Although tuberculosis is a major health problem globally, concomitant cryptococcosis has rarely been reported, especially in immunocompetent individuals. This report describes a case involving an otherwise healthy young woman who was diagnosed with a concomitant infection and emphasizes the importance of considering more than one infection occurring simultaneously in patients with significant comorbidities or immunodeficiency.
Keywords: Coinfection, Cryptococcosis, Cryptococcus gattii, Immunocompetent, Tuberculosis
Abstract
A case of Cryptococcus gattii (pulmonary and central nervous system) and Mycobacterium tuberculosis (pulmonary) coinfection in an otherwise healthy young woman is reported. The patient presented with a two-month history of dry cough. She had an unremarkable medical history. Both tuberculosis and cryptococcosis were diagnosed following bronchoscopy, and a subsequent lumbar puncture revealed C gattii in the cerebrospinal fluid. There is evidence that both M tuberculosis and C gattii may have suppressive effects on the host immune system. This suggests a mechanism by which an otherwise healthy individual developed these two infections.
Abstract
Les auteurs rendent compte d’un cas de co-infection par le Cryptococcus gattii (système pulmonaire et système nerveux central) et le Mycobacterium tuberculosis (système pulmonaire) chez une jeune femme autrement en santé. La patiente s’est présentée en raison d’une toux sèche depuis deux mois. Ses antécédents médicaux étaient sans histoire. On a diagnostiqué à la fois une tuberculose et une cryptococcose après la bronchoscopie, et une ponction lombaire subséquente a révélé un C gattii dans le liquide céphalorachidien. Des données probantes indiquent que le M tuberculosis et le C gattii auraient tous deux des effets suppresseurs sur le système immunitaire de l’hôte, ce qui laisse supposer un mécanisme par lequel une personne autrement en santé a contracté ces deux infections.
An 18-year-old university student presented with a two-month history of dry cough. She was otherwise healthy, with an unremarkable medical history. She took no regular medications, but had just completed a seven-day course of clarithromycin with no improvement in her symptomatology. Her travel history included two trips to India, one and three years before presentation. She had not travelled to Vancouver Island (British Columbia), a known epicentre for Cryptococcus gattii. She immigrated to Canada from Sri Lanka at eight years of age and, before immigration, received Bacille Calmette-Guérin vaccination. Her family history was unremarkable and she did not have any known exposures to tuberculosis (TB).
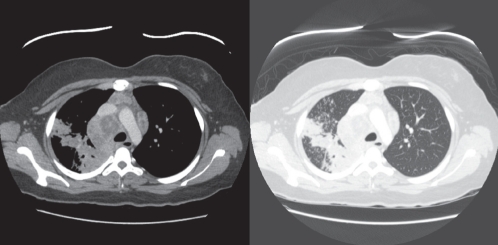
A physical examination revealed right lower lobe crackles but was otherwise normal. Her body mass index was 24 kg/m2. Initially, a chest x-ray revealed a large area of mass-like opacification in the mediastinum and several areas of consolidation in the right lung. A subsequent computed tomography scan of her chest confirmed the presence of large, necrotic mediastinal lymph nodes and areas of consolidation throughout the right lung (Figure 1). A diagnosis of concomitant TB and cryptococcosis was made following a bronchoscopy. Bronchial alveolar lavage specimens were acid-fast bacilli (AFB) negative; however, special stains and cytological features were consistent with Cryptococcus species. Bronchial brushings showed acute granulomatous inflammation with no definite organisms. A transbronchial biopsy specimen from the right upper lobe demonstrated necrotizing granulomatous inflammation with large numbers of acid-fast bacteria. A fine-needle aspirate of one of the mediastinal lymph nodes showed cytological features consistent with Cryptococcus species and rare Cryptococcus organisms. Cultures from the bronchoscopy specimens grew fully drug-sensitive Mycobacterium tuberculosis, but were negative for Cryptococcus. Sputum samples were AFB positive and grew TB. A lumbar puncture was performed and cerebrospinal fluid (CSF) cultures were positive for Cryptococcus gattii subtype VGIIa. India ink staining was not performed on the CSF, but the Gram-stain and AFB were negative. The other CSF parameters were as follows: opening pressure of 12 cmH2O; total white blood cell count of 2×106/L; protein level of 2.76 g/L; and a glucose level of 3.1 mmol/L. Both serum and CSF cryptococcal antigen titres were also positive (serum titre 1:512, and CSF titre 1:32). HIV 1 and 2 serologies were negative, and the patient’s CD4 count was normal (620×106 cells/L).
