Efficacy End Points
The primary efficacy endpoints in this study were HBeAg seroconversion defined as combination of HBeAg loss and development of hepatitis B e antibody accompanied by HBV DNA negative(< 300 copies/ml). The secondary efficacy endpoints are HBeAg loss and viral response (VR) defined as HBV DNA negative.
Statistical Analyses
Descriptive statistics such as the mean, median, and standard deviation for each continuous variable and frequencies for each categorical variable were used to summarize the data as well as detect outliers and missing values. The associations of baseline and on-treatment parameters with virological, serological and biochemical responses were assessed using Chi-square tests or Fisher's exact tests. All p values were calculated with two-sided significance level of 0.05. Data analyses were performed using SAS 9.2 (SAS Institute, Inc, Cary, North Carolina).
Results
1. Baseline characteristics of demographic, clinical and laboratory data
Except for 1 woman who withdrew because of pregnance at week 104, 47 patients completed 5 years' follow up. All patients were ethnically Chinese and HBV genotype C positive. The baseline demographic, clinical and virological data are shown in Table 1.
Table 1.
Baseline characteristics of demographic, clinical and laboratory data
| variables | |
|---|---|
| Age in years, mean ± SD | 31.7 ± 6.6 |
| Male, % | 85.1% |
| HBV genotype C, % | 100% |
| Baseline ALT, IU/L | 216.3 ± 169.7 |
| Baseline HBV DNA(log10copies/ml) | 8.5 ± 0.8 |
| Baseline HBeAg(log10) | 2.8 ± 0.4 |
| BaselineHBsAg(log10) | 3.7 ± 0.5 |
2. Virological, serological and biochemical response
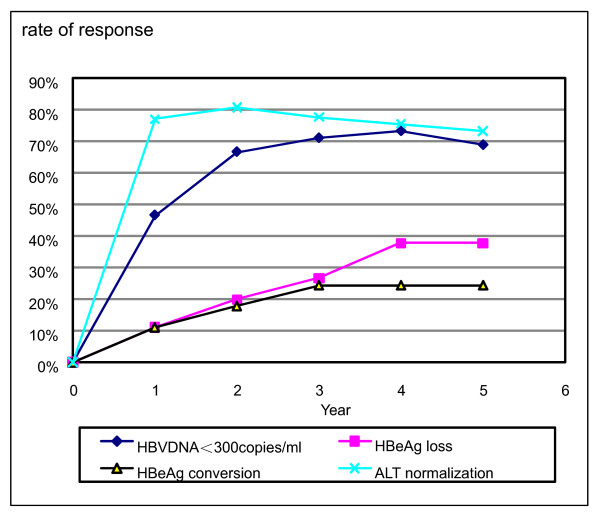
Of 47 patients, the rates of VR, accumulative HBeAg loss, HBeAg seroconversion and ALT normalization in the end of the fifth year were 66.0%, 36.2%, 23.4% and 70.2%, respectively. The rates of HBeAg loss increased over time, and the proportion of patients with ALT normalization and HBV DNA < 300 copies/ml achieved maximal values after 2 and 4 years respectively (Figure 1). Virus breakthrough was found in 4 patients but ADV correlated variants were detectable only in 3 of them.
Figure 1.
Virological, serological and biochemical response over time. The rates of VR, accumulative HBeAg loss, HBeAg seroconversion increased with time.
3. Relationship between baseline parameters and 5-year response
We evaluated baseline characteristics such as ALT, HBV DNA, HBsAg and HBeAg level in prediction of 5-year response and found baseline ALT was a potential predictor of outcome. Patients with baseline ALT ≥5 × ULN were more likely to have HBeAg seroconversion, HBeAg loss and VR than patients with ALT < 5 × ULN (Table 2), while in patients with HBeAg < 800 s/co or HBsAg < 5000 IU/ml higher rates of HBeAg loss were achieved. The baseline HBeAg level predicted VR too, as 88.9% patients with baseline HBeAg < 800 s/co became HBV DNA negative, whereas baseline HBV DNA level alone was not a significant predictor for HBeAg seroconversion, HBeAg loss or VR.
Table 2.
