Abstract
In vivo exposure of rodents to ethanol leads to a long-lasting increase in Fyn kinase activity in the dorsomedial striatum (DMS) (Wang et al. 2010). Here we set out to identify a molecular mechanism that contributes to the enhancement of Fyn activity in response to ethanol in the DMS. Protein tyrosine phosphatase α (PTPα) positively regulates the activity of Fyn, and we found that repeated systemic administration or binge drinking of ethanol results in an increase in the synaptic localization of PTPα in the DMS, the same site where Fyn resides. We also demonstrate that binge drinking of ethanol leads an increase in Fyn activity and to the co-localization of Fyn and PTPα in lipid rafts in the DMS. Finally, we show that the level of tyrosine phosphorylated (and thus active) PTPα in the synaptic fractions is increased in response to contingent or non-contingent exposure of rats to ethanol. Together, our results suggest that the redistribution of PTPα in the DMS into compartments where Fyn resides is a potential mechanism by which the activity of the kinase is increased upon ethanol exposure. Such neuroadaptations could be part of a mechanism that leads to the development of excessive ethanol consumption.
Keywords: PTPα, Fyn, Dorsal Striatum, ethanol, lipid rafts, Tyrosine Phosphatase, Dorsal Striatum
Introduction
Fyn is a kinase that belongs to the Src family of non-receptor protein tyrosine kinases (PTKs) (Resh 1998, Ingley 2008). Fyn is highly expressed in the adult brain in regions such as cortex, hippocampus and cerebellum as well as in the striatum (Umemori et al. 1992, Yagi et al. 1993). In the central nervous system (CNS) Fyn is involved in mechanisms underlying myelin formation, serotonin receptor function, synaptic plasticity and learning and memory ( Grant et al. 1992, Umemori et al. 1994, Kojima et al. 1997, Lu et al. 1999, Sperber et al. 2001, Yun et al. 2007, Isosaka et al. 2008, Kramer-Albers & White 2011). In addition, one of the best-characterized substrates of Fyn in the brain is the NR2B subunit of the NMDA receptor (NR2B-NMDAR) (Nakazawa et al. 2001, Yaka et al. 2003a, Thornton et al. 2004, Salter & Kalia 2004, Abe et al. 2005). Specifically, the phosphorylation of the C-terminus of the NR2B-NMDAR by Fyn results in the enhancement of channel activity (Suzuki & Okumura-Noji 1995, Yaka et al. 2002, Yaka et al. 2003a, Salter & Kalia 2004, Wu et al. 2007, Bjarnadottir et al. 2007, Yang et al. 2011) and enhances its binding to spectrin and post synaptic density protein 95 (PSD-95) (Rong et al. 2001).
Fyn’s structure consists of a unique N-terminal region that contains lipid modification sites, SH3 and SH2 domains, and a catalytic kinase domain (Engen et al. 2008, Ingley 2008). The activity of Fyn is regulated by intramolecular interactions that depend on the phosphorylation state of two tyrosine residues (Engen et al. 2008, Ingley 2008). In its inactive conformation, the regulatory tyrosine 531 (Y531) at the C-terminus is phosphorylated and interacts with the SH2 domain locking the catalytic domain in an inactive conformation. Dephosphorylation of Y531 results in the dissociation of the tyrosine residue from the SH2 domain, and once the inhibition is removed, Fyn undergoes autophosphorylation at tyrosine 420 (Y420) in the activation loop of the catalytic domain of the kinase, stabilizing it in its active conformation (Engen et al. 2008, Ingley 2008).
The phosphatase responsible for the dephosphorylation of Y531 site, and the activation of Fyn, is Protein Tyrosine Phosphatase α (PTPα), and PTPα was shown to play essential role in the activation of Fyn and Src (den Hertog et al. 1993, Bhandari et al. 1998, Maksumova et al. 2005, Vacaresse et al. 2008, Wang et al. 2009, van Eekelen et al. 2010). PTPα is a transmembrane phosphatase that belongs to the family of receptor-like protein tyrosine phosphatases (Sap et al. 1990, Paul & Lombroso 2003). PTPα is highly expressed in several brain regions such as hippocampus, cortex and striatum (Sap et al. 1990, Sahin et al. 1995). PTPα knockout (KO) mice have reduced activity of Fyn and Src in mouse brain and primary embryonic fibroblasts, and increased level of the inactive form of the kinases (Ponniah et al. 1999, Su et al. 1999). Furthermore, Fyn-mediated tyrosine phosphorylation of the NR2B subunit is reduced in hippocampal tissue (Petrone et al. 2003), and in synaptosomal membranes from PTPα KO mice (Le et al. 2006). In addition, the introduction of inhibitory antibodies against PTPα reduces NMDAR-mediated currents in hippocampal neurons (Lei et al. 2002). Finally, PTPα KO mice, like Fyn KO mice (Grant 1996), show deficits in NMDAR-dependent processes, such as long-term potentiation (LTP), and learning and memory (Lei et al. 2002, Skelton et al. 2003).
Studies using the Fyn gene knockout (KO) mice revealed that the kinase plays an important role in ethanol-induced behaviors. For example, Fyn KO mice display higher sensitivity to the acute sedative hypnotic effects of ethanol compared to wild-type (WT) littermates (Miyakawa et al. 1997, Yagi 1999, Boehm et al. 2003, Yaka et al. 2003c). Boehm and collaborators reported that Fyn KO mice were more sensitive to the anxiolytic effects of ethanol a reduced preference for ethanol in a two-bottle choice paradigm compared to WT mice (Boehm et al. 2003), although Fyn overexpressing mice also exhibited lower consumption and preference for ethanol (Boehm et al. 2004). Fyn Overexpression of Fyn also resulted in reduced withdrawal symptoms after chronic ethanol intake (Stork et al. 2002). In addition, we, and others, showed that Fyn-mediated phosphorylation of the NR2B subunit of the NMDAR is required for ethanol-induced sedation in mice and for the development of acute tolerance to ethanol in hippocampal slices (Yagi 1999, Yaka et al. 2003b, Yaka et al. 2003c). In addition, we previously showed that ex vivo and in vivo exposure of rodents to ethanol results in increased Fyn activity in the dorsal striatum, and more specifically, in the DMS (Wang et al. 2007, Wang et al. 2010). We also reported that Fyn is required for ethanol-mediated long-term facilitation (LTF) of NMDAR activity in the dorsal striatum (Wang et al. 2007). Most recently we observed that repeated systemic administration of ethanol and withdrawal as well as a history of excessive ethanol consumption in rats results in a long-lasting increase in the activity of Fyn, increased levels of phosphorylation and synaptic localization of NR2B subunit of the NMDAR in the DMS (Wang et al. 2010, Wang et al. 2011). Furthermore, the inhibition of Fyn activity specifically in the DMS, but not in the dorsolateral striatum (DLS) or the nucleus accumbens (NAc) reduces operant self-administration of ethanol in rats (Wang et al. 2007, Wang et al. 2010). Together, these results show that Fyn activity specifically in the DMS is required for the neuroadaptations that underlie the development and/or maintenance of ethanol intake.
The mechanism(s) that mediate(s) the activation of Fyn kinase in the DMS in response to ethanol exposure is unknown. Here we set out to test the possibility that the altered protein expression and/or localization of PTPα in the DMS of rats in response to ethanol enable(s) the activation of Fyn. We found that in vivo ethanol exposure causes a subcellular redistribution of active PTPα into compartments where Fyn is localized, thus strengthening the positive regulatory mechanism leading to increased Fyn activity.
Materials and Methods
Reagents
Anti-Flotillin-1 antibodies were obtained from BD Transduction Laboratories (San Diego, CA). Anti-PSD95 and anti-PTPα antibodies were obtained from Millipore (Billerica, MA). Anti-CREB and anti-[pY789]-PTPα antibodies were obtained from Cell Signaling Technology (Danvers, MA). Anti-Actin, anti-GAPDH, rabbit polyclonal anti-Fyn, mouse monoclonal anti-Fyn, anti-Synapsin 1a/b, and the secondary antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA). Anti-[pY418/420] Src/Fyn (for measuring active Fyn), anti-[pY529/531] Src/Fyn (for measuring inactive Fyn), and anti-Transferrin receptor (TrfR) antibodies, NuPAGE 10% Bis-Tris gels, NuPAGE 4–12% Bis-Tris gradient gels, and protein G agarose were obtained from Invitrogen (Carlsbad, CA). Bicinchoninic acid (BCA)™ protein assay kit was obtained from Pierce (Rockford, IL). Phosphatase inhibitor cocktails 1 and 2 were obtained from Sigma (St. Louis, MO). Complete™ mini, EDTA-free protease inhibitor cocktail was purchased from Roche (Indianapolis, IN). Enhanced Chemiluminescence (ECL)™ Plus was purchased from GE Healthcare Biosciences (Pittsburg, PA).