Figure 1).
Computed tomography scan images showing necrotic mediastinal lymphadenopathy and consolidation in the right lung
The patient was successfully treated with a combination of anti-TB and antifungal therapy. Her initial anti-TB medication regimen consisted of a combination of isoniazid (300 mg daily), rifampin (600 mg daily), pyrazinamide (1500 mg daily) and ethambutol (1200 mg daily). After one week of therapy, the patient developed drug-induced hepatitis and, thus, isoniazid, rifampin and pyrazinamide were discontinued and moxifloxacin (400 mg daily) was added. These medications were gradually reintroduced, and the ethambutol and moxifloxacin were discontinued. She received a total of three weeks of moxifloxacin, six weeks of ethambutol, eight weeks of pyrazinamide, and eight months of isoniazid and rifampin (total duration of therapy was eight months). Her antifungal therapy initially consisted of amphotericin B and flucytosine. However, she subsequently developed nephrotoxicity; consequently, these were discontinued and fluconazole was initiated. After six weeks in hospital, she was discharged home and, to date, has continued to do well.
DISCUSSION
Since the late 1990s C gattii has emerged in the Pacific Northwest region of North America as an increasingly common cause of pulmonary and central nervous system (CNS) infections (1). Unlike Cryptococcus neoformans, it most commonly infects immunocompetent individuals (1). TB remains a major global health problem.
Concomitant TB and cryptococcosis has rarely been reported in HIV-infected individuals (2). Coinfection with TB and Cryptococcus in immunocompetent individuals appears to be an even rarer entity. The first report of concomitant TB and cryptococcosis was reported in 1966 (3). The patient was a 61-year-old man who was being treated for pulmonary TB when he presented with C neoformans meningitis. Since that initial report, there have been several other case reports of coinfection with C neoformans and TB in HIV-negative patients. These include a case of concomitant M tuberculosis and C neoformans meninigits in a patient with TB epididymitis, a case of C neoformans osteomyelitis and abscess in a patient with TB lymphadenitis, and a case of C neoformans meningitis in a patient suspected of having miliary TB (4–6). Most recently, a case of concurrent severe CNS infection with C neoformans and M tuberculosis (CNS and pulmonary involvement) in an otherwise healthy 25-year-old Chinese woman was reported (7). Our case appears to be the only reported case of coinfection with C gattii and TB. In the present case, it is impossible to know whether infection with TB preceded infection with Cryptococcus or vice versa. It may be that infection with one predisposes to additional infections by way of immune system downregulation and altering of host defenses. There is some evidence that both TB and Cryptococcus have immunomodulatory effects on host defenses.
Three recent studies have explored the effects of TB on several different aspects of host immunity (8–10). Two of them (8,10) used cells isolated from bronchoalveolar lavage fluid to study the expression of immune mediators in patients with TB. The third study (9) used induced sputum samples from TB patients, patients with other lung diseases and healthy controls to do the same. Collectively, the results of these studies suggest that expression of immunosuppressive mediators inhibit host defenses against TB. The immunosuppressive mediators identified as being upregulated in patients with active TB include both intracellular (eg, suppressors of cytokine signalling, and interleukin receptor associated) and extracellular (interleukin [IL]-10, transforming growth factor-beta RII, IL-1Rn, IDO and CD163) molecules (8–10).
