Abstract
Primary hepatic leiomyosarcoma are rare tumors with less than 30 cases reported in the English literature. Non specific presentations and often diagnosis delayed until they reach a large size, is the norm with therapy leading to an often dismal prognosis. A 67-year-old man presented complaining of abdominal pain and a palpable abdominal mass since Jan 2010. Abdominal ultrasonography and abdominal computed tomography revealed a large tumor in the left lobe of the liver. Surgical exploration was undertaken and an extended left hepatectomy with extension onto the dorsal part of segment 8 preserving the MHV with partial resection of segment 6 was undertaken. The weight of the resected specimen was 1300 g of the left lobectomy specimen and 8 g of the segment 6 partial resection specimen. The pathology report confirmed the diagnosis of leiomyosarcoma. On immunohistochemistry, the tumor cells were positive for smooth muscle actin stain. The patient is on regular follow up and is currently 9 mo post resection with no evidence of recurrence. We report the case of a resected primary hepatic leiomyosarcoma and emphasize the need for a global database for these rare tumors to promote a better and broader understanding of this less understood subject.
Keywords: Primary hepatic leiomyosarcoma, Smooth muscle actin, Smooth muscle, Hepatectomy
INTRODUCTION
Primary hepatic leiomyosarcoma are rare tumors with less than 30 cases reported in the English literature. Non-specific presentations and diagnosis often delayed until they reach a large size is the norm with therapy leading to an often dismal prognosis. The rarity of these tumors has precluded our understanding of them and therefore the standard of care has not been well defined. We herein report a case of primary hepatic leiomyosarcoma, which was treated surgically, and review the English literature with an emphasis on management outcomes.
CASE REPORT
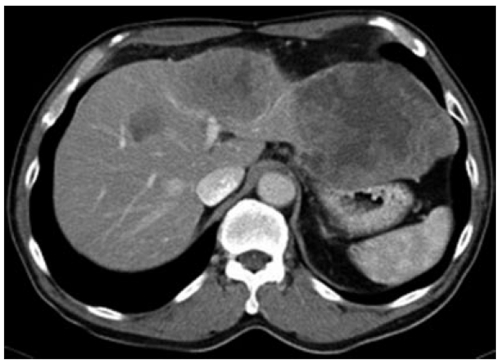
A 67-year-old man was referred to our department with chief complaining of pain in the abdomen and a palpable abdominal mass since Jan 2010. He had no history of liver disease or alcohol abuse. His past medical history and family history were unremarkable. Physical examination revealed a marked hepatomegaly extending 6 cm below the right costal margin. Laboratory analysis revealed normal liver function tests including serum albumin level and prothrombin time. White blood cell count, platelet, α-fetoprotein, CA 19-9 and carcinoembryonic antigen, were normal. Antibody to hepatitis C virus and hepatitis B surface antigen was negative. Impedance cardiogram (ICG) clearance at 15 min was 10%. Abdominal ultrasonography revealed a hypoechoic mass, measuring 14 cm in diameter, in the hepatic left lobe. Abdominal computed tomography (CT) showed a hypodense lesion on plain scans, heterogenous enhancing lesion on arterial phase and delayed washout on portal venous phase occupying segments 2, 3, 4 and 8 (Figure 1). CT arterio-portography revealed a hypodense lesion. Selective angiography of the celiac trunk and superior mesenteric artery showed a faint tumor stain in the left lobe of the liver and stenosis of the left portal vein. Chest CT, upper gastrointestinal and lower gastrointestinal endoscopy were within normal limits.
Figure 1.
Computed tomography scan showing a large heterogeneously enhancing tumor in the left lobe of the liver.