Animals
Male Sprague Dawley (3–4 weeks old, 80–120 g at the time of purchase) and Long Evans rats (3–4 weeks old, 80–120 g at the time of purchase) were obtained from Harlan (Indianapolis, IN). Animals were housed under a light:dark cycle of 12 hrs, with lights on at 7:00 a.m. and food and water available ad libitum. All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Systemic administration of ethanol
Male Sprague Dawley (5 to 6 weeks of age) and Long Evans rats (8 to 10 weeks of age) were systemically treated intraperitoneally (i.p.) with ethanol (20% v/v, 2 g/kg) or saline once a day for 7 consecutive days. Sixteen hrs after the last administration, the DMS, DLS and the NAc were dissected.
Intermittent-access to 20% ethanol 2-bottle choice drinking paradigm
Male Long-Evans rats (8 to 10 weeks of age) were trained to voluntarily consume high levels of ethanol using the intermittent-access to 20% ethanol (v/v) 2-bottle choice drinking paradigm. Animals were given 24 hrs access to one bottle of 20% ethanol (v/v) and one bottle of water concurrently. Drinking sessions started at 10:00 a.m. on Monday, Wednesday, and Friday, with 24 or 48 hrs (weekend) of ethanol deprivation between the drinking sessions. The water and ethanol bottles were weighed at the beginning and at the end of each session. After achieving a stable baseline of ethanol consumption (average ethanol consumption was 6.23±0.413 g/kg/24hr), on the last ethanol-drinking session rats were allowed to drink only for 30 min, a period in which rats drink approximately 25% of the total daily ethanol intake (Carnicella et al. 2009). This procedure resulted in an ethanol intake of 1.21±0.14 g/kg/30min, which corresponds to a blood ethanol concentration (BEC) of 80 mg% (Carnicella et al. 2008, Carnicella et al. 2009), suggesting that the rats were highly motivated to consume ethanol for its pharmacological effects, thus exhibiting “binge-like” drinking behavior.
Synaptosomal membrane preparation
Crude synaptosomal membranes were prepared as described in (Wang et al. 2010). Briefly, immediately after being collected, tissue was homogenized in a glass homogenizer containing 500 µl of ice-cold Krebs-sucrose buffer (in mM: 125 NaCl, 1.2 KCl, 1.2 MgSO4, 1.2 CaCl2, 22 Na2CO3, 1.2 NaH2PO4, 10 glucose, and 320 sucrose, pH 7.4) in the presence of protease and phosphatase inhibitors. The homogenate was centrifuged at 1,000 g for 10 min at 4°C to pellet heavy membranes and debris (P1). The supernatant (S1) was collected and was centrifuged at 16,000 g at 4°C for 20 min to pellet the crude synaptosomal membrane fraction (P2). P2 was re-suspended in 100 µl RIPA buffer. Total protein concentration was determined using BCA™ protein assay kit.
Immunoprecipitation
DMS tissue was homogenized in 1x immunoprecipitation (IP) buffer (in mM: 150 NaCl, 10 Tris-HCl pH 7.4, 1 EDTA, 1 EGTA, and 1% Triton X-100) in the presence of protease and phosphatase inhibitor cocktails. DMS homogenates were pre-cleared by incubation with protein G agarose for 1 hr at 4°C. The samples were centrifuged and protein quantity was determined using BCA™ protein assay kit. IPs were performed by combining 5 µg of the appropriate antibody with approximately 1 mg protein diluted in 1× IP buffer to a total volume of 1 ml. Following overnight incubation at 4°C, protein G agarose was added, and the mixture was incubated at 4°C for 4 hrs. The protein G was washed extensively with 1x IP buffer and pellets were re-suspended in 25 µl of 2x Laemmli buffer and incubated at 95°C for 10 min. The resulting supernatant was resolved in NuPAGE 10% Bis-Tris gels.
Lipid rafts isolation
Lipid rafts were isolated as described in (Kosugi et al. 1999) with minor modifications. Briefly, immediately after being collected, DMS tissue was homogenized with 20 strokes in a glass homogenizer containing 2 ml of ice-cold MES-buffered saline (MBS; in mM: 25 MES pH 6.5, and 150 NaCl) containing 0.25% Triton X-100, and protease and phosphatase inhibitors. The lysate was mixed with equal volume of 80% sucrose (w/v) in MBS, and placed in the bottom of a 14 × 89 mm clear centrifuge tube (Beckman, Brea, CA). The samples were then overlaid with 4 ml of 30% sucrose followed by 4 ml 5% sucrose in MBS and ultracentrifuged at 200,000 g using a SW-41 Ti rotor (Beckman, Brea, CA) at 4°C for 18 hrs. Samples were divided into twelve 1000 µl fractions starting from the top of the gradient. Total protein concentration was determined using BCA™ protein assay kit.
Western blot analysis
Equal amount of samples (25 µg) was denatured with Laemmli buffer, heated at 95°C for 15 min and resolved on NuPAGE 4–12% Bis-Tris gradient gels. After overnight transfer at 4°C onto nitrocellulose membranes, membranes were blocked in 5% non-fat milk in Tris Buffer Saline/0.1% Tween-20 (TBS-T) before probing with specific antibodies for 1 hr at room temperature. After extensive washing with TBS-T, bound primary antibodies were detected with horseradish peroxidase-conjugated (HRP)-conjugated secondary antibody and visualized by ECL™ plus.
Statistical analysis
All data are expressed as mean ± SEM. The optical density of the relevant immunoreactive band was quantified using NIH ImageJ 1.44c software. Bar graphs in Figs. 1–3 and 6, were generated by the ratio of the intensity of the immunoreactive band of interest to GAPDH which was determined for each sample to normalize for the amount of protein loaded. Bar graph in Fig. 4 was generated by quantifying the intensity of [pY418/420]Src/Fyn, or [pY529/531]Src/Fyn band to the intensity of the total IP-ed Fyn band. Data were analyzed using one-tail or two-tail Student’s t-test as indicated in the figure legends.
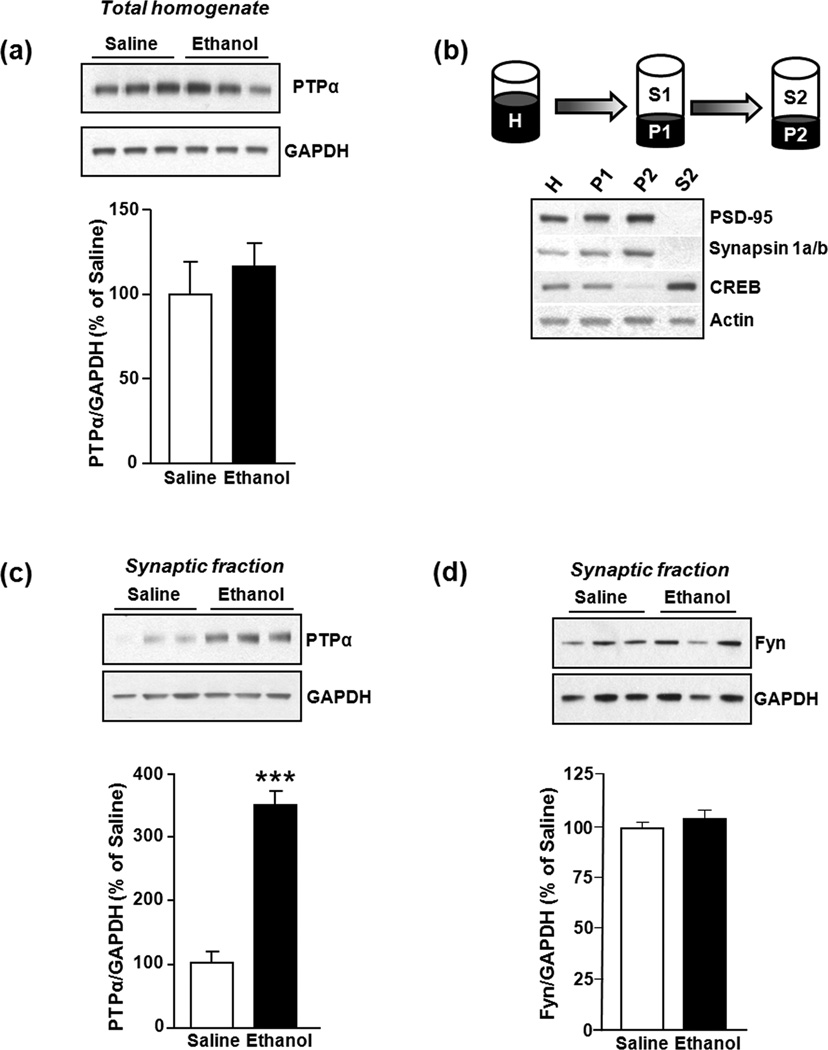
Figure 1. Repeated systemic administration of ethanol does not alter total protein levels of PTPα in the DMS of Sprague Dawley Rats.
