Abstract
Background
The epidermal growth factor receptor (EGFR) is a promising therapeutic target in cancer, but its clinical value in breast cancer remains controversial. Our previous studies have found that quantitative analysis of biomarkers with quantum dot-based nanotechnology had better detection performance than conventional immunohistochemistry. The present study was undertaken to investigate the prognostic value of EGFR in breast cancer using quantum dot-based quantitative spectral analysis.
Methods
EGFR expression in 65 breast cancer specimens was detected by immunohistochemistry and quantum dot-immunohistochemistry, and comparisons were made between the two methods. EGFR expression in tissue microarrays of 240 breast cancer patients was then detected by quantum dot-immunohistochemistry and spectral analysis. The prognostic value of EGFR immunofluorescence area (EGFR area) for five-year recurrence-free survival was investigated.
Results
The same antigen localization, high correlation of staining rates (r = 0.914), and high agreement of measurement (κ = 0.848) of EGFR expression in breast cancer were found by quantum dot-immunohistochemistry and immunohistochemistry. The EGFR area showed significant differences by tumor grade, lymph node status, HER2 status, and hormone receptor status (all P < 0.05). Patients in the large EGFR area (≥30.51) group had a significantly higher five-year recurrence rate (47.2% versus 27.4%, P = 0.002) and worse five-year recurrence-free survival (log-rank test, P = 0.0015) than those in the small EGFR area (<30.51) group. In the subgroups, EGFR area was an independent prognosticator in the HER2-positive and lymph node-positive subgroups.
Conclusion
Quantum dot-based quantitative detection demonstrates the prognostic value of EGFR area in the HER2-positive and lymph node-positive subgroups of invasive breast cancer.
Keywords: quantum dots, breast cancer, epidermal growth factor receptor, quantitative analysis, recurrence-free survival, prognosis
Introduction
Breast cancer is the most common cancer in women, both in terms of incidence and mortality.1 Understanding its biological behavior and identifying objective prognosticators and biologic targets could help improve the outcome. The epidermal growth factor receptor (EGFR) is a member of the ErbB receptor family and is a new therapeutic target in solid tumors.2 To date, four members of the ErbB receptor family have been identified, including EGFR (HER1/ErbB-1), HER2 (ErbB-2/neu), HER3 (ErbB-3), and HER4 (ErbB-4). These ErbB receptors are widely expressed in several mammalian tissues and cell types, particularly those of epithelial, mesenchymal, and neuronal origin. They participate actively in physiological functions, such as cell proliferation and differentiation, cell– cell interaction, cytokine signaling and stress responses, and in oncological activities, such as cancer cell proliferation, survival, and metastasis.2,3 Structurally, EGFR is a transmembrane protein with an extracellular epidermal growth factor-binding domain, a transmembrane region, and an intracellular domain with ligand-activated tyrosine kinase activity. EGFR has been identified as a key cell surface receptor involved in a complex signaling network, with a binding capacity to various classes of agonists, including epidermal growth factor, transforming growth factor alpha, heparin-binding epidermal growth factor-like growth factor, amphiregulin, epiregulin, epigen, betacellulin, and neuregulin 2β.4 The clinical value of EGFR in breast cancer remains controversial.5–9 Some researchers have demonstrated a significant negative impact of EGFR overexpression on both relapse-free survival and overall survival,5–7 while others have failed to establish such a link.8,9 The causes for discrepancies among these studies may not only be due to differences in sample size and durations of follow-up, but also due to use of differing analytical procedures with different cutoff levels. Currently, the most commonly used method to detect EGFR in breast cancer specimens is immunohistochemistry. This conventional staining method may not be appropriate to investigate the role of EGFR in breast cancer because of its technical shortcomings, such as being prone to interfering factors, unstable sensitivity, high discrepancy between laboratories, and subjective interpretation.9,10
Quantum dots are semiconductor nanocrystals with a core/shell structure and a large spectral band gap, with unique photodynamic properties such as size-tunable symmetric emission bands, strong light absorbance, high fluorescent intensity, and high photostability.11 Quantum dot fluorescence can be separated from background autofluorescence in biological specimens, including cells and tissues.12 These properties facilitate integration of nanotechnology and biology, contributing to major advances in medical diagnostics, targeted therapeutics, and cellular and molecular biological studies.11–13 Notably, bioconjugation of quantum dots with functional molecules like antigens and antibodies offers a new pathway to enhanced sensing and imaging technologies.13 Our previous study of molecular targeted imaging of cancer cells and molecules14–16 has demonstrated the advantages of quantum dot-based molecular pathology, such as higher fluorescent efficiency over organic fluorescent dyes, better signal clarity, and a higher sensitivity and accuracy compared with conventional immunohistochemistry techniques. Therefore, quantum dot-based nanotechnology opens a new window to gain better insights into tumor biology. This study was undertaken to investigate the prognostic value of EGFR in breast cancer using quantum dot-based quantitative nanotechnology and spectrum analysis.