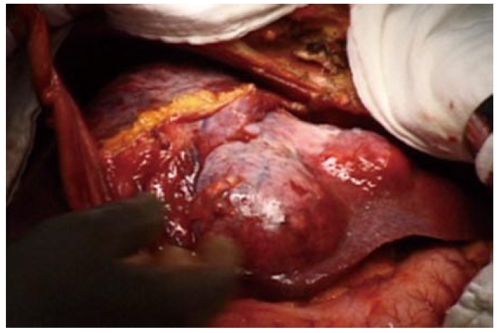
Preoperative diagnosis was unconfirmed, written informed consent was obtained, and surgical exploration was undertaken. A huge tumor, measured 17 cm × 7 cm × 14 cm, occupying almost the whole left lobe of the liver was found (Figure 2). Intraoperative ultrasound revealed that the tumor also extended into the dorsal aspect of segment 8 and another small hypoechoic lesion in segment 6. An extended left hepatectomy with extension onto the dorsal part of segment 8 preserving the MHV with partial resection of segment 6 was undertaken. The pringle time was 60 min and the operative blood loss was 520 mL. The weight of the resected specimen was 1300 g of the left lobectomy specimen and 8 g for the segment 6 partial resection specimen. Careful inspection of the abdominal and pelvic contents did not reveal any other masses or lesions. Grossly, the tumor was lobulated, well encapsulated, and prominent in fibrotic bands. The pathology report confirmed the diagnosis of leiomyosarcoma. Light microscopy demonstrated the typical pattern of growth of leiomyosarcoma, predominantly fascicular, with tumor bundles intersecting each other at wide angles and merging of tumor cells with blood vessel walls, an important diagnostic clue (Figure 3). The individual cells had elongated, blunted nuclei and acidophilic fibrillary cytoplasm. Numerous mitoses were present. On immunohistochemistry, the tumor cells were positive for the smooth muscle actin (SMA) stain (Figure 4). The patient is on regular follow up and is currently 9 mo post resection with no evidence of recurrence.
Figure 2.
Intraoperative photo showing the large left lobe tumor.
Figure 3.
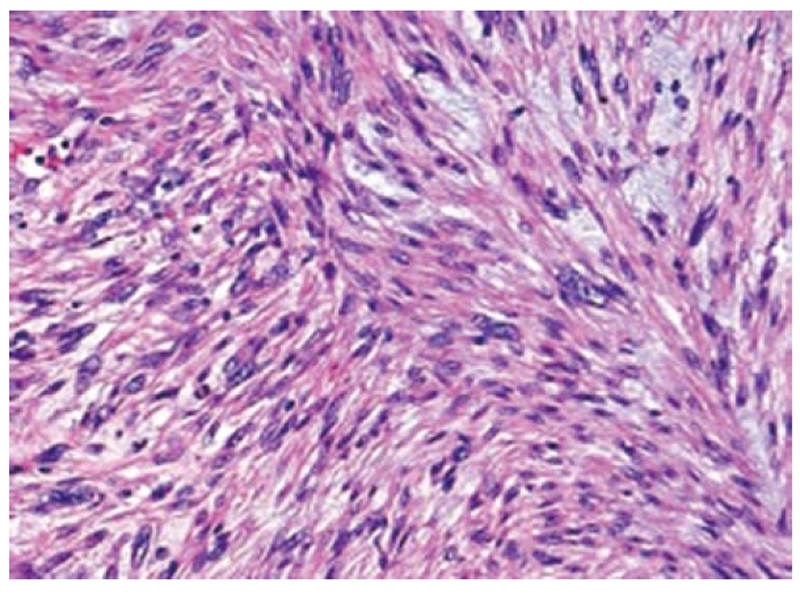
Histomicrograph showing fascicular growth pattern with tumor bundles intersecting each other at wide angles and merging of tumor cells with blood vessel walls.
Figure 4.