Rats were treated with saline or ethanol (2 g/kg, i.p.) once a day for 7 days. DMS was dissected 16 hrs after the last injection. (a) PTPα in total homogenates was detected with anti-PTPα antibodies (1:1000). Images are representative of n=6 (saline), n=6 (ethanol). (b) Schematic representation of the synaptosomal fractionation procedure. Anti-PSD95 (1:10,000) and anti-Synapsin 1a/b antibodies (1:2000) were used as synaptosomal membrane markers; anti-CREB antibody (1:1000) was used as a non-synaptic membrane marker; and anti-Actin antibody (1:2000) was used as loading control. H: Total homogenate, P1: Nuclei and large debris, P2: Crude synaptosomal membrane, S2: Cytosol and light membranes. (c) Repeated systemic administration of ethanol alters the protein levels of PTPα in the synaptosomal fraction. Images are representatives of n=8 (saline), n=8 (ethanol). (d) Repeated systemic administration of ethanol does not alter the protein levels of Fyn (polyclonal anti-Fyn antibodies, 1:500) in the synaptosomal fraction. Image is representative of n=3 (saline), n=3 (ethanol). Bar graphs summarize the averaged change in protein levels of PTPα in total homogenates (a), PTPα in synaptosomal fractions (c), and Fyn in synaptosomal fractions (d). Band intensity of PTPα or Fyn were normalized to the level of GAPDH and plotted as percentage of saline-treated rats. Results are expressed as mean ± SEM. ***P < 0.001, vs. saline (two-tailed Student’s t-test).
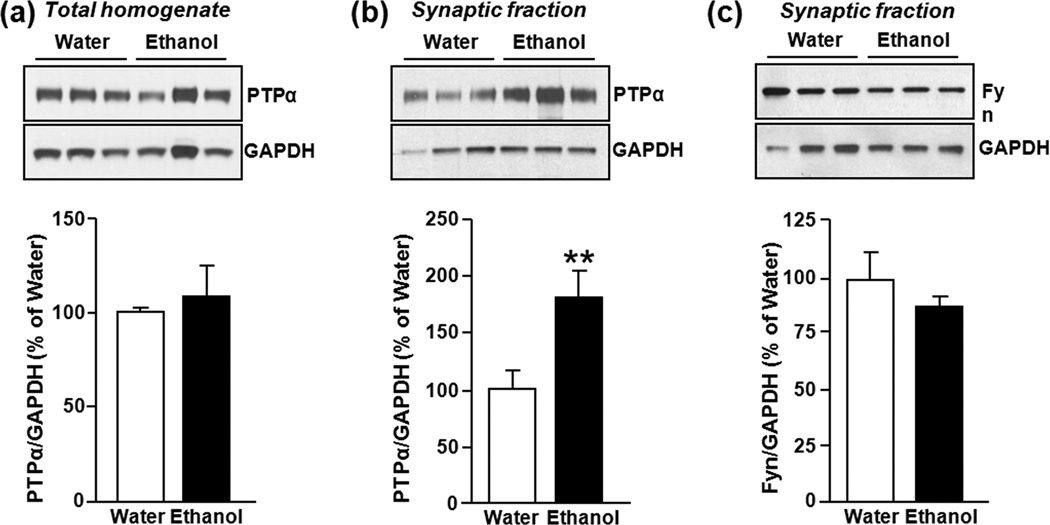
Figure 3. Binge drinking of ethanol increases protein levels of PTPα in synaptic membranes in the DMS of Long Evans rats.
Rats underwent an intermittent access to 20% ethanol in a 2-bottle choice paradigm for 7–8 weeks. Thirty minutes after the initiation of the last drinking session, DMS tissue was dissected and protein levels of PTPα in homogenates (a), and synaptic membranes (b) as well as the level of Fyn in the synaptosomal fraction were measured. (a) Image is representative of n=9 (water), n=9 (ethanol). (b) Images are representative of n=8 (water), n=6 (ethanol). (c) Image is representative of n=3 (water), n=3 (ethanol). Bar graphs summarize the averaged changes in protein levels of PTPα in total homogenates (a), as well as PTPα (b) and Fyn (c) in the synaptosomal fraction. Band intensity of PTPα or Fyn was normalized to the level of GAPDH and plotted as percentage of water-only drinking rats. Results are expressed as mean ± SEM. **P < 0.01 vs. water (two-tailed Student’s t-test).
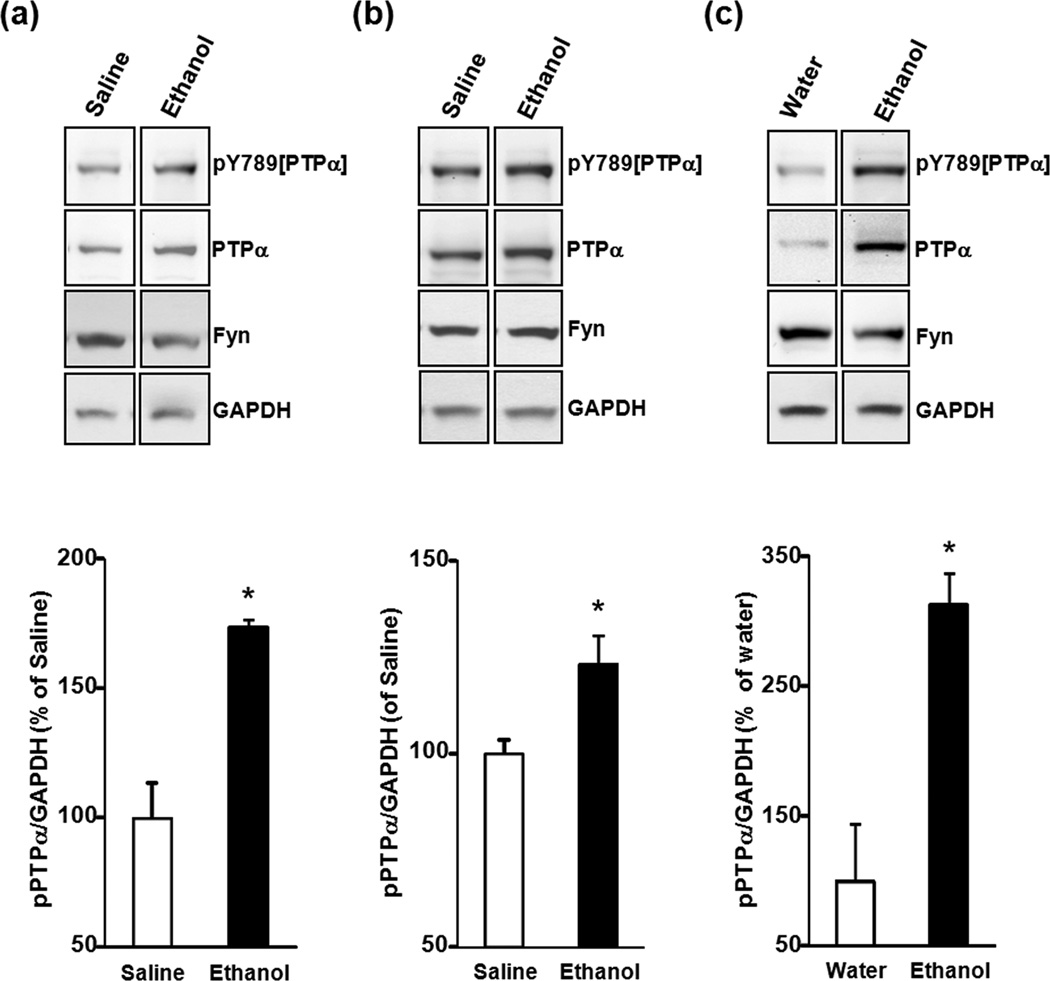
Figure 6. Repeated systemic injections and binge drinking of ethanol increase the level of tyrosine phosphorylation of PTPα in DMS synaptic membranes of Sprague Dawley and Long Evans rats.