Materials and methods
Patients and specimens
Complete information on the clinicopathological characteristics of patients and fabrication of tissue microarrays has been detailed in our previous study.16 Briefly, tumor specimens from 240 patients aged 29–78 (median 48) years with invasive breast cancer were collected from Hubei Cancer Hospital, Wuhan, China, from January 2002 to December 2006. Two tissue cores representing two different invasive areas were obtained for each specimen, giving 480 cores for 240 specimens. Seven tissue microarray blocks were constructed, six containing 70 cores and one containing 60 cores. Consecutive sections (4 μm in thickness) of the tissue microarray blocks were cut to make tissue microarray slides. Major pathological parameters were available, including tumor size, location and number, lymph node status, histological grade, hormone receptor status, and HER2 status, as determined by conventional immunohistochemistry. All the patients were on a regular follow-up schedule. Written informed consent was obtained from the patients, and the study protocol was approved by the ethics committee.
The primary end point was recurrence-free survival, defined as the time interval from breast cancer surgery to the first evidence of recurrence (local, regional, or distant).16 If without recurrence, patients were censored on the last follow-up. In this study, we only selected the five-year data for analysis.
EGFR testing
The antibodies were purchased from Zhongshan Golden Bridge Biotechnology Co Ltd, Beijing, China. The primary antibody was rabbit antihuman monoclonal antibody against EGFR (clone SP9, 1:120 dilution) and the control group antibody was rabbit IgG (1:120 dilution). Biotinylated goat antirabbit IgG (1:400 dilution) was the secondary antibody. The three-step avidin-biotin-horseradish peroxidase complex method (ABC, MaiXin Bio Co Ltd, Fuzhou, China) was used for immunohistochemistry. A quantum dot-conjugated streptavidin probe (1:200 dilution) with a 605 nm emission wavelength was obtained from Wuhan Jiayuan Quantum Dots Co Ltd, Wuhan, China.
Quantum dot-immunohistochemistry was similar to conventional immunohistochemistry, with the major procedures described in our previous study,14–16 including the following brief steps: deparaffinizing → antigen retrieval → blocking → primary antibody (rabbit IgG for control group) → washing → blocking → biotinylated secondary antibody → washing → blocking → quantum dot-conjugated streptavidin probes → washing → mounting and observation.
This study was divided into two parts, ie, a feasibility study and a confirmation study. In the feasibility study, 65 specimens randomly selected from 240 breast cancer specimens were detected by both conventional immunohistochemistry and quantum dot-immunohistochemistry. One hundred tumor cells from five representative fields of each specimen at high magnification (400×) were counted for EGFR-positive cells. The EGFR positivity rates detected by quantum dot-immunohistochemistry and immunohistochemistry staining were compared. In the confirmation study, quantum dot-based immunofluorescent imaging of EGFR was conducted using the 605 nm quantum dot-conjugated streptavidin probe on the abovementioned tissue microarray slides. The slides were examined under an Olympus BX51 fluorescence microscope equipped with an Olympus DP72 camera (Olympus Optical Co Ltd, Tokyo, Japan) and a multispectral imaging Nuance system (Cambridge Research and Instrumentation Inc, Woburn, MA). The 605 quantum dots were excited by blue light (excitation wavelength 450–480 nm). Images of the quantum dots were captured using a DP72 camera. The spectral cube for each core of tissue microarray containing complete spectral information at 10 nm wavelength intervals from 520 to 680 nm was collected by the Nuance system. All the cubes were captured under the same conditions at low magnifications (40×), because this technique could obtain the entire images for each core, making it more accurate and representative in the tumor marker assay. Quantum dot fluorescence signal information for every core was analyzed by the analysis software package (Nuance version 2.8) within the Nuance system.
EGFR fluorescence signals and distribution area in the tumor were calculated numerically based on spectral unmixing. The distribution area of internal cytokeratin imaging was measured using the same method and conditions as in our previous study,16 and defined as the total area of tumor cells. The ratio of EGFR distribution area to cytokeratin area was calculated and defined as a percentage of EGFR-positive area. The EGFR area was calculated by the following equation:
The acquired total fluorescence areas of EGFR were defined as the sum of EGFR fluorescence areas on the two cores of tissue microarray.