Baseline predictors and 5-year outcomes
| VR % | P-value | HBeAg loss % | P-value | HBeAg seroconversion % | P-value | |
|---|---|---|---|---|---|---|
| ALT≥5 × ULN | 75.0% | 0.035 | 43.9% | 0.017 | 37.9% | 0.035 |
| ALT < 5 × ULN | 43.8% | 13.8% | 13.8% | |||
| HBeAg < 800 s/co | 88.9% | 0.027 | 77.8% | 0.045 | 27.8% | 0.831 |
| HBeAg≥800 s/co | 47.6% | 38.0% | 19.0% | |||
| HBsAg < 5000 IU/ml | 80.0% | 0.132 | 80.0% | 0.026 | 27.8% | 0.200 |
| HBsAg≥ 5000 IU/ml | 59.3% | 48.1% | 24.1% | |||
| HBV DNA < 9 log10 copies/ml | 71.9% | 0.494 | 61.8% | 0.621 | 26.5% | 0.702 |
| HBV DNA≥ 9 log10copies/ml | 69.2% | 53.9% | 15.4% |
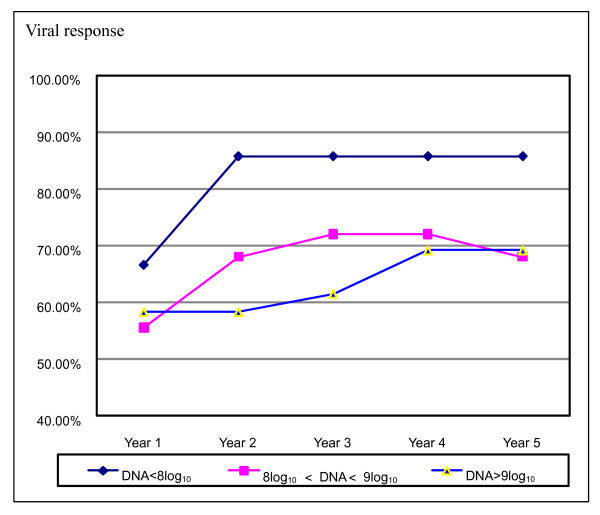
Even so, there was a trend that the rate of VR in the 5 years decreased with the higher levels of baseline viral load (Figure 2). In subgroup analyses, we found in patients with baseline HBV DNA < 109 copies/ml, ALT was positively associated with VR and HBeAg seroconversion. The rate of HBeAg seroconversion was significantly higher in patients with ALT ≥3 × ULN when compared to the patients with ALT < 3 × ULN (40.9% vs 8.7%, p = 0.012).
Figure 2.
Viral response in patients with different levels of baseline viral load. The rate of VR in the 5 years decreased with the higher levels of baseline viral load although no statistical significance was observed.
4. Impact of on-treatment HBV DNA on outcomes
The relationships between on-treatment HBV DNA levels and 5-year outcomes were presented in Table 3. Patients with serum HBV DNA level < 4 log10 copies/ml at week 24 had higher rates of HBeAg seroconversion, HBeAg loss and VR compared to the patients with serum HBV DNA ≥4 log10 copies/ml. The drop of the serum HBV DNA ≥3 log10 copies/ml at week 4 and HBV DNA undetectable at week 52 can predict VR and HBeAg loss while the drop of the serum HBV DNA ≥4 log10 copies/ml at week 12 was significantly associated with the increased rates of HBeAg loss and HBeAg seroconversion.
Table 3.