Sprague Dawley Rats (a) or Long Evans rats (b,c) were systemically treated with saline or ethanol (a,b) or underwent an ethanol binge-drinking session (c) as described in Figs. 1 and 3, respectively. Phosphorylated PTPα was detected using anti-pY789[PTPα] antibodies (1:250). Protein levels of PTPα (1:500), Fyn (1:500) and GAPDH (1:25,000) were also measured. Images are representative of n=3 (saline), n=3 (ethanol) (a,b), n=2 (water), n=2 (ethanol) (c). Bar graphs summarize the averaged change in phosphorylated levels of PTPα in synaptosomal membranes. Band intensities of pY789[PTPα] were normalized to the level of GAPDH and plotted as percentage of saline (a,b) or water (c) controls. Results are expressed as mean ± SEM. *P < 0.05 vs. control (one-tailed Student’s t-test).
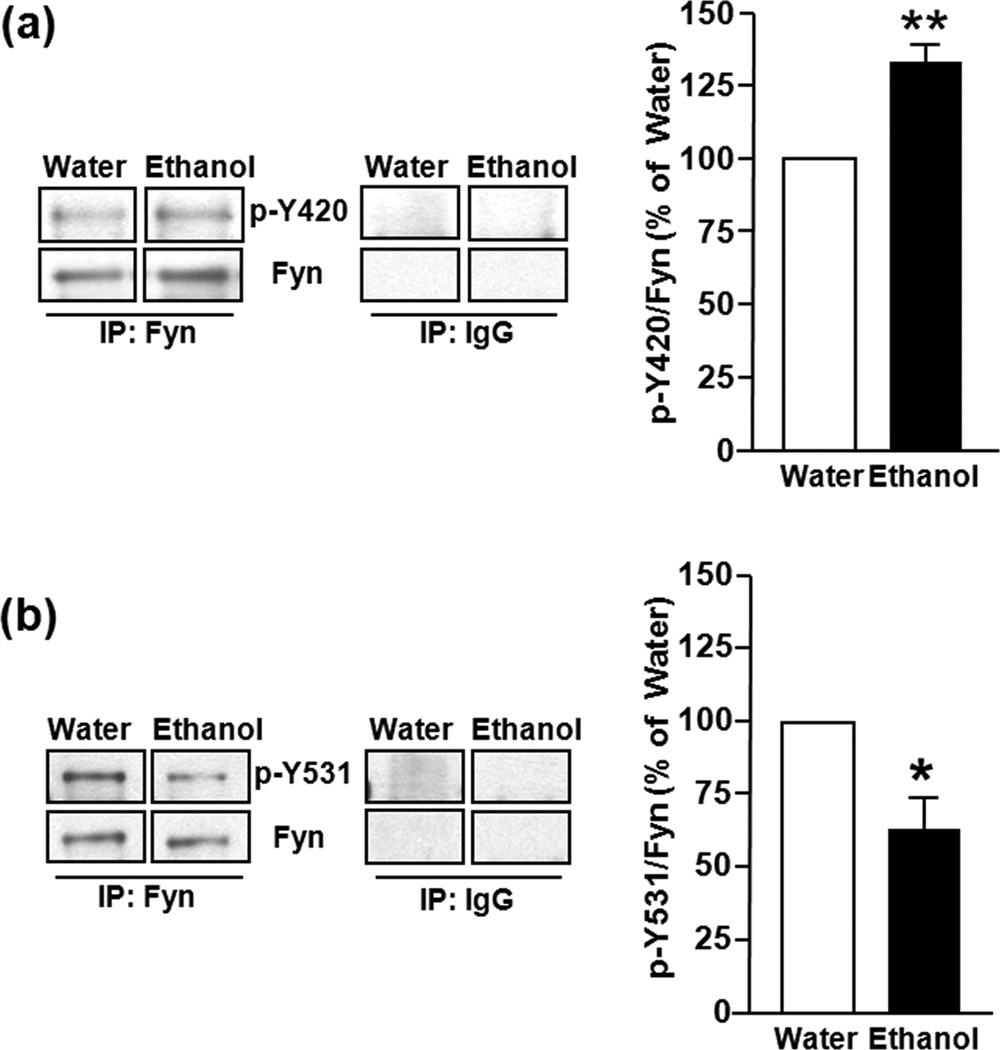
Figure 4. Binge drinking of ethanol increases Fyn activation in the DMS of Long Evans rats.
Rats underwent ethanol drinking procedue as described in Fig. 3, thirty minutes after the initiation of the last drinking session, DMS tissue was dissected and Fyn total was IP-ed from homogenates with mouse monoclonal anti-Fyn antibodies, and anti-mouse IgG antibodies as a control. Active Fyn was detected by the anti-pY420/418[Fyn/Src] antibodies (1:500) (a). In a separate membrane, the anti-pY531/529 [Fyn/Src] antibodies (1:1000) detected inactive Fyn kinase (b). Both membranes were stripped and re-probed with polyclonal anti-Fyn antibodies to measure the level of IP-ed Fyn. Images are representative of n=3 (water), n=3 (ethanol). Bar graphs summarize the averaged effect of ethanol treatment on the levels of active and inactive Fyn by quantification of the level of pY420/418 or pY531/529 to total IP-ed Fyn. Data are expressed as percentage of water control, and are expressed as mean ± SEM. **P < 0.01, *P < 0.05 vs. water-drinking rats (two-tailed Student’s t-test).
Results
Repeated systemic administration of ethanol results in a long-lasting increase in PTPα in synaptosomal membranes of the DMS
We previously showed that non-contingent exposure of Sprague Dawley rats to ethanol (20% v/v, 2 g/kg, i.p.) once daily for 7 consecutive days results in a long-lasting increase in Fyn activity in the DMS of rats that was observed 16 hrs after the last ethanol treatment (Wang et al. 2007, Wang et al. 2010). Since PTPα is a positive regulator of Fyn activity (den Hertog et al. 1993, Bhandari et al. 1998, Maksumova et al. 2005, Vacaresse et al. 2008, Wang et al. 2009, van Eekelen et al. 2010), we hypothesized that repeated systemic ethanol administration and withdrawal leads to a change in the total protein level of PTPα, and/or increased localization of the phosphatase at the site where Fyn is localized. However, as shown in Fig. 1a, repeated systemic administration of ethanol and withdrawal did not result in a significant change in the total protein levels of PTPα in the DMS compared to saline-treated rats (P = 0.18, t10 = 1.43). Next, we tested the possibility that ethanol exposure and withdrawal leads to a redistribution of PTPα to the synaptic membranes, where Fyn and its substrate, NR2B, reside. Synaptosomal membranes from the DMS were isolated 16 hrs after the 7th systemic administration of saline or ethanol, and the levels of PTPα in the synaptosomal fraction (P2) were measured. The effectiveness of the fractionation procedure was confirmed by using subcellular protein markers. Specifically, the levels of PSD-95 and Synapsin 1a/b were higher in the crude synaptosomal fraction (P2) compared to total homogenate (H), while absent in the cytoplasmic fraction (S2) (Fig. 1b). Likewise, protein levels of CREB, a non-synaptosomal marker, were higher in the cytoplasmic and total homogenate fractions and absent in the crude synaptosomal membrane (Fig. 1b). As shown in Fig. 1c, repeated systemic administration of ethanol results in a long-lasting increase in the protein levels of PTPα in DMS synaptosomal fractions of ethanol-treated as compared to saline-treated rats (P < 0.001, t9 = 5.23). Although Fyn is localized in the synaptosomal fractions, ethanol treatment did not alter its localization (P=0.31, t4 =1.16, Fig. 1d). These results suggest that repeated administration of ethanol and withdrawal results in increased redistribution of PTPα to synaptic membranes where Fyn resides.