Statistical analysis
The Spearman’s rho correlation, consistency (κ) check and Chi-square test were used to compare the results for quantum dot-immunohistochemistry and immunohistochemistry. Differences in EGFR areas were assessed using the Mann– Whitney U test or Kruskal–Wallis H test. Receiver-operating characteristic (ROC) curve analysis was conducted to evaluate the predictive value of the EGFR area for five-year recurrence-free survival. The optimal point with the highest sum value of sensitivity and specificity was defined as the cutoff. The Kaplan–Meier method was used to assess for differences in five-year recurrence-free survival. A multivariate Cox regression model was used to select independent predictors of five-year recurrence-free survival. Statistical analyses were performed using SPSS 13.0 software (SPSS Inc, Chicago, IL). Two-tailed P < 0.05 was considered to be statistically significant.
Results
EGFR expression in feasibility study
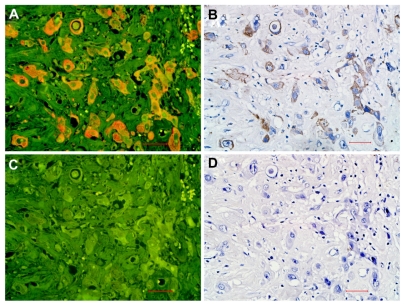
In the feasibility study, EGFR expression was observed on the same location on the membranes and in the cytoplasm by quantum dot-immunohistochemistry (Figure 1A) and immunohistochemistry (Figure 1B). The isotype control group (rabbit IgG) did not show any positive expression on breast cancer cells (Figure 1C and D). EGFR staining rates detected by quantum dot-immunohistochemistry and immunohistochemistry were 0%–85% and 0%–82%, respectively. Median EGFR staining rates by quantum dot-immunohistochemistry and immunohistochemistry were 15% (25%–75% interquartile range, 8%–38.5%) and 13% (25%–75% interquartile range, 5%–35.5%), respectively. Spearman’s rho correlation analysis showed that the correlation coefficient of EGFR staining rates by the two methods was 0.914. According to the EGFR PharmaDx Interpretation Manual,17 with a EGFR staining rate of 30% set as cutoff, 20 (30.8%) cases were positive by quantum dot- immunohistochemistry and 19 (29.2%) cases were positive by immunohistochemistry (P = 0.848). Of 65 cases, 19 cases were concurrently positive by two methods (κ = 0.848, consistency check). Both methods revealed the same antigen distribution and measurement consistency. These results suggest that quantum dot-immunohistochemistry had performance equal to that of conventional immunohistochemistry.
Figure 1.
EGFR expression on breast cancer cells by quantum dot-immunohistochemistry and immunohistochemistry. EGFR-positive expression on breast cancer cells imaged under Olympus DP72 camera (400×) by quantum dot-immunohistochemistry (A) and immunohistochemistry (B), control group (rabbit IgG) showed no any positive expression on breast cancer cells by quantum dot-immunohistochemistry (C), and immunohistochemistry (D). Scale bar: 25 μm for (A, B, C, and D).
Abbreviation: EGFR, epidermal growth factor receptor.
EGFR area in 240 cases
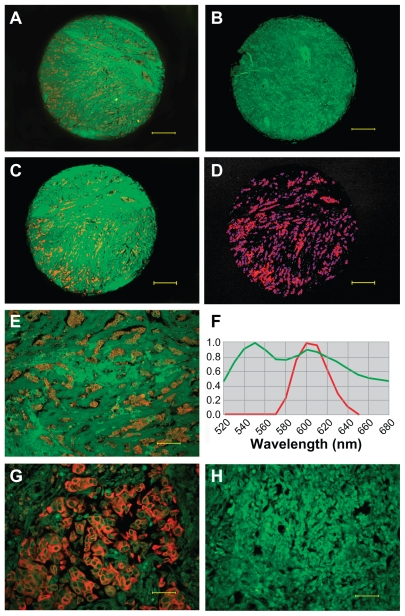
Among 240 breast cancer specimens, EGFR expression was observed in 235 tumors (Figure 2A, E, and G), and five tumors did not show any EGFR signal (Figure 2B). Quantum dot signals of EGFR were obtained from cubes by spectral unmixing (Figure 2C, D, and F). The control group showed no EGFR expression (Figure 2H). The EGFR total fluorescence areas of the 235 samples ranged from 171 to 176,629. The median EGFR area of all the breast cancer patients was 33.08 (25%–75% interquartile range, 11.79–59.77), after adjustment for the area of cytokeratin (internal control).
Figure 2.
EGFR determination and quantitative analysis. EGFR positive expression (A) and EGFR-negative expression (B) imaged under DP72 camera (40×); EGFR signal distribution (C) and EGFR signal locating (D) in the core (40×) unmixed by the Nuance multispectral imaging system; EGFR-positive expression imaged under DP72 camera (200×) (E), EGFR signal analysis by Nuance multispectral imaging systems (F), EGFR positive expression (G) and negative control group (H) imaged under DP72 camera (400×). Scale bar: 250 μm for (A–D), 50 μm for (E), 25 μm for (G and H).
Abbreviation: EGFR, epidermal growth factor receptor.