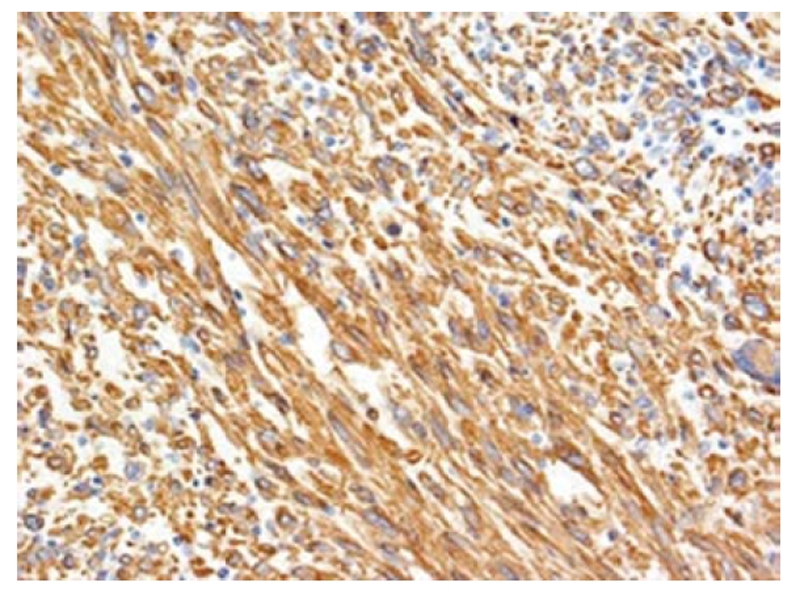
Tumor cells are positive for smooth muscle actin by immunohistochemical stain (× 100).
DISCUSSION
Primary hepatic mesenchymal tumors are rare tumors. Sarcomas constitute only 1% to 2% of all primary malignant tumors of the liver with the majority being either hepatocellular carcinoma or cholangiocarcinoma[1]. Nearly all primary sarcomas of the liver are angiosarcomas, epitheloid hemangioendotheliomas, or undifferentiated embryonal sarcoma constituting nearly 70% with leiomyosarcoma being a modest 8% to 10% of all sarcomas[2]. Previous reports of primary hepatic leiomyosarcoma, published in the English literature are summarized in Table 1. Most hepatic leiomyosarcoma are metastatic from other sites of leiomyosarcoma including gastrointestinal tract, uterus, retroperitoneum and lung[3]. So, exclusion of metastatic leiomyosarcoma in the liver is an essential event in diagnosing a primary lesion.
Table 1.
Management outcomes of published cases of hepatic leiomyosarcoma
| Author (yr) | Age (yr) | Sex | Risk factor | Treatment | Follow up |
| Wilson (1971)[12] | 49 | F | Wedge resection | Died at 18 mo | |
| Fong (1974)[12] | 62 | M | …. | Died at 20 mo | |
| Masur (1975)[12] | 62 | F | Chemotherapy | Autopsy diagnosis | |
| Masur (1975)[12] | 66 | F | Lobectomy | Died at 49 mo | |
| Yoshikawa (1977)[12] | 58 | F | Wedge resection | Died at 11 d postoperatively | |
| Bloustein (1978)[12] | 12 | F | Trisegmentectomy, chemotherapy | NED at 6 yr | |
| O’Leary (1982)[12] | 69 | M | Alive at 24 mo | ||
| Chen (1983)[12] | 78 | M | Chemotherapy, radiation | Died at 10 mo | |
| Maki (1987)[12] | 86 | F | Surgery | NED at 5 mo | |
| Paraskevopoulos (1991)[12] | 62 | M | Lobectomy | NED at 5 mo | |
| Baur (1993)[12] | 69 | F | Surgery | Recurrence after 10 yr | |
| Holloway (1996)[5] | 63 | M | Conservative | ||
| Soyer (1996)[12] | 67 | F | Surgery | ||
| Sato (2000)[12] | 62 | F | Autopsy diagnosis | ||
| Tsuji (2000)[11] | 68 | M | Hepatitis C | Autopsy diagnosis | |
| Lordanidis (2002)[12] | 25 | M | Surgery | Died at 3 mo | |
| Fujita (2002)[9] | 33 | F | Prior renal transplant | Right posterior segmentectomy | NED at 24 mo |
| Lee (2002)[13] | 64 | F | Right lobectomy + wedge resection of left lobe | NED at 24 mo | |
| Almogy (2004)[12] | 58 | F | Surgery + chemotherapy | Died at 4 mo | |
| 63 | F | Surgery + chemotherapy | Died at 12 mo | ||
| Watanabe (2008)[12] | 63 | Autopsy diagnosis | |||
| 49 | F | Autopsy diagnosis | |||
| Matthei (2009)[17] | 19 | F | Liver transplant | Died at 73 mo | |
| 64 | F | Surgery | NED 181 mo | ||
| 53 | F | Surgery | Died 21 mo | ||
| 55 | M | Surgery | NED 133 mo | ||
| 51 | M | Liver transplant | NED 144 mo | ||
| 59 | M | Surgery | Died 45 mo | ||
| 63 | F | Surgery | NED 133 mo | ||
| Giuliante (2009)[10] | 26 | M | Hodgkin’s lymphoma | Right lobectomy + wedge resection of seg III | Died at 25 mo |
| Liang (2009)[20] | 44 | F | Liver transplant | Died 34 mo | |
| Shamseddine (2010)[12] | 25 | F | Right lobectomy | Died 22 mo | |
| 39 | M | Extended right lobectomy | Died 19 d | ||
| 30 | M | Right lobectomy + chemotherapy | NED at 12 mo |
NED: No evidence of disease.