Previously, we observed that the increase in Fyn activity in response to ethanol was specific for the dorsal striatum and was not observed in the NAc (Wang et al. 2007, Wang et al. 2010). We further showed that NR2B phosphorylation, and the enhancement of LTF of the NR2B-NMDARs function in response to ethanol is localized in the DMS but not in the DLS or in the NAc (Wang et al. 2007, Wang et al. 2010). Thus, we tested whether the ethanol-mediated increases in the level of synaptic PTPα are specific for the DMS. No changes in the level of synaptic PTPα in the DLS (P = 0.96, t10 = 0.63) (Fig. 2a) or in the NAc (P = 0.54, t10 = 0.63) (Fig. 2b) were detected after repeated ethanol administration compared to saline-treated rats. These results suggest a positive association between the activation of Fyn and the redistribution of PTPα protein levels to synaptic membranes specifically in the DMS.
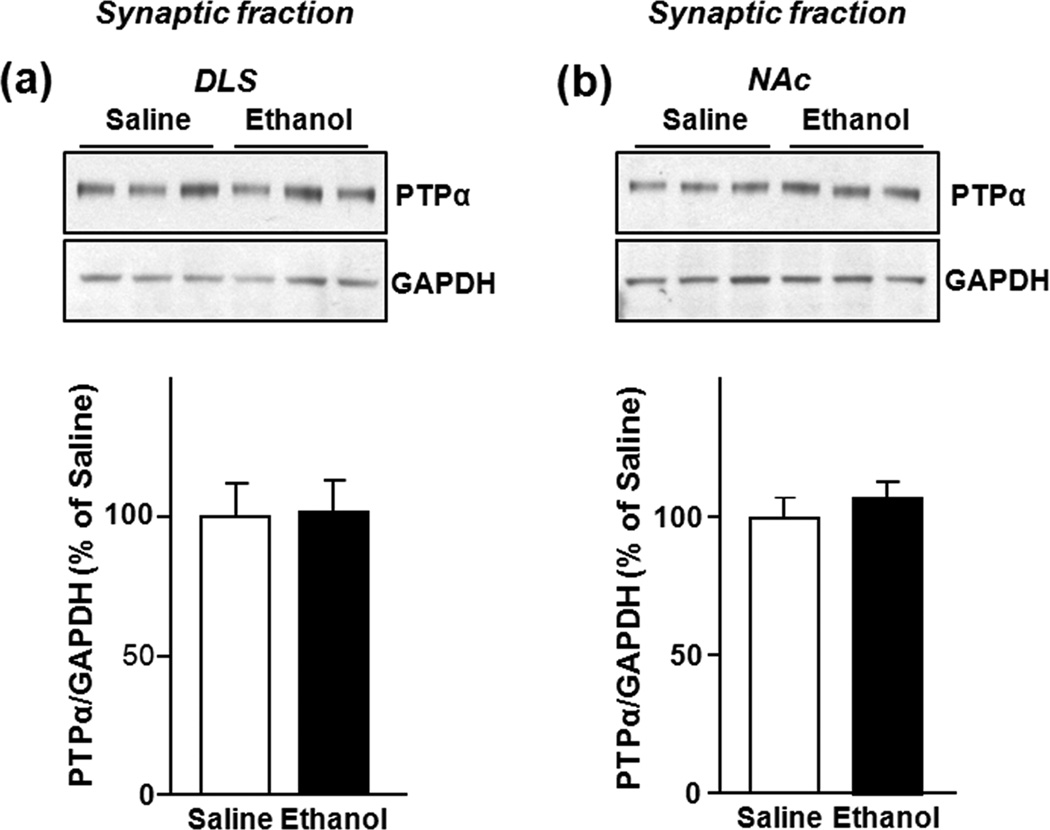
Figure 2. Repeated systemic administration of ethanol does not alter PTPα protein levels in synaptosomal membranes in the DLS and NAc of Sprague Dawley Rats.
Rats were treated as in Fig. 1, the DLS and NAc were dissected 16 hrs after the last injection and the level of PTPα in synaptosomal membranes was determined. Repeated systemic administration of ethanol did not alter the protein levels of synaptic PTPα in the DLS (a) and in the NAc (b). Images are representative of n=6 (saline), n=6 (ethanol). Bar graphs summarize the averaged change in protein levels of PTPα in synaptic membranes. Band intensity of PTPα was normalized to the level of GAPDH and plotted as percentage of saline-treated rats. Results are expressed as mean ± SEM. P > 0.05 vs. saline (two-tailed Student’s t-test).
Binge drinking of ethanol increases the localization of PTPα to synaptic membranes
Long Evan rats that undergo cycles of voluntary alcohol intake and withdrawal develop a binge ethanol consumption phenotype. Specifically, rats consume large quantities of alcohol in a short period of time that results in a blood alcohol concentration (BAC) of 80.9 ± 7 mg%, 30 min after the beginning of an alcohol drinking session (Carnicella et al. 2008, Carnicella et al. 2009). This BAC corresponds to human binge drinking as defined by the National Institute on Alcohol Abuse and Alcoholism. We therefore examined whether the levels and/or subcellular localization of the PTPα are altered in the DMS after a session of binge drinking of ethanol. Long Evans rats underwent an intermittent-access to 20% ethanol (v/v) 2-bottle choice drinking paradigm for a period of 8 weeks, the DMS tissue was dissected 30 min after the initiation of the ethanol-drinking session, and the protein levels of PTPα were measured in total homogenates and synaptosomal fractions. Consistent with what we observed following repeated systemic administration of ethanol (Fig. 1a), the total protein levels of PTPα did not change in rats consuming ethanol (P = 0.61, t16 = 0.5204) (Fig. 3a). However, the imunoreactivity of synaptic PTPα was increased in the DMS of ethanol-treated rats compared to the DMS from water only-drinking animals (P = 0.009, t12 = 3.1091) (Fig. 3b). Binge drinking of ethanol did not however alter the localization of synaptic Fyn (P=0.34, t4=1.08, Fig. 3c). These results suggest that binge drinking of ethanol results in a redistribution of PTPα to synaptic membranes in the DMS of rats with a history of excessive ethanol intake.
Binge drinking of ethanol results in increased Fyn kinase activity
Next, we tested the possibility that the increase in the amount of PTPα in the synaptic membranes after an ethanol binge drinking session correlates with an increase in the activity of Fyn in the DMS. Long Evans rats underwent the same paradigm described above, and DMS tissue was dissected 30 min after the start of the ethanol-drinking session. Fyn was then IP-ed, and its activity detected using phospho-specific antibodies that recognize the active (pY420[Fyn]) and inactive (pY531[Fyn]) forms of the kinase. We found that the levels of active Fyn were increased in the DMS of ethanol-consuming rats but not in water only-drinking animals (P < 0.01, t4 = 6.67) (Fig. 4a). Additionally, we found reduced levels of inactive Fyn in the DMS after ethanol binge drinking (P = 0.03, t4 = 3.28) (Fig. 4b). Together, the data suggest that binge drinking leads to the activation of Fyn in the DMS of rats with a history of excessive ethanol intake.