There is also evidence that C gattii can negatively affect the host immune system (1,11–13). The main virulence factors of C gattii is an outer polysaccharide capsule composed primarily of glucuronoxylomannan (GXM) and galactoxylomannan (GalXM). GXM has been shown to have several effects on the host immune system including induction of suppressor T cells (which inhibit cell-mediated immunity), direct inhibition of T cell responses and inhibition of leukocyte movement into inflammatory sites (1,11). A recent study demonstrated the ability of GXM to induce macrophage apoptosis in rats, both in vivo and in vitro (12). This result was also documented in a study that used peritoneal macrophages to show that both GXM and GalXM can cause macrophage apoptosis (13).
The present case report emphasizes the importance of considering more than one infection occurring simultaneously in patients without significant comorbidities or immunodeficiency. M tuberculosis and C gattii can suppress the host immune system. This may be the mechanism by which this otherwise healthy woman developed these two infections.
Footnotes
CONFLICTS OF INTERESTS: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Dixit A, Carroll SF, Qureshi ST. Cryptococcus gattii: An emerging cause of fungal disease in North America. Interdiscip Perspect Infect Dis. 2009:131–44. doi: 10.1155/2009/840452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawat D, Capoor MR, Nair D, Deb M, Aggarwal P. Concomitant TB and cryptococcosis in HIV-infected patients. Trop Doct. 2008;38:251–2. doi: 10.1258/td.2007.070295. [DOI] [PubMed] [Google Scholar]
- 3.Chomicki J. Coexistence of pulmonary tuberculosis with pulmonary and meningeal cryptococcosis. Chest. 1966;50:214–6. doi: 10.1378/chest.50.2.214. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Aranda F, Lopez-Dominguez JL, Munoz Malaga A, Blanco Ollero A. Meningitis simultaneously due to Cryptococcus neoformans and Mycobacterium tuberculosis. Clin Infect Dis. 1993;16:588–9. doi: 10.1093/clind/16.4.588-a. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq JA, Ghandour J. Cryptococcus neoformans abscess and osteomyelitis in an immunocompetent patient with tuberculous lymphadenitis. Infection. 2007;35:377–82. doi: 10.1007/s15010-007-6109-9. [DOI] [PubMed] [Google Scholar]
- 6.Aydemir H, Piskin N, Oztoprak N, Celebi G, Tekin OI, Akduman D. Cryptococcus neoformans meningitis in a HIV negative miliary tuberculosis-suspected patient. Mikrobiyol Bul. 2008;42:519–24. [PubMed] [Google Scholar]
- 7.Manfredi R, Calza L. Severe brain co-infection with Cryptococcus neoformans and Mycobacterium tuberculosis in a young, otherwise healthy student recently immigrated from China. Int J Infect Dis. 2008;12:438–41. doi: 10.1016/j.ijid.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Bonecini-Almeida MG, Ho JL, Boéchat NL, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor ß (TGF-ß) and analysis of TGF-ß receptors I and II in active tuberculosis. Infect Immun. 2004;72:2628–34. doi: 10.1128/IAI.72.5.2628-2634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida AS, Lago PM, Boechat N, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol. 2009;183:718–31. doi: 10.4049/jimmunol.0801212. [DOI] [PubMed] [Google Scholar]
- 10.Raju B, Hoshino Y, Belitskaya-Lévy I, et al. Gene expression profiles of bronchoalveolar cells in pulmonary TB. Tuberculosis. 2008;88:39–51. doi: 10.1016/j.tube.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellerbroek PM, Walenkamp AM, Hoepelman AI, Coenjaerts FE. Effects of the capsular polysaccharides of Cryptococcus neoformans on phagocyte migration and inflammatory mediators. Curr Med Chem. 2004;11:253–66. doi: 10.2174/0929867043456188. [DOI] [PubMed] [Google Scholar]
- 12.Chiapello LS, Baronetti JL, Garro AP, Spesso MF, Masih DT. Cryptococcus neoformans glucuronoxylomannan induces macrophage apoptosis mediated by nitric oxide in a caspase-independent pathway. Int Immunol. 2008;20:1527–41. doi: 10.1093/intimm/dxn112. [DOI] [PubMed] [Google Scholar]
- 13.Villena SN, Pinheiro RO, Pinheiro CS, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008;10:1274–85. doi: 10.1111/j.1462-5822.2008.01125.x. [DOI] [PubMed] [Google Scholar]