Relationship between EGFR area and clinicopathological features
The association between EGFR area and major clinicopathological features, including age, tumor size, lymph node status, tumor grade, hormone receptor status, and HER2 status was evaluated. EGFR area was significantly correlated with lymph node status, hormone receptor status, HER2 status, and tumor grading. No significant relationship was found between EGFR area and the other clinicopathological features (Table 1).
Table 1.
Main clinicopathological features and EGFR area of 240 patients with breast cancer
| Items | n (%) | EGFR area, median (IQR) | P value |
|---|---|---|---|
| Age (years) | |||
| ≤50 | 149 (62.1) | 32.28 (11.91, 59.09) | 0.927a |
| >50 | 91 (37.9) | 34.82 (11.72, 60.76) | |
| Tumor size (cm) | |||
| T1 (T ≤ 2) | 35 (14.6) | 24.46 (4.71, 55.23) | 0.102b |
| T2 (2 < T ≤ 5) | 162 (67.5) | 30.90 (7.57, 63.49) | |
| T3 (T > 5) | 43 (17.9) | 41.33 (27.50, 59.94) | |
| Lymph node status | |||
| Positive | 131 (54.6) | 37.81 (15.43, 65.90) | 0.009a |
| Negative | 109 (45.4) | 24.04 (5.29, 55.31) | |
| Tumor grade | |||
| Grade 1 | 40 (16.7) | 29.70 (9.12, 59.35) | 0.031b |
| Grade 2 | 141 (58.8) | 26.16 (6.18, 58.84) | |
| Grade 3 | 59 (24.6) | 45.31 (27.50, 62.45) | |
| HR status | |||
| Positive | 152 (63.3) | 29.28 (6.74, 58.03) | 0.049a |
| Negative | 88 (36.7) | 44.02 (14.47, 65.73) | |
| HER2 status | |||
| Positive | 123 (51.3) | 46.61 (17.42, 66.35) | 0.040a |
| Negative | 117 (48.7) | 29.04 (9.41, 58.59) | |
Notes: Mann–Whitney U test;
Kruskal–Wallis H test.
Abbreviations: EGFR, epidermal growth factor receptor; HR, hormone receptor; IQR, interquartile range.
ROC analysis of EGFR area by five-year recurrence-free survival
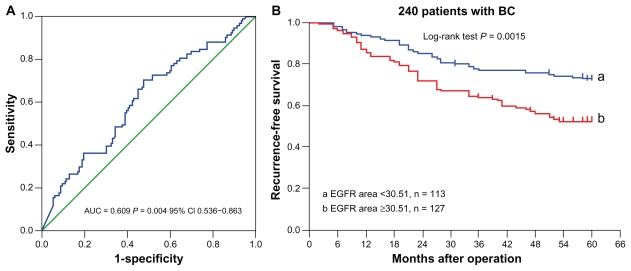
ROC analysis of EGFR area by five-year recurrence is shown in Figure 3A, which indicates that the quantum dot-based EGFR area could predict five-year recurrence. According to the optimal sensitivity and specificity of the ROC curve by five-year recurrence status, 30.51 were defined as the optimal cutoff for EGFR area, with sensitivity of 65.9% and specificity of 55%.
Figure 3.
Receiver-operating characteristic analysis of EGFR area by five-year recurrent status of 240 cases (A) and the five-year recurrence-free survival of patients with different EGFR area (B).
Abbreviation: EGFR, epidermal growth factor receptor.
EGFR area and five-year recurrence-free survival
In this study, the five-year recurrence rate was 37.9% (91/240), with 20 local recurrences and 71 distant recurrences. Based on the cutoff value of 30.51 for EGFR fluorescence area, the 240 breast cancer tumors were classified into two subgroups, ie, tumors with a small EGFR area (<30.51, n = 113) and those with a large EGFR area (≥30.51, n = 127). The five-year recurrence rate was 47.2% in patients with a large EGFR area, and 27.4% in those with a small EGFR area (P = 0.002). The five-year recurrence-free survival of the two groups showed a significant difference (P = 0.0015, log-rank test, Figure 3B).
Univariate analysis indicated that lymph node status, tumor grade, tumor size, HER2 status, EGFR area, and hormone receptor status had significant correlations with five-year recurrence-free survival (all P < 0.05, Table 2). However, among all the above factors, multivariate analysis using a Cox regression model revealed that only lymph node status, tumor size, tumor grade, and HER2 status were independent prognosticators, whereas the other factors, including EGFR area, were not in the equation.
Table 2.