Leiomyosarcomas are malignant neoplasms that arise from smooth muscle. Hepatic leiomyosarcoma may arise from intrahepatic vascular structures[4], bile ducts[5] or ligamentum teres[6]. Tumors arising from the hepatic veins may develop Budd-Chiari syndrome and have a worse prognosis with tumors arising from the ligamentum teres having a better prognosis due to its increased resectability[6]. No underlying etiologic factors are known, although Thorotrast, acquired immunodeficiency syndrome[7], Epstein-Barr virus[8], prior history of immunosuppression in the form of post renal transplant[9] and previously treated Hodgkin’s lymphoma[10], and the rare association with Hepatitis C virus liver cirrhosis which was not directly implicated[11], have all been described in the literature. The median age of diagnosis is 58 years with sporadic occurrence of the tumor in the younger age group[12].
Primary hepatic leiomyosarcoma presents a clinical dilemma: not only are they unusual and rare with less than 50 cases described in the literature, but they are often asymptomatic until they become large, and even then they produce nonspecific symptoms[5-8]. Patients may be afflicted with a wide spectrum of symptoms, such as abdominal pain, weight loss, anorexia, vomiting, jaundice and rarely acute intraabdominal bleeding secondary to tumor rupture[13]. Tenderness of the upper abdomen, hepatomegaly, and mass may be the main signs. Some patients may have abnormal liver function tests but essentially the α-fetoprotein and other serological markers are normal. The non-specific nature of symptoms and the lack of serological markers make the diagnosis of hepatic leiomyosarcoma challenging.
Histological pre operative diagnosis of hepatic leiomyosarcoma is controversial as with other liver tumors, as most of the tumors are treated presuming to be hepatocellular carcinoma with its inherent propensity for needle track seeding. Histological examination reveals tumor composed of intersecting bundles of spindle-shaped cells. Immunohistochemistry is positive for desmin, vimentin and SMA but negative for keratin, S-100 protein and neuron-specific enolase and FNA biopsy will allow for specific FNA diagnosis in most cases[14].
CT findings of primary hepatic leiomyosarcoma have been described as a large, well-defined, heterogeneous-hypodensity mass with internal and peripheral enhancement or cystic mass with an enhancing thick wall. Cystic variant of leiomyosarcoma may be misdiagnosed as hydatid cyst or liver abscess[15]. On MR imaging the tumor displays homogenous or heterogeneous hypointensity on Tl-weighted images and hyperintensity on T2-weighted images with occasional observation of encapsulation[16].
Due to the rarity of primary hepatic sarcomas in general, and primary hepatic leiomyosarcoma in particular, the standard of care has not been defined. However surgical resection followed by adjuvant chemotherapy is being widely followed in an empirical manner[2].
Resection surgery forms the cornerstone of successful management of primary hepatic leiomyosarcoma with an intention of R0 resection. All patients with potentially resectable tumors with adequate remnant liver volume should undergo surgical exploration and liver resection. Criteria for inoperability would include extrahepatic tumor spread, diffuse intrahepatic tumor making the patient not a candidate for complete tumor removal, and impaired liver function precluding the planned hepatic resection. Resections in multifocal tumors should respect anatomical planes as enucleation might result in a R1 resection. The role of partial resection in multifocal tumors has not been defined. The surgical outcome for R0 resection extrapolated from 2 large series was 67% disease specific survival at 5 years with 0% 3 years survival for patients who underwent R1+ resection[2,17]. Age was another major prognostic factor with patients less than 50 years achieving better survival.