Impact of on-treatment HBV DNA on 5-year outcomes
| N | VR | P-value | HBeAg loss | P-value | HBeAg seroconversion | P-value | |
|---|---|---|---|---|---|---|---|
| drop of HBV DNA ≥3 log10copies/ml at week 4 | 12 | 91.7% | 0.06 | 91.7% | 0.013 | 41.7% | 0.083 |
| drop of HBV DNA < 3 log10copies/ml at week 4 | 35 | 62.6% | 51.4% | 17.1% | |||
| drop of HBV DNA ≥4 log10copies/ml at week 12 | 20 | 85.0% | 0.366 | 80.0% | 0.028 | 45.0% | 0.006 |
| drop of HBV DNA < 4 log10copies/ml at week 12 | 27 | 60.0% | 48.0% | 7.4% | |||
| DNA level < 4 log10copies/ml at week 24 | 25 | 96.0% | < 0.001 | 84.0% | 0.001 | 36.0% | 0.030 |
| DNA level ≥ 4 log10copies/ml at week 24 | 22 | 40.9% | 36.3% | 9.1% | |||
| DNA negative at week 52 | 27 | 100% | < 0.001 | 88.9% | < 0.001 | 33.3% | 0.062 |
| DNA positive at week 52 | 20 | 45.0% | 20.0% | 10.0% |
5. Resistance to ADV
Four patients experienced viral breakthrough at week 104, 184, 208, 260 respectively. HBV DNA rebounded to 2.2 × 104- 6.5 × 107 copies/ml, and ALT flares were found in 3 patients but none of them developped liver decompensation. ADV correlated mutations were detected in 3 of the 4 patients (1 of them with N236T and another 2 with combination of A181T/V and N236T mutations in HBV DNA polymerase). None of the patients who achieved HBV DNA < 4 log copies/ml at week 24 experienced viral breakthrough. All four patients were then given additional LAM for combination therapy.
6. Safety analysis
Four patients had an increase in serum creatinine of more than the peak value of normal (1.5 mg/dl), but the abnormality was not confirmed by a consecutive sample test. Three patients had slightly elevated serum uric acid in incontinuous samples. No action was taken regarding study medication dosing.
Discussion
The response of ADV monotherapy for HBeAg positive CHB patients is less satisfying comparing with that of other oral NAs, but in many Asian countries like China, tenofovir is unavailable, ADV is still the only nucleotide analogue without cross resistance to other NAs to date [12-14]. The optimization of ADV therapy according to patients' baseline and on-treatment parameters may be a feasible strategy to increase the response. It was ever reported that ADV might provide additional benefits for HBeAg seroconversion in patients with pre-treatment HBV DNA levels between 107 and 108 copies/ml [15]. Subgroup analysis of GLOBE Study with telbivudine revealed that patients with ALT≥2 × ULN and HBV DNA < 109 copies/ml had more chance of VR, HBeAg seroconversion and ALT nomalization at year 2 [16]. There were also similar reports about long term LAM therapy [10]. In our study baseline factors such as ALT predicted long term response to ADV therapy. Patients with higher baseline ALT (≥5 × ULN) had more chance of optimal outcomes, and lower levels of HBeAg (< 800 s/co) and HBsAg(< 5000 IU/ml) also predicted HBeAg loss. In addition, there was a trend that patients with lower HBV DNA level might have higher rates of VR, HBeAg loss and HBeAg seroconversion although no statistical significance was observed in our study. Our findings may be limited by the relatively small sample size. When we combined baseline ALT and HBV DNA, we found that patients with baseline HBV DNA < 109 copies/ml and ALT ≥3 × ULN had more favorable outcomes. An elevated ALT level indicates increased clearance to HBV infected hepatocytes. It was reported that Chinese patients with ALT > 5 × ULN had high rate of spontaneous HBeAg seroconversion even without treatment [17], but treatment with anti-virus medicaments can improve the chance of HBeAg seroconversion and decrease the disease progression which has been proved in many clinical studies [2,18-20]. Similarly, HBeAg level may reflect the clearance capability of a HBV infected body to HBV infected hepatocytes [21,22]. The significance of HBsAg in treatment of patients with CHB is being paid close attention to recently. HBsAg may reflect the level of cccDNA in liver and the decrease during treatment with pegylated interferon alfa-2a may predict the sustained viral response [23,24]. More studies are needed to elucidate HBsAg variations during treament with NAs. In our study patients with lower level of baseline HBeAg and HBsAg had more chance of HBeAg loss which indicaties that baseline HBeAg and HBsAg may be regarded as subsidiary parameters in pretreatment evaluation.