Factors correlated with five-year recurrence-free survival of patients with breast cancer
| Patient group | Univariate analysis | Multivariate analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wald | P | RR | 95% CI | Wald | P | RR | 95% CI | |||
| Lower | Upper | Lower | Upper | |||||||
| Total cases (n = 240) | ||||||||||
| Age | 0.204 | 0.652 | 1.102 | 0.724 | 1.677 | NE | NE | NE | NE | NE |
| Grade | 61.983 | 0.000 | 4.439 | 3.063 | 6.433 | 30.419 | 0.000 | 3.074 | 2.062 | 6.045 |
| Lymph node status | 35.862 | 0.000 | 5.035 | 2.966 | 8.545 | 20.555 | 0.000 | 3.512 | 2.040 | 6.323 |
| Tumor size | 24.190 | 0.000 | 2.542 | 1.753 | 3.686 | 5.283 | 0.022 | 1.547 | 1.066 | 2.246 |
| HER2 status | 18.255 | 0.000 | 2.603 | 1.678 | 4.038 | 5.034 | 0.025 | 1.218 | 1.025 | 1.448 |
| HR status | 12.799 | 0.000 | 0.472 | 0.313 | 0.712 | NE | NE | NE | NE | NE |
| EGFR area | 9.576 | 0.002 | 1.984 | 1.286 | 3.063 | NE | NE | NE | NE | NE |
| Lymph node-positive subgroup (n = 131) | ||||||||||
| Age | 0.005 | 0.946 | 1.016 | 0.639 | 1.616 | NE | NE | NE | NE | NE |
| Grade | 23.691 | 0.000 | 2.879 | 1.888 | 4.447 | 16.688 | 0.000 | 2.428 | 1.586 | 3.716 |
| Tumor size | 10.134 | 0.001 | 1.900 | 1.280 | 2.820 | NE | NE | NE | NE | NE |
| HER2 status | 25.065 | 0.000 | 3.367 | 2.093 | 5.416 | 18.599 | 0.000 | 2.897 | 1.787 | 4.699 |
| HR status | 20.782 | 0.000 | 0.342 | 0.216 | 0.543 | NE | NE | NE | NE | NE |
| EGFR area | 6.654 | 0.010 | 1.930 | 1.171 | 3.182 | 5.174 | 0.023 | 1.789 | 1.084 | 2.953 |
| HER2-positive subgroup (n = 123) | ||||||||||
| Age | 0.802 | 0.371 | 1.274 | 0.750 | 2.162 | NE | NE | NE | NE | NE |
| Grade | 34.649 | 0.000 | 4.256 | 2.627 | 6.893 | 13.277 | 0.000 | 2.507 | 1.529 | 4.110 |
| Tumor size | 6.296 | 0.011 | 1.900 | 1.155 | 3.125 | NE | NE | NE | NE | NE |
| Lymph node status | 27.219 | 0.000 | 9.624 | 4.111 | 22.532 | 16.738 | 0.000 | 6.316 | 2.612 | 15.272 |
| HR status | 7.501 | 0.006 | 0.474 | 0.277 | 0.809 | NE | NE | NE | NE | NE |
| EGFR area | 6.269 | 0.012 | 2.044 | 1.168 | 3.578 | 4.495 | 0.034 | 1.843 | 1.047 | 3.245 |
Abbreviations: RR, relative risk; NE, not in the equation; HR, hormone receptor; EGFR, epidermal growth factor receptor; CI, confidence interval.
The 240 patients were further classified into subgroups according to lymph node status, tumor size, HER2 status, and hormone receptor status. Univariate analysis and multivariate analysis indicated that EGFR area was an independent prognosticator in the lymph node-positive and HER2-positive subgroups (Table 2), whereas in other subgroups, EGFR area did not enter into the equation of multivariate analysis.
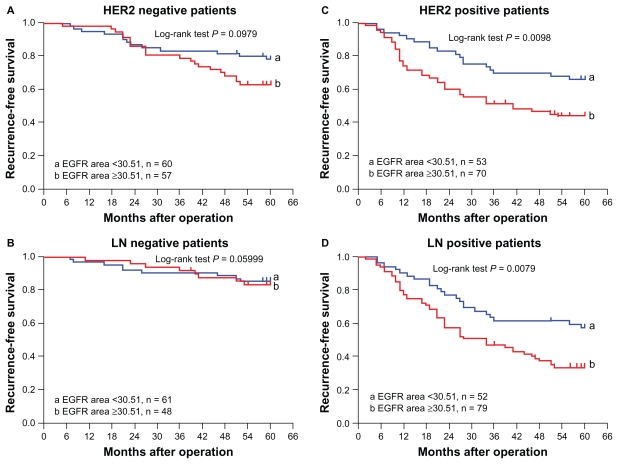
Five-year recurrence rate and recurrence-free survival were investigated in the HER2 and lymph node subgroups. The differences in five-year recurrence rate and recurrence-free survival between patients with a large EGFR area and a small EGFR area in the HER2-negative and lymph node-negative subgroups did not reach statistical significance (both P > 0.05, Figure 4A and B).