The role of adjuvant chemotherapy/chemo radiotherapy is not well defined. Adjuvant chemotherapy in the form of doxorubicin and ifosfamide seems to slow the course of the disease and may prolong survival in R1 resections but the evidence is lacking as the data are extrapolated from the unresectable or metastatic leiomyosarcoma setting[18].
Liver transplant has been attempted sporadically in primary hepatic leiomyosarcoma but is not as well-defined as in the setting of primary hepatic epithelioid hemangioendothelioma[19]. The outcome for liver transplant in primary hepatic leiomyosarcoma has been varied with all cases developing recurrent or metastatic disease and only one case showing long term survival after undergoing resection for local chest wall recurrence[20-22]. Immune status manipulation may play an important role in prolonging survival in the post transplant period by preventing recurrence[12].
In conclusion, primary hepatic leiomyosarcoma is a rare tumor with often delayed diagnosis and poor prognosis. To have a better understanding of these rare tumors we need to have a global database to analyze and understand the outcomes of different therapies. This case highlights the diagnostic and surgical therapy of this rare tumor.
Footnotes
Peer reviewer: Imtiaz Ahmed Wani, MD, Amira Kadal, Srinagar, Kashmir 190009, India
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Maki HS, Hubert BC, Sajjad SM, Kirchner JP, Kuehner ME. Primary hepatic leiomyosarcoma. Arch Surg. 1987;122:1193–1196. doi: 10.1001/archsurg.1987.01400220103020. [DOI] [PubMed] [Google Scholar]
- 2.Weitz J, Klimstra DS, Cymes K, Jarnagin WR, D'Angelica M, La Quaglia MP, Fong Y, Brennan MF, Blumgart LH, Dematteo RP. Management of primary liver sarcomas. Cancer. 2007;109:1391–1396. doi: 10.1002/cncr.22530. [DOI] [PubMed] [Google Scholar]
- 3.Cioffi U, Quattrone P, De Simone M, Bonavina L, Segalin A, Masini T, Montorsi M. Primary multiple epithelioid leiomyosarcoma of the liver. Hepatogastroenterology. 1996;43:1603–1605. [PubMed] [Google Scholar]
- 4.Civardi G, Cavanna L, Iovine E, Buscarini E, Vallisa D, Buscarini L. Diagnostic imaging of primary hepatic leiomyosarcoma: a case report. Ital J Gastroenterol. 1996;28:98–101. [PubMed] [Google Scholar]
- 5.Holloway H, Walsh CB, Thomas R, Fielding J. Primary hepatic leiomyosarcoma. J Clin Gastroenterol. 1996;23:131–133. doi: 10.1097/00004836-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi J, Azuma T, Fujioka H, Tanaka K, Furui J, Tomioka T, Kanematsu T. Leiomyosarcoma occurring in the ligamentum teres of the liver: a case report and a review of seven reported cases. Hepatogastroenterology. 1996;43:1051–1056. [PubMed] [Google Scholar]
- 7.Ross JS, Del Rosario A, Bui HX, Sonbati H, Solis O. Primary hepatic leiomyosarcoma in a child with the acquired immunodeficiency syndrome. Hum Pathol. 1992;23:69–72. doi: 10.1016/0046-8177(92)90014-t. [DOI] [PubMed] [Google Scholar]
- 8.Brichard B, Smets F, Sokal E, Clapuyt P, Vermylen C, Cornu G, Rahier J, Otte JB. Unusual evolution of an Epstein-Barr virus-associated leiomyosarcoma occurring after liver transplantation. Pediatr Transplant. 2001;5:365–369. doi: 10.1034/j.1399-3046.2001.00022.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujita H, Kiriyama M, Kawamura T, Ii T, Takegawa S, Dohba S, Kojima Y, Yoshimura M, Kobayashi A, Ozaki S, et al. Primary hepatic leiomyosarcoma in a woman after renal transplantation: report of a case. Surg Today. 2002;32:446–449. doi: 10.1007/s005950200073. [DOI] [PubMed] [Google Scholar]