PTPα and Fyn kinase co-localized in lipid rafts after binge drinking of ethanol
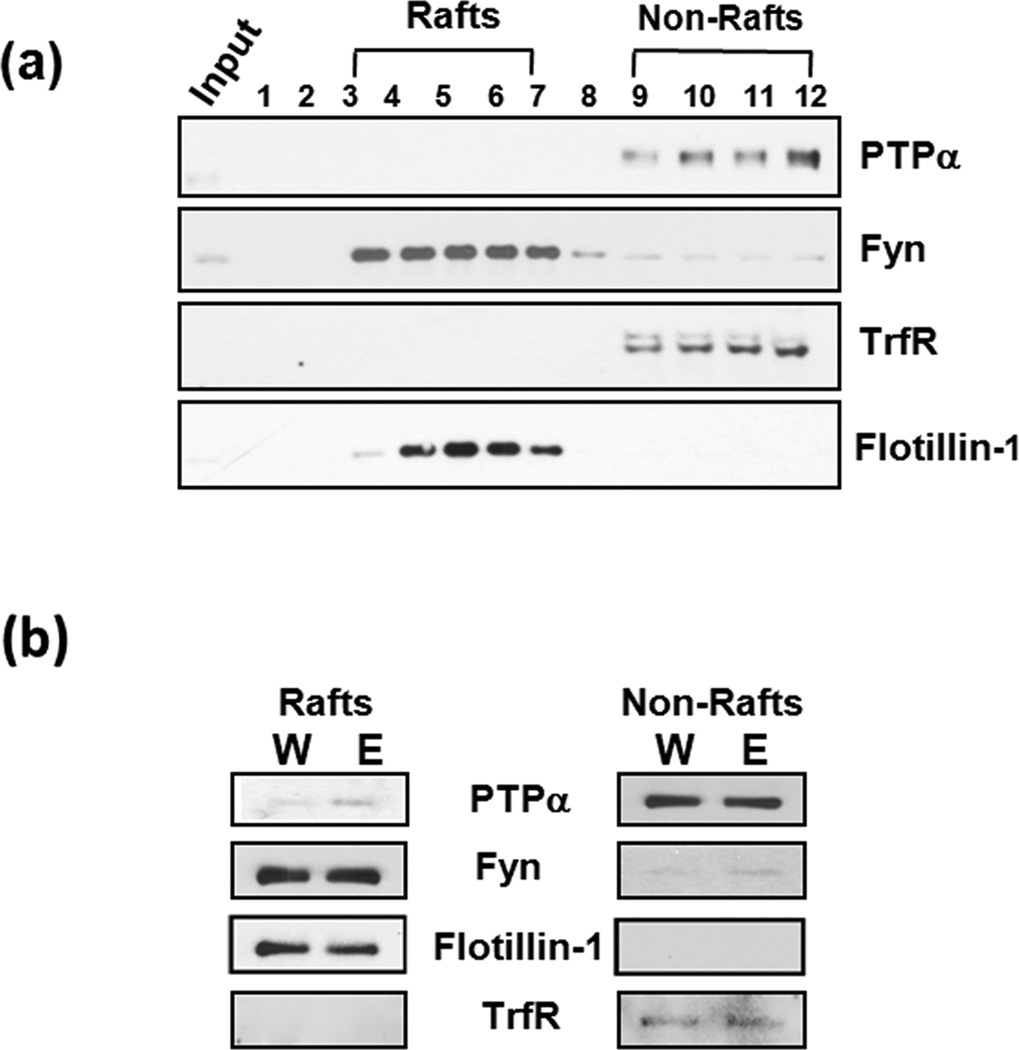
High levels of Fyn and a portion of PTPα are found in lipid raft microdomains (Yasuda et al. 2002, Besshoh et al. 2005, Maksumova et al. 2005). Lipid rafts are detergent insoluble membrane microdomains enriched in glycosphingolipids, cholesterol, and glycophosphatidylinositol (GPI)-anchored proteins that compartmentalize signal transduction molecules to either dampen or facilitate signaling (Allen et al. 2007). PTPα was shown to regulate the activity of Fyn in rafts (Maksumova et al. 2005, Vacaresse et al. 2008) suggesting that PTPα in lipid rafts compartment is required, at least in part, for Fyn kinase activity. Thus, we tests whether binge drinking of ethanol alters the localization of PTPα to Fyn in lipid rafts. To do so, lipid rafts were isolated from DMS homogenates, and the effectiveness of the procedure was confirmed by analyzing individual fractions for the presence of flotillin-1 (as a lipid rafts marker), and Transferrin receptor (TrfR), (a non-raft marker). Flotillin-1 immunoreactivity was higher in fractions 4 to 7 and TrfR was most abundant in fractions 9 to 12 (Fig. 5a). As shown in Fig. 5a, under non-stimulated conditions, PTPα is mainly localized in the non-rafts fractions, and the majority of Fyn immunoreactivity is detected in lipid rafts, although a portion of the kinase is also detected in the non-rafts fractions 3 and 8–12. However, as shown in Fig 5b, a session of binge drinking of ethanol leads to a detectible increase in a portion of PTPα in DMS raft fractions in the DMS compared to water controls. Taken together, these results suggest that ethanol-binge drinking leads to a redistribution of a portion of PTPα to lipid rafts where Fyn is localized.
Figure 5. Binge drinking of ethanol results in redistribution of PTPα, but not Fyn to lipid rafts in the DMS of Long Evans rats.
(a) Characterization of the distribution of PTPα and Fyn in raft and non-raft fractions under non-stimulated conditions. Anti-Flotillin-1 antibody (1:1000), was used as a lipid rafts marker; and anti-TrfR antibody (1:500) was used as a non-lipid rafts marker. (b) Ethanol-binge drinking increases PTPα protein levels in lipid rafts in the DMS. Anti-Flotillin-1 and anti-TrfR antibodies were used as fractionation markers. The image is a representative of 2 independent experiments.
Repeated systemic administration and binge drinking of ethanol increase the levels of PTPα phosphorylation in synaptic membranes of the DMS
In order for Fyn to be active it must undergo dephosphorylation of Y531 by PTPα (Bhandari et al. 1998). However, for this dephosphorylation to take place PTPα itself must also be active. The tyrosine 789 (Y789) residue of PTPα is phosphorylated (den Hertog et al. 1994, Chen et al. 2006), and the phosphorylation plays an important role in the activation of Src PTKs including Fyn (Zheng et al. 2000, Maksumova et al. 2007). Thus, we sought to determine whether ethanol exposure changes the level of phosphorylated PTPα in synaptic factions. Because Sprague Dawley and Long Evans were used in this study, we tested whether comparable changes can be observed in the DMS of both strains of rats following repeated systemic administration of ethanol. Similar to what is shown in Fig. 1c, we observed increased levels of PTPα in synaptic fractions obtained from the DMS of ethanol-treated Sprague Dawley (Fig. 6a) and Long Evans rats (Fig. 6b) which, importantly, was accompanied by a significant increase in the levels of tyronise phosphorylated phosphoatase (pY789[PTPα]) in the synaptic fraction (P=0.013, t4= 4.247, Fig. 6a) and in Long Evans rats (P=0.049, t4= 2.7867 Fig. 6b). Finally, we tested whether similar increases can be observed following an episode of ethanol binge drinking in Long Evans rats. As shown in Fig. 6c, binge drinking of ethanol also resulted in increased levels of both total PTPα and pY789[PTPα] in synaptic fractions from the DMS (P=0.041, t2=4.76 Fig. 6c). Together, these results suggest that tyrosine phosphoylated and thus active PTPα is localized in close proximity to Fyn in response to ethanol exposure.
Discussion
In this study we show that repeated systemic administration of ethanol or an episode of binge ethanol consumption results in the redistribution of PTPα to the synaptic membranes and lipid rafts compartments where Fyn resides. We also show that the redistribution of PTPα in response to ethanol is specific for the DMS and is not observed in other subregions of the striatum. Finally, we observed that ethanol exposure leads to an increase in the tyrosine phosphorylation of synaptic PTPα suggesting that the phosphatase is indeed active. Together, these findings suggest that the redistribution of PTPα by ethanol is, at least part, of the molecular mechanism that enables Fyn to be activated in the DMS in response to in vivo exposure of rats to ethanol.
Ethanol-mediated PTPα localization and phosphorylation is observed in Sprague Dawley and Long Evans rats
We found that repeated daily systemic administration of ethanol for 7 days leads to a long-lasting increase in the localization of active PTPα to synaptic membranes in the DMS of both Sprague Dawley and Long Evans rats. Additionally, we found increased Fyn activity and synaptic localization of active PTPα in the DMS of Long Evans rats that underwent a session of binge ethanol consumption. Sprague Dawley rats are commonly used for electrophysiological measurements and Long Evans rats are used for ethanol drinking paradigms. Therefore, the fact that similar findings can be observed in both strains of rats is of importance. The results also correlate well with our previous results in which we found similar biochemical and electrophysiological changes in NR2B-NMDAR phosphorylation, localization and activity in the DMS of Sprague Dawley and Long Evan rats in response to ethanol (Wang et al. 2010, Wang et al. 2011). Taken together, these results strongly imply that both strains of rats can be used in combination to study the molecular physiological and behavioral effects of ethanol.