Figure 4.
The EGFR area and five-year recurrence-free survival in HER2 and lymph node subgroups.
Abbreviations: EGFR, epidermal growth factor receptor; LN, lymph node.
In the HER2-positive subgroup, the five-year recurrence rate was 55.7% (39/70) in patients with a large EGFR area and 34.0% (18/53) in those with a small area (P = 0.017). The median five-year recurrence-free survival in patients with a large EGFR area was 41.0 months (95% confidence interval [CI] 16.4–65.6 months), but the median five-year recurrence-free survival in those with small EGFR area was not reached yet (P = 0.0098, log-rank test, Figure 4C). In the lymph node-positive subgroup, the five-year recurrence rate was 65.8% (52/79) in patients with a large EGFR area and 42.3% (22/52) in those with a small EGFR area (P = 0.008). The median five-year recurrence-free survival was 34.0 months (95% CI 19.9–28.1) in patients with a large EGFR area, but was not reached in those with a small EGFR area (P = 0.0079, log-rank, Figure 4D).
Discussion
In this study, quantum dot-immunohistochemistry showed good correlation and consistency with conventional immunohistochemistry in detecting EGFR, and better image quality and sensitivity than conventional immunohistochemistry in detecting biomarkers, as previously reported.14 Taking advantage of the optical properties of quantum dots, which overcome the limitations associated with tissue autofluorescence, allow accurate determination of biomarkers, and quantify the biomarkers because of sharper and more photostable fluorescent signals of quantum dots than organic dyes,11–13 we quantified the expression of EGFR in breast cancer specimens using a quantum dot-based immunofluorescence probe. From a new perspective, ie, the EGFR fluorescence area, we investigated the prognostic value of EGFR in breast cancer. Patients with a large EGFR area demonstrated a significantly higher five-year recurrence rate and worse recurrence-free survival. It was a prognostic predictor of five-year recurrence-free survival for the entire study population in univariate analysis, but not in multivariate analysis. However, in the HER2-positive and lymph node-positive subgroups, EGFR area was an independent prognostic predictor both in univariate analysis and multivariate analysis.
The prognostic significance of EGFR in breast cancer has been investigated for over 20 years, yet no agreement has been reached. Sainsbury et al5 investigated 139 patients with breast cancer and found that recurrence-free survival and overall survival were significantly worse for patients with EGFR-positive tumors compared with EGFR-negative tumors, that EGFR was the most important variable in predicting recurrence-free survival and overall survival in lymph node-negative patients, and that this was the second most important variable in lymph node-positive patients. However, Ferrero et al8 investigated 780 consecutive breast cancer patients using a specific ligand-binding assay and found that there was no link between tumor size, grade, node status, and EGFR tumor levels. There was a constant and significant decrease in EGFR tumor levels according to patient age, and a significant inverse relationship between estrogen receptor status and EGFR. Tsutsui et al9 investigated 1029 patients with primary breast cancer by immunohistochemistry and found that EGFR was an independent prognostic factor for recurrence-free survival and overall survival in all patients, but the value of EGFR was somewhat insufficient to achieve statistical significance for both recurrence-free survival and overall survival in the subgroups according to nodal status. These confusing and even contradictory findings justify the need to re-evaluate EGFR using other molecular methods in different patient populations.
With the recent advances in biomedical science, targeted therapies and personalized medicine hold the future in clinical practice. EGFR has been studied from different perspectives and has been considered as a new therapeutic target in solid tumors. Cetuximab, gefitinib, erlotinib, and lapatinib17–22 are agents targeting the EGFR pathway, and have been approved for the treatment of advanced colorectal cancer, squamous cell carcinoma of the head and neck, advanced non-small cell lung cancer, pancreatic cancer, and breast cancer,23 which has led investigators to restudy EGFR from different perspectives.24
In the ErbB family, HER2 is the most extensively studied member, and its overexpression is closely correlated with aggressive behavior and poor prognosis of breast cancer. The relationship between EGFR and HER2 in breast cancer has been extensively investigated. Recently, McIntyre et al25 investigated all receptors and ligands in the ErbB family using immunohistochemistry and found that the heterogeneity in expression of receptor and ligands was unexpectedly high, with HER2 ranking first and EGFR ranking second. Yonemori et al26 investigated the immunohistochemistry expression of EGFR, HER3, and HER4 in HER2-positive patients with breast cancer treated with trastuzumab-containing neoadjuvant chemotherapy, and found that only EGFR was a negative prognosticator of pathologic complete response in this HER2-positive population. With regard to the mechanism, one study reported that both EGFR and HER2 act synergistically to cause aberrant tumor growth, leading to early onset of metastasis and death.27 Overexpression of HER2 can potentiate EGFR signaling and contribute to EGFR-mediated transformation and tumor progression.28 Further, HER2/ EGFR heterodimers showed ineffective endocytosis and destruction of ligand-bound EGFR, in contrast with EGFR homodimers.29 Breast cancer with coexpression of EGFR and HER2 has been shown to have the worst prognosis, whereas the prognostic value of HER2 was stronger than that of EGFR in breast cancer.30 In accordance with these reports, our study confirmed that HER2 was an independent prognosticator of breast cancer. EGFR and HER2 had additive adverse effects on prognosis. Patients with both positive HER2 and a large EGFR area had worse recurrence-free survival, and a large EGFR area significantly increased five-year recurrence rate by 21.7% compared with a small EGFR area. Therefore, we suggest that simultaneous detection of HER2 and EGFR could improve the predictive value and enable better treatment decisions. Quantum dot-based concurrent labeling technology provides such potential.31
Lymph node status is an important prognostic predictor of breast cancer. We observed that the EGFR area was significantly higher in our lymph node-positive subgroup than in our lymph node-negative subgroup. In the lymph node-positive subgroup, a large EGFR area significantly increased five-year recurrence rate by 23.5% comparing with a small EGFR area. In patients with simultaneous lymph node positivity and a large EGFR area, the median five-year recurrence-free survival was only 34.0 months, which implies that EGFR detection was more meaningful in patients with lymph node-positive breast cancer.