Early HBV DNA reduction may also contribute to VR and HBeAg seroconversion in some studies [11,15,25]. It's generally accepted that therapy can be optimized by monitoring of serum HBV DNA levels during treatment with oral NAs and week 24 is the most important time point recommended to assess efficacy in LAM and telbivudine therapy [26], but whether it's reasonable in treatment of suboptimal antiviral drug like ADV remains controversial. We selected week 4, 12, 24 and 52 as time points in our study and found that serum HBV DNA < 4 log10 copies/ml at week 24 was predictive for VR, HBeAg loss and HBeAg seroconversion after 5-year treatment, The drop of HBV DNA from baseline ≥3 log10 copies/ml at week 4, ≥ 4 log10 copies/ml at week 12 and HBV DNA turning negative at week 52 can also predict favorable outcomes although not as optimal as HBV DNA < 4 log10 copies/ml at week 24. None of the patients who achieved HBV DNA < 4 log10 copies/ml at week 24 experienced viral breakthrough. Accordingly we recommend serum HBV DNA level < 4 log10copies/ml at week 24 as one of the major predictors for long term outcomes in patients with ADV therapy.
Our study has some limitations because of its small sample size and lack of a control group. However, in our study most patients completed 5 years' follow-up, and the detection method was advanced. The findings may give some evidence to manage HBeAg positive patients who intend to accept or have already accepted ADV therapy.
Conclusions
ADV treatment may be initiated to patients with baseline ALT≥5 × ULN or patients with ALT≥3 × ULN and HBV DNA < 109 copies/ml, lower level of HBeAg(< 800 s/co) or HBsAg (< 5000 IU/ml) may be regarded as referenced factors. Patients will be followed up regularly and long term outcome may be predicted according to on-treatment HBV DNA. For patients with HBV DNA level < 104 copies/ml at week 24 therapy should continue and favorable outcomes may be achieved in 5 years or longer. For others with HBV DNA ≥104 copies/ml at week 24, some modifications for the therapeutic regimen such as addition or switch to another agent should be considered to enhance the long term response.
List of abbreviations
ADV: Adefovir Dipivoxil; ALT: alanine aminotransferase; CHB: chronic hepatitis B; HBeAg: hepatitis B e antigen; HBV: hepatitis B virus; LAM: lamivudine; NAs: nucleos(t)ide analogues; ULN: upper limit of normal; VR: viral response.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HL conceived of the study, participated the design, carried out the study and drafted the manuscript. DYG carried out laboratory work, FS and JYZ participated in following up of patients and data collecting. BL participated in the design of the study and performed the statistical analysis. LXM participated in the design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hui Lu, Email: lvhui_6@126.com.
Da Ying Geng, Email: jmyu163@126.com.
Fei Shen, Email: graceshenfei@sina.com.
Jing Yao Zhang, Email: zhangjingyao73@163.com.
Bing Lu, Email: nhblu@channing.harvard.edu.
Li Xian Ma, Email: lxmdoctor@126.com.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (NSFC No.81071340) and Natural Science Foundation of Shandong Province (No. Y2008C68).
References
- Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, Fry J, Brosgart CL. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- Marcellin P, Chang TT, Lim S, Sievert W, Tong M, Arterburn S, Borroto-Esoda K, Frederick D, Rousseau F. Long-term efficacy and safety of adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2008;48:750–758. doi: 10.1002/hep.22414. [DOI] [PubMed] [Google Scholar]
- Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, Paik SW, Liaw YF, Button P, Popescu M. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47(2):428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]
- Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30(3):770–774. doi: 10.1002/hep.510300313. [DOI] [PubMed] [Google Scholar]
- Liaw YF. On-treatment outcome prediction and adjustment during chronic hepatitis B therapy: now and future. Antivir Ther. 2009;14:13–22. [PubMed] [Google Scholar]
- Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai MY, Brunetto MR, Farci P, Popescu M, McCloud P. Predicting response to peginterferon alpha-2a, lamivudine and the two Combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699–705. doi: 10.1136/gut.2005.089722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N. Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. Engl J Med. 2005;352(26):2682–2695. doi: 10.1056/NEJMoa043470. [DOI] [PubMed] [Google Scholar]