Ethanol-mediated Fyn activity is associated with alterations in PTPα localization
Our results suggest that PTPα’s ability to activate Fyn depends on the subcellular localization of the phosphatase, in close proximity or away from Fyn. The compartmentalization of kinases and phosphatases depends on their interactions with scaffolding/adaptor proteins (Pawson & Scott 1997, Pawson & Scott 2010). In its inactive form, Fyn binds to the scaffolding protein, RACK1 (Yaka et al. 2002, Thornton et al. 2004, Wang et al. 2007), and it is plausible that the PTPα-mediated activation of Fyn requires the association of both enzymes with another adaptor protein. One possibility of such a protein is contactin, a neuronal cell adhesion molecule which was shown to bring PTPα and Fyn into close proximity (Zeng et al. 1999). Specifically, contactin, which interacts with the extracellular domain of PTPα can transiently activate Fyn upon aggregation of the cell adhesion protein (Zeng et al. 1999). Similarly, the intracellular domain of the neural cell adhesion molecule (NCAM) 140 (NCAM140) interacts with the catalytic domain of PTPα and Fyn, and the NCAM140-Fyn complex is disrupted in PTPα KO mice, suggesting that the three proteins exist in a complex (Bodrikov et al. 2005). Finally, in neuronal cells, the ability of the scaffolding protein PSD-95 to interact with Fyn and PTPα, may facilitate the activation of a subpopulation of Fyn that is part of the NMDAR complex at the synaptic membrane (Lei & McBain 2002). Thus, it is conceivable that the increase in synaptic PTPα in the DMS requires its interactions with these or other scaffolding/adaptor proteins.
We also found that ethanol binge drinking results in the distribution of a fraction of PTPα into the lipid rafts compartment. We observed in water controls that only a small fraction of Fyn is localized in non-lipid fractions, whereas a small portion of PTPα is found in lipid rafts. These results are similar to previous studies reporting that a large portion of PTPα in the brain are located in non lipid-raft environments, and the majority of Fyn kinase was localized in lipid rafts (Yasuda et al. 2002, Vacaresse et al. 2008). However, in response to stimulation such as NCAM activation, there is a redistribution of NCAM-associated PTPα into lipid rafts where Fyn is localized (Bodrikov et al. 2005). Furthermore, directed overexpression of PTPα in lipid raft microdomains results in activation of lipid rafts-associated Fyn kinase (Vacaresse et al. 2008). Together, these results imply that ethanol-mediated recruitment of PTPα to synaptic membranes and lipid rafts compartment, which could explain, at least in part, the mechanism that underlies ethanol-mediated activation of Fyn.
Ethanol exposure leads to increased phosphorylation of PTPα
PTPα contains several Serine and Tyrosine residues that were found to be phosphorylated. Specifically, PTPα was shown to be constitutively phosphorylated at serine residues 180 and 204, and protein kinase C (PKC) phosphorylation at these sites enhances PTPα activaty (Tracy et al. 1995, den Hertog et al. 1995). However, PKC-mediated phosphorylation of PTPα has not been directly linked to the redistribution of the phosphotase to membranal compartments or to Src PTKs activity. PTPα is also be phosphorylated on tyrosine 789 (den Hertog et al. 1994, Chen et al. 2006), and this phosphorylation was shown to be crucial for the dephosphorylation of Src PTKs at the inhibitory site leading to kinase activation (Chen et al. 2006, Zheng et al. 2000). We found that ethanol exposure results in an increase in tyrosine phosphorylated PTPα in synaptic fractions in the DMS. The presence of increased levels of active PTPα upon repeated systemic administration of ethanol and upon binge drinking session correlates with the increase in Fyn activity in the DMS following both paradigms as reported in Wang et al., 2010 and in this study, respectively. Thus, these results provide a direct link between PTPα and Fyn activities in response to ethanol.
Implications
We previously showed that repeated intermittent administration of ethanol or excessive ethanol intake in rats results in a long-lasting phosphorylation of the NR2B-NMDAR, leading to an increase in the activity of the channel in the DMS (Wang et al. 2010, Wang et al. 2011). Furthermore, the inhibition of the NR2B-NMDARs in the DMS reduced ethanol operant self-administration (Wang et al. 2010), suggesting that the activity of NR2B-NMDARs is required for neuroadaptations that underlie the maintenance of ethanol intake. PTPα, Fyn and NMDAR subunits are localized in synaptic membranes (Yaka et al. 2002, Dunah & Standaert 2003, Le et al. 2006,); and the phosphorylation state of Fyn and NR2B, have been shown to be altered in PTPα KO mice (Petrone et al. 2003, Le et al. 2006). Our data suggest that ethanol exposure causes a selective alteration in PTPα subcellular localization to synaptic membranes and lipid raft microdomains where Fyn resides, thus strengthening the positive regulatory mechanism resulting in the enhancement of Fyn kinase and NMDAR function. A long-lasting increase in PTPα in synaptic membranes in the DMS would facilitate PTPα-mediated Fyn activation after ethanol exposure, which would result in the long-lasting increased activity of the NMDAR.
Acknowledgements
The authors thank Vincent Walnault for technical assistance. This research was supported by NIAAA (R01AA 013438) (D.R.) and by funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (D.R.).
Footnotes
The authors of the manuscript have no conflict of interest.
References
- Abe T, Matsumura S, Katano T, et al. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- Besshoh S, Bawa D, Teves L, Wallace MC, Gurd JW. Increased phosphorylation and redistribution of NMDA receptors between synaptic lipid rafts and post-synaptic densities following transient global ischemia in the rat brain. J Neurochem. 2005;93:186–194. doi: 10.1111/j.1471-4159.2004.03009.x. [DOI] [PubMed] [Google Scholar]
- Bhandari V, Lim KL, Pallen CJ. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J Biol Chem. 1998;273:8691–8698. doi: 10.1074/jbc.273.15.8691. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodrikov V, Leshchyns'ka I, Sytnyk V, Overvoorde J, den Hertog J, Schachner M. RPTPalpha is essential for NCAM-mediated p59fyn activation and neurite elongation. J Cell Biol. 2005;168:127–139. doi: 10.1083/jcb.200405073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Peden L, Chang R, Harris RA, Blednov YA. Deletion of the fyn-kinase gene alters behavioral sensitivity to ethanol. Alcohol Clin Exp Res. 2003;27:1033–1040. doi: 10.1097/01.ALC.0000075822.80583.71. [DOI] [PubMed] [Google Scholar]
- Boehm SL, 2nd, Peden L, Jennings AW, Kojima N, Harris RA, Blednov YA. Over-expression of the fyn-kinase gene reduces hypnotic sensitivity to ethanol in mice. Neurosci Lett. 2004;372:6–11. doi: 10.1016/j.neulet.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chen SC, Pallen CJ. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J Biol Chem. 2006;281:11972–11980. doi: 10.1074/jbc.M600561200. [DOI] [PubMed] [Google Scholar]
- den Hertog J, Pals CE, Peppelenbosch MP, Tertoolen LG, de Laat SW, Kruijer W. Receptor protein tyrosine phosphatase alpha activates pp60c-src and is involved in neuronal differentiation. EMBO J. 1993;12:3789–3798. doi: 10.1002/j.1460-2075.1993.tb06057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog J, Sap J, Pals CE, Schlessinger J, Kruijer W. Stimulation of receptor protein-tyrosine phosphatase alpha activity and phosphorylation by phorbol ester. Cell Growth Differ. 1995;6:303–307. [PubMed] [Google Scholar]
- den Hertog J, Tracy S, Hunter T. Phosphorylation of receptor protein-tyrosine phosphatase alpha on Tyr789, a binding site for the SH3-SH2-SH3 adaptor protein GRB-2 in vivo. EMBO J. 1994;13:3020–3032. doi: 10.1002/j.1460-2075.1994.tb06601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Subcellular segregation of distinct heteromeric NMDA glutamate receptors in the striatum. J Neurochem. 2003;85:935–943. doi: 10.1046/j.1471-4159.2003.01744.x. [DOI] [PubMed] [Google Scholar]
- Engen JR, Wales TE, Hochrein JM, Meyn MA, 3rd, Banu Ozkan S, Bahar I, Smithgall TE. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65:3058–3073. doi: 10.1007/s00018-008-8122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SG. Analysis of NMDA receptor mediated synaptic plasticity using gene targeting: roles of Fyn and FAK non-receptor tyrosine kinases. J Physiol Paris. 1996;90:337–338. doi: 10.1016/s0928-4257(97)87914-4. [DOI] [PubMed] [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Isosaka T, Hattori K, Kida S, Kohno T, Nakazawa T, Yamamoto T, Yagi T, Yuasa S. Activation of Fyn tyrosine kinase in the mouse dorsal hippocampus is essential for contextual fear conditioning. Eur J Neurosci. 2008;28:973–981. doi: 10.1111/j.1460-9568.2008.06405.x. [DOI] [PubMed] [Google Scholar]
- Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci U S A. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi A, Saitoh S, Noda S, Yasuda K, Hayashi F, Ogata M, Hamaoka T. Translocation of tyrosine-phosphorylated TCRzeta chain to glycolipid-enriched membrane domains upon T cell activation. Int Immunol. 1999;11:1395–1401. doi: 10.1093/intimm/11.9.1395. [DOI] [PubMed] [Google Scholar]
- Kramer-Albers EM, White R. From axon-glial signalling to myelination: the integrating role of oligodendroglial Fyn kinase. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-010-0616-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le HT, Maksumova L, Wang J, Pallen CJ. Reduced NMDA receptor tyrosine phosphorylation in PTPalpha-deficient mouse synaptosomes is accompanied by inhibition of four src family kinases and Pyk2: an upstream role for PTPalpha in NMDA receptor regulation. J Neurochem. 2006;98:1798–1809. doi: 10.1111/j.1471-4159.2006.04075.x. [DOI] [PubMed] [Google Scholar]
- Lei G, Xue S, Chery N, et al. Gain control of N-methyl-D-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J. 2002;21:2977–2989. doi: 10.1093/emboj/cdf292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, Matsui H. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- Maksumova L, Le HT, Muratkhodjaev F, Davidson D, Veillette A, Pallen CJ. Protein tyrosine phosphatase alpha regulates Fyn activity and Cbp/PAG phosphorylation in thymocyte lipid rafts. J Immunol. 2005;175:7947–7956. doi: 10.4049/jimmunol.175.12.7947. [DOI] [PubMed] [Google Scholar]
- Maksumova L, Wang Y, Wong NK, Le HT, Pallen CJ, Johnson P. Differential function of PTPalpha and PTPalpha Y789F in T cells and regulation of PTPalpha phosphorylation at Tyr-789 by CD45. J Biol Chem. 2007;282:20925–20932. doi: 10.1074/jbc.M703157200. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Paul S, Lombroso PJ. Receptor and nonreceptor protein tyrosine phosphatases in the nervous system. Cell Mol Life Sci. 2003;60:2465–2482. doi: 10.1007/s00018-003-3123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson CT, Scott JD. Signal integration through blending, bolstering and bifurcating of intracellular information. Nat Struct Mol Biol. 2010;17:653–658. doi: 10.1038/nsmb.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- Petrone A, Battaglia F, Wang C, et al. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 2003;22:4121–4131. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S, Wang DZ, Lim KL, Pallen CJ. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr Biol. 1999;9:535–538. doi: 10.1016/s0960-9822(99)80238-3. [DOI] [PubMed] [Google Scholar]
- Resh MD. Fyn, a Src family tyrosine kinase. Int J Biochem Cell Biol. 1998;30:1159–1162. doi: 10.1016/s1357-2725(98)00089-2. [DOI] [PubMed] [Google Scholar]
- Rong Y, Lu X, Bernard A, Khrestchatisky M, Baudry M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- Sahin M, Dowling JJ, Hockfield S. Seven protein tyrosine phosphatases are differentially expressed in the developing rat brain. J Comp Neurol. 1995;351:617–631. doi: 10.1002/cne.903510410. [DOI] [PubMed] [Google Scholar]
- Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- Sap J, D'Eustachio P, Givol D, Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci U S A. 1990;87:6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Ponniah S, Wang DZ, Doetschman T, Vorhees CV, Pallen CJ. Protein tyrosine phosphatase alpha (PTP alpha) knockout mice show deficits in Morris water maze learning, decreased locomotor activity, and decreases in anxiety. Brain Res. 2003;984:1–10. doi: 10.1016/s0006-8993(03)02839-7. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21:2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork O, Kojima N, Stork S, Kume N, Obata K. Resistance to alcohol withdrawal-induced behaviour in Fyn transgenic mice and its reversal by ifenprodil. Brain Res Mol Brain Res. 2002;105:126–135. doi: 10.1016/s0169-328x(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Su J, Muranjan M, Sap J. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr Biol. 1999;9:505–511. doi: 10.1016/s0960-9822(99)80234-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Okumura-Noji K. NMDA receptor subunits epsilon 1 (NR2A) and epsilon 2 (NR2B) are substrates for Fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- Thornton C, Tang KC, Phamluong K, Luong K, Vagts A, Nikanjam D, Yaka R, Ron D. Spatial and temporal regulation of RACK1 function and NMDA receptor activity through WD40 motif-mediated dimerization. J Biol Chem. 2004 doi: 10.1074/jbc.M402316200. [DOI] [PubMed] [Google Scholar]
- Tracy S, van der Geer P, Hunter T. The receptor-like protein-tyrosine phosphatase, RPTP alpha, is phosphorylated by protein kinase C on two serines close to the inner face of the plasma membrane. J Biol Chem. 1995;270:10587–10594. doi: 10.1074/jbc.270.18.10587. [DOI] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signalling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Specific expressions of Fyn and Lyn, lymphocyte antigen receptor-associated tyrosine kinases, in the central nervous system. Brain Res Mol Brain Res. 1992;16:303–310. doi: 10.1016/0169-328x(92)90239-8. [DOI] [PubMed] [Google Scholar]
- Vacaresse N, Moller B, Danielsen EM, Okada M, Sap J. Activation of c-Src and Fyn kinases by protein-tyrosine phosphatase RPTPalpha is substrate-specific and compatible with lipid raft localization. J Biol Chem. 2008;283:35815–35824. doi: 10.1074/jbc.M807964200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eekelen M, Runtuwene V, Overvoorde J, den Hertog J. RPTPalpha and PTPepsilon signaling via Fyn/Yes and RhoA is essential for zebrafish convergence and extension cell movements during gastrulation. Dev Biol. 2010;340:626–639. doi: 10.1016/j.ydbio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, Janak PH, Lovinger DM, Ron D. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Ron D. Ethanol-mediated long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum. Channels (Austin) 2011;5 doi: 10.4161/chan.5.3.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–10198. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284:33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HY, Hsu FC, Gleichman AJ, Baconguis I, Coulter DA, Lynch DR. Fyn-mediated phosphorylation of NR2B Tyr-1336 controls calpain-mediated NR2B cleavage in neurons and heterologous systems. J Biol Chem. 2007;282:20075–20087. doi: 10.1074/jbc.M700624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi T. Molecular mechanisms of Fyn-tyrosine kinase for regulating mammalian behaviors and ethanol sensitivity. Biochem Pharmacol. 1999;57:845–850. doi: 10.1016/s0006-2952(98)00309-8. [DOI] [PubMed] [Google Scholar]
- Yagi T, Shigetani Y, Okado N, Tokunaga T, Ikawa Y, Aizawa S. Regional localization of Fyn in adult brain; studies with mice in which fyn gene was replaced by lacZ. Oncogene. 1993;8:3343–3351. [PubMed] [Google Scholar]
- Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem. 2003a;278:9630–9638. doi: 10.1074/jbc.M209141200. [DOI] [PubMed] [Google Scholar]
- Yaka R, Phamluong K, Ron D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J Neurosci. 2003b;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003c;27:1736–1742. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HB, Yang X, Cao J, Li S, Liu YN, Suo ZW, Cui HB, Guo Z, Hu XD. cAMP-dependent protein kinase activated Fyn in spinal dorsal horn to regulate NMDA receptor function during inflammatory pain. J Neurochem. 2011;116:93–104. doi: 10.1111/j.1471-4159.2010.07088.x. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Nagafuku M, Shima T, et al. Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J Immunol. 2002;169:2813–2817. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- Yun HM, Kim S, Kim HJ, Kostenis E, Kim JI, Seong JY, Baik JH, Rhim H. The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem. 2007;282:5496–5505. doi: 10.1074/jbc.M606215200. [DOI] [PubMed] [Google Scholar]
- Zeng L, D'Alessandri L, Kalousek MB, Vaughan L, Pallen CJ. Protein tyrosine phosphatase alpha (PTPalpha) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XM, Resnick RJ, Shalloway D. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. Embo J. 2000;19:964–978. doi: 10.1093/emboj/19.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]