A couple of studies have reported a significant inverse relationship between EGFR and hormone receptor status.5,6,8 Consistent with these reports, we also observed an inverse relationship between EGFR area and hormone receptor status, and the EGFR area was significantly higher in the hormone receptor-negative group than in the hormone receptor-positive group.
This study has several limitations. First, it was a retrospective study using a nonrandomized database. Second, it included only 240 patients with breast cancer, and the median follow-up was only five years, so the results may have been more accurate with a larger sample size and longer follow up. Third, the treatment was not controlled, and we assumed that all 240 patients with breast cancer received optimal treatment after surgery, which might have introduced some bias in the results. Nevertheless, quantum dot-based nanotechnology provides a new insight into this elusive biomarker.
In conclusion, quantum dot-immunohistochemistry demonstrated a performance at least equivalent to that of immunohistochemistry in detecting EGFR in breast cancer specimens. Quantitative analysis with quantum dot- based technology indicated that EGFR area has a negative prognostic value in patients with HER2-positive and lymph node-positive breast cancer.
Acknowledgments
This study was supported by the grants from the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (No. 20621502 and 20921062), the Science and Research Program of Health Department of Hubei Province (No. JX5B69), the Fundamental Research Funds for the Central Universities (No. 4103005), and the Scholarship Award for Excellent Doctoral Student awarded by the Ministry of Education (2010).
Footnotes
Disclosure
The authors report no conflicts of interest in this work
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Saxena R, Dwivedi A. ErbB family receptor inhibitors as therapeutic agents in breast cancer: current status and future clinical perspective. Med Res Rev. 2010 Oct 25; doi: 10.1002/med.20209. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 3.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8(1):11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 4.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103(2):211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 5.Sainsbury JR, Farndon JR, Needham GK, Malcolm AJ, Harris AL. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- 6.Klijn JG, Berns PM, Schmitz PI, Foekens JA. The clinical significance of epidermal growth factor receptor (EGF-R) in human breast cancer: a review on 5232 patients. Endocr Rev. 1992;13(1):3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 7.Tovey SM, Witton CJ, Bartlett JM, Stanton PD, Reeves JR, Cooke TG. Outcome and human epidermal growth factor receptor (HER) 1–4, status in invasive breast carcinomas with proliferation indices evaluated by bromodeoxyuridine labelling. Breast Cancer Res. 2004;6(3):R246–251. doi: 10.1186/bcr783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrero JM, Ramaioli A, Largillier R, et al. Epidermal growth factor receptor expression in 780 breast cancer patients: a reappraisal of the prognostic value based on an eight-year median follow-up. Ann Oncol. 2001;12(6):841–846. doi: 10.1023/a:1011183421477. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsui S, Ohno S, Murakami S, Hachitanda Y, Oda S. Prognostic value of epidermal growth factor receptor (EGFR) and its relationship to the estrogen receptor status in 1029 patients with breast cancer. Breast Cancer Res Treat. 2002;71(1):67–75. doi: 10.1023/a:1013397232011. [DOI] [PubMed] [Google Scholar]