- Gish RG, Lau DT, Schmid P, Perrillo R. A pilot study of extended duration of peginterferon alfa-2a for patients with hepatitis B e antigen-negative chronic hepatitis B. Am J Gastroenterol. 2007;102:2718–2723. doi: 10.1111/j.1572-0241.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, Chutaputti A, Rasenack J, Hou J, O'Brien C, Nguyen TT, Jia J, Poynard T, Belanger B, Bao W, Naoumov NV. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51(1):11–20. doi: 10.1016/j.jhep.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Yuen MF, Fung J, Seto WK, Wong DK, Yuen JC, Lai CL. Combination of baseline parameters and on-treatment hepatitis B virus DNA levels to start and continue patients with lamivudine therapy. Antivir Ther. pp. 679–685. [PubMed]
- Llop E, De la Revilla J, Pons F, Peñas B, Martínez JL, Abreu L, Calleja JL. Decrease in viral load at weeks 12 and 24 in patients with chronic hepatitis B treated with lamivudine or adefovir predicts virological response at week 48. Rev Asp Enferm Dig. 2009;101(11):763–767. doi: 10.4321/s1130-01082009001100003. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254–265. doi: 10.1002/hep.21698. [DOI] [PubMed] [Google Scholar]
- Qi X, Xiong S, Yang H, Miller M, Delaney WE. Invitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355–362. [PubMed] [Google Scholar]
- Reijnders JG, Leemans WF, Hansen BE, Pas SD, de Man RA, Schutten M, Janssen HL. On-treatment monitoring of adefovir therapy in chronic hepatitis B: virologic response can be assessed at 24 weeks. J Viral Hepat. 2009;16:113–120. doi: 10.1111/j.1365-2893.2008.01053.x. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Buti M, Gane EJ. Baseline parameters predict both early virologic response and longer term outcomes for telbivudine-treated patients with chronic hepatitis B. The GLOBE study. Hepatology. 2007;46(Suppl 1):681 A. [Google Scholar]
- Yuen M-F, Yuan H-J, Hui C-K, Wong D-K, Wong WM, Chan AO, Wong BC, Lai CL. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52(3):416–419. doi: 10.1136/gut.52.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T, Ngo Y, Marcellin P, Hadziyannis S, Ratziu V, Benhamou Y. Adefovir Dipivoxil 437 and 438 Study Groups. Impact of adefovir dipivoxil on liver fibrosis and activity assessed with biochemical markers (FibroTest-ActiTest) in patients infected by hepatitis B virus. J Viral Hepat. 2009;16(3):203–213. doi: 10.1111/j.1365-2893.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Cirrhosis Asian Lamivudine Multicentre Study Group. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Leung N, Guan R, Lau GK, Merican I, McCaughan G, Gane E, Kao JH, Omata M. Asian-Pacific consensus update working party on chronic hepatitis B. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2005 update. Liver Int. 2005;25(3):472–489. doi: 10.1111/j.1478-3231.2005.01134.x. [DOI] [PubMed] [Google Scholar]
- Brunetto MR, Oliveri F, Rocca G, Criscuolo D, Chiaberge E, Capalbo M, David E, Verme G, Bonino F. Natural course and response to interferon of chronic hepatitis B accompanied by antibody to hepatitis B e antigen. Hepatology. 1989;10(2):198–202. doi: 10.1002/hep.1840100213. [DOI] [PubMed] [Google Scholar]
- Chu CM, Hung SJ, Lin J, Tai DI, Liaw YF. Natural history of hepatitis B e antigen to antibody seroconversion in patients with normal serum aminotransferase levels. Am J Med. 2004;116(12):829–834. doi: 10.1016/j.amjmed.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Nguyen T, Iser D, Ayres A, Jackson K, Littlejohn M, Slavin J, Bowden S, Gane EJ, Abbott W, Lau GK, Lewin SR, Visvanathan K, Desmond PV, Locarnini SA. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–1944. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- Brunetto MR, Moriconi F, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Luo K, Wang Y, Hadziyannis S, Wolf E, McCloud P, Batrla R, Marcellin P. Hepatitis B virus surface antigen levels: a guide to sustained response to peginterferon alfa-2a in HBeAg-negative chronic hepatitis B. Hepatology. 2009;49(4):1141–1150. doi: 10.1002/hep.22760. [DOI] [PubMed] [Google Scholar]
- Tseng KC, Cheng PN, Wu IC, Chang CK, Chou AL, Liu WC, Chang TT. HBV DNA level as an important determinant of e antigen seroconversion of chronic hepatitis B during adefovir dipivoxil therapy. Hepatogastroenterology. 2009;56(91-92):813–818. [PubMed] [Google Scholar]
- Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6(12):1315–1341. doi: 10.1016/j.cgh.2008.08.021. quiz 1286. [DOI] [PubMed] [Google Scholar]