- 10.Giuliante F, Sarno G, Ardito F, Pierconti F. Primary hepatic leiomyosarcoma in a young man after Hodgkin's disease: diagnostic pitfalls and therapeutic challenge. Tumori. 2009;95:374–377. doi: 10.1177/030089160909500318. [DOI] [PubMed] [Google Scholar]
- 11.Tsuji M, Takenaka R, Kashihara T, Hadama T, Terada N, Mori H. Primary hepatic leiomyosarcoma in a patient with hepatitis C virus-related liver cirrhosis. Pathol Int. 2000;50:41–47. doi: 10.1046/j.1440-1827.2000.00999.x. [DOI] [PubMed] [Google Scholar]
- 12.Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, Soubra A, Shamseddine A. Unusually young age distribution of primary hepatic leiomyosarcoma: case series and review of the adult literature. World J Surg Oncol. 2010;8:56. doi: 10.1186/1477-7819-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong TY, Kim YS, Park KJ, Lee JS, Huh JG, Ryu SH, Lee JH, Moon JS. [A case of primary leiomyosarcoma of the liver presenting with acute bleeding] Korean J Gastroenterol. 2008;51:194–198. [PubMed] [Google Scholar]
- 14.Smith MB, Silverman JF, Raab SS, Towell BD, Geisinger KR. Fine-needle aspiration cytology of hepatic leiomyosarcoma. Diagn Cytopathol. 1994;11:321–327. doi: 10.1002/dc.2840110403. [DOI] [PubMed] [Google Scholar]
- 15.Gates LK, Cameron AJ, Nagorney DM, Goellner JR, Farley DR. Primary leiomyosarcoma of the liver mimicking liver abscess. Am J Gastroenterol. 1995;90:649–652. [PubMed] [Google Scholar]
- 16.Yu RS, Chen Y, Jiang B, Wang LH, Xu XF. Primary hepatic sarcomas: CT findings. Eur Radiol. 2008;18:2196–2205. doi: 10.1007/s00330-008-0997-7. [DOI] [PubMed] [Google Scholar]
- 17.Matthaei H, Krieg A, Schmelzle M, Boelke E, Poremba C, Rogiers X, Knoefel WT, Peiper M. Long-term survival after surgery for primary hepatic sarcoma in adults. Arch Surg. 2009;144:339–44; discussion 344. doi: 10.1001/archsurg.2009.30. [DOI] [PubMed] [Google Scholar]
- 18.Oosten AW, Seynaeve C, Schmitz PI, den Bakker MA, Verweij J, Sleijfer S. Outcomes of first-line chemotherapy in patients with advanced or metastatic leiomyosarcoma of uterine and non-uterine origin. Sarcoma. 2009;2009:348910. doi: 10.1155/2009/348910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrabi A, Kashfi A, Schemmer P, Sauer P, Encke J, Fonouni H, Friess H, Weitz J, Schmidt J, Büchler MW, et al. Surgical treatment of primary hepatic epithelioid hemangioendothelioma. Transplantation. 2005;80:S109–S112. doi: 10.1097/01.tp.0000186904.15029.4a. [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Xiao-Min S, Jiang-Ping X, Jie-Yu Y, Xiao-Jun Z, Zhi-Ren F, Guo-Shan D, Rui-Dong L. Liver transplantation for primary hepatic leiomyosarcoma: a case report and review of the literatures. Med Oncol. 2010;27:1269–1272. doi: 10.1007/s12032-009-9372-z. [DOI] [PubMed] [Google Scholar]
- 21.Husted TL, Neff G, Thomas MJ, Gross TG, Woodle ES, Buell JF. Liver transplantation for primary or metastatic sarcoma to the liver. Am J Transplant. 2006;6:392–397. doi: 10.1111/j.1600-6143.2005.01179.x. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Paul MC, Gugenheim J, Hofman P, Arpurt JP, Fabiani P, Michiels JF, Fujita N, Goubeaux B, Loubière R, Delmont J. [Leiomyosarcoma of the liver: a case treated by transplantation] Gastroenterol Clin Biol. 1993;17:218–222. [PubMed] [Google Scholar]