- 10.Foley J, Nickerson NK, Nam S, et al. EGFR signaling in breast cancer: Bad to the bone. Semin Cell Dev Biol. 2010;21(9):951–960. doi: 10.1016/j.semcdb.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azzazy HM, Mansour MM, Kazmierczak SC. From diagnostics to therapy: prospects of quantum dots. Clin Biochem. 2007;40(13–14):917–927. doi: 10.1016/j.clinbiochem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Salata O. Applications of nanoparticles in biology and medicine. J Nanobiotechnology. 2004;2(1):3. doi: 10.1186/1477-3155-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medintz IL, Mattoussi H, Clapp AR. Potential clinical applications of quantum dots. Int J Nanomedicine. 2008;3(2):151–167. doi: 10.2147/ijn.s614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Peng J, Xia HS, et al. Quantum dots-based immunofluorescence technology for the quantitative determination of HER2 expression in breast cancer. Biomaterials. 2009;30(15):2912–2918. doi: 10.1016/j.biomaterials.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Chen C, Peng J, Xia H, et al. Quantum-dot-based immunofluorescent imaging of HER2 and ER provides new insights into breast cancer heterogeneity. Nanotechnology. 2010;1(9):95–101. doi: 10.1088/0957-4484/21/9/095101. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Xia HS, Gong YP, et al. The quantitative detection of total HER2 load by quantum dots and the identification of a new subtype of breast cancer with different five-year prognosis. Biomaterials. 2010;31(33):8818–8825. doi: 10.1016/j.biomaterials.2010.07.091. [DOI] [PubMed] [Google Scholar]
- 17.DakoEGFR PharmDxTM Interpretation ManualAvailable from: http://www.dako.com/08052_egfr_pharmdx_interpretation_manual.pdfAccessed August 8, 2011.
- 18.Kotsakis A, Georgoulias V. Targeting epidermal growth factor receptor in the treatment of non-small-cell lung cancer. Expert Opin Pharmacother. 2010;11(14):2363–2389. doi: 10.1517/14656566.2010.498581. [DOI] [PubMed] [Google Scholar]
- 19.Pao W, Chmielecki J. Rational, biologically based treatment of EGFRmutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10(11):760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7(4):301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Cripps C, Winquist E, Devries MC, Stys-Norman D, Gilbert R. Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer. Curr Oncol. 2010;17(3):37–48. doi: 10.3747/co.v17i3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu CW, Nozawa H, Hanahan D. Survival benefit with proapoptotic molecular and pathologic responses from dual targeting of mammalian target of rapamycin and epidermal growth factor receptor in a preclinical model of pancreatic neuroendocrine carcinogenesis. J Clin Oncol. 2010;28(29):4425–4433. doi: 10.1200/JCO.2010.28.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77(6):400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 24.Higa GM, Singh V, Abraham J. Biological considerations and clinical applications of new HER2-targeted agents. Expert Rev Anticancer Ther. 2010;10(9):1497–1509. doi: 10.1586/era.10.129. [DOI] [PubMed] [Google Scholar]
- 25.McIntyre E, Blackburn E, Brown PJ, Johnson CG, Gullick WJ. The complete family of epidermal growth factor receptors and their ligands are co-ordinately expressed in breast cancer. Breast Cancer Res Treat. 2010;122(1):105–110. doi: 10.1007/s10549-009-0536-5. [DOI] [PubMed] [Google Scholar]
- 26.Yonemori K, Tsuta K, Shimizu C, et al. Immunohistochemical expression of HER1, HER3, and HER4 in HER2-positive breast cancer patients treated with trastuzumab-containing neoadjuvant chemotherapy. J Surg Oncol. 2010;101(3):222–227. doi: 10.1002/jso.21486. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks BS, Orr G, Wells A, Wiley HS, Lauffenburger DA. Parsing ERK activation reveals quantitatively equivalent contributions from epidermal growth factor receptor and HER2 in human mammary epithelial cells. J Biol Chem. 2005;280(7):6157–6169. doi: 10.1074/jbc.M410491200. [DOI] [PubMed] [Google Scholar]
- 28.Gross ME, Shazer RL, Agus DB. Targeting the HER-kinase axis in cancer. Semin Oncol. 2004;31(1 Suppl 3):9–20. doi: 10.1053/j.seminoncol.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Baulida J, Kraus MH, Alimandi M, Di Fiore PP, Carpenter G. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271(9):5251–5257. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- 30.Tsutsui S, Ohno S, Murakami S, Kataoka A, Kinoshita J, Hachitanda Y. Prognostic value of the combination of epidermal growth factor receptor and c-erbB-2 in breast cancer. Surgery. 2003;133(2):219–221. doi: 10.1067/msy.2003.32. [DOI] [PubMed] [Google Scholar]
- 31.Peng CW, Liu XL, Chen C, et al. Patterns of cancer invasion revealed by QDs-based quantitative multiplexed imaging of tumor microenvironment. Biomaterials. 2011;32(11):2907–2917. doi: 10.1016/j.biomaterials.2010.12.053. [DOI] [PubMed] [Google Scholar]