Abstract
Objectives
Cholesterol-rich nanoemulsions (LDE) bind to low-density lipoprotein (LDL) receptors and after injection into the bloodstream concentrate in aortas of atherosclerotic rabbits. Association of paclitaxel with LDE markedly reduces the lesions. In previous studies, treatment of refractory cancer patients with etoposide associated with LDE had been shown devoid of toxicity. In this study, the ability of etoposide to reduce lesions and inflammatory factors in atherosclerotic rabbits was investigated.
Methods
Eighteen New Zealand rabbits were fed a 1% cholesterol diet for 60 days. Starting from day 30, nine animals were treated with four weekly intravenous injections of etoposide oleate (6 mg/kg) associated with LDE, and nine control animals were treated with saline solution injections.
Results
LDE-etoposide reduced the lesion areas of cholesterol-fed animals by 85% and intima width by 50% and impaired macrophage and smooth muscle cell invasion of the intima. Treatment also markedly reduced the protein expression of lipoprotein receptors (LDL receptor, LDL-related protein-1, cluster of differentiation 36, and scavenger receptor class B member 1), inflammatory cytokines (interleukin-1β and tumor necrosis factor-α), matrix metallopeptidase-9, and cell proliferation markers (topoisomerase IIα and tubulin).
Conclusion
The ability of LDE-etoposide to strongly reduce the lesion area and the inflammatory process warrants the great therapeutic potential of this novel preparation to target the inflammatory-proliferative basic mechanisms of the disease.
Keywords: atherosclerosis treatment, drug delivery, LDL-receptors
Atherosclerosis is currently considered a chronic inflammatory-proliferative disease.1,2 The quest for anti-inflammatory antiproliferative agents that effectively achieve reduction of atherogenesis has been growing.3 Among other strategies, chemotherapeutic agents that are used in cancer treatment have also been tested in experimental models of atherosclerosis. Compounds such as paclitaxel and etoposide were shown to promote reduction of lesions in rabbits made atherosclerotic by high cholesterol feeding.4 However, those agents also convey high toxicity levels that discourage their potential use in cardiovascular therapeutics.
In previous studies, we have shown that a cholesterol-rich nanoemulsion, termed LDE, that roughly resembles the lipidic structure of low-density lipoprotein (LDL) is taken up by the LDL receptors after injection into the bloodstream.5 Although made without protein, in contact with plasma, LDE acquires several exchangeable apolipoproteins, such as apo E that is recognized by LDL receptors or by LDL-related protein (LRP)-1 receptors;6 LDE is then internalized into cells by the receptor-mediated endocytic mechanism. As most cancer cell lines show LDL receptor upregulation, LDE can be used as a vehicle to direct antineoplastic drugs to those cells, as demonstrated in breast and ovary carcinoma tissues7,8 as well as in hematologic cancers such as acute myelocytic leukemia and multiple myeloma.9,10 Association to LDE of drugs such as carmustine, etoposide, and paclitaxel pronouncedly reduces the toxicity of those agents while preserving or even increasing the antineoplastic activity, as shown in tumor-implanted rats and mice.11–13
In a subsequent study, we showed that LDE can also concentrate in the lesioned aortas of cholesterol-fed rabbits after injection into the blood circulation. Treatment of the atherosclerotic rabbits with the association of paclitaxel to LDE resulted in marked reduction of the atherosclerotic lesions.14 Concentration in the lesioned aorta segments occurs because LDL-receptor overexpression is not restricted to neoplastic tissues: it possibly takes place whenever a greater cell input of cholesterol and other lipids for membrane building is required to assist the increased mitosis rates in proliferative processes. Zhu et al observed overexpression of LDL receptors in smooth muscle cells of the hyperplastic intima.15
Reduction of toxicity of chemotherapeutic agents by associating them to LDE was confirmed at clinical level. In trials enrolling patients with advanced cancers treated with LDE-carmustine,10 LDE-paclitaxel,16 and LDE-etoposide,17 the toxicity of high-dose treatments was minimal or absent. Together with the demonstration that LDE can concentrate in atherosclerotic lesions, those findings paved the way for experiments aiming to test the powerful antiproliferative weaponry in atherosclerosis therapeutics.
In this study, the effects of the association to LDE of etoposide on atherosclerotic lesions and on the protein expression of inflammatory markers were tested in cholesterol-fed rabbits. Etoposide is a semisynthetic derivative of podophyllotoxin that causes double strand deoxyribonucleic acid (DNA) brakes by an association with topoisomerase IIα and DNA, leading to cell cycle block and cell death.18 Similarly to paclitaxel, etoposide is poorly incorporated into nanoemulsions. To optimize the yield of the incorporation to LDE and the stability of the formed association, lipophilicity of etoposide was increased by derivatizing the agent with an oleyl group.
Methods
Animals and experimental protocols
Eighteen male New Zealand White rabbits weighing 3.4 ± 0.3 kg (mean ± standard deviation [SD]) were housed in individual cages in a temperature-controlled room (20°C–22°C) and on a 12-hour light–dark cycle during the experimental period. The animals were fed a diet consisting of regular feed with added 1% cholesterol (w/w) for 60 days.
On day 30, animals were separated into two groups. Nine animals were treated with four weekly intravenous injections of LDE-etoposide (6 mg/kg and 30 mg of total lipids), and nine animals received four weekly intravenous saline solution in equivalent volume. One week after the last dose of the treatment, the euthanasia was performed by a lethal intravenous dose of 80 mg/kg of sodium pentobarbital.
The amount of feed was restricted to 150 g. The food intake was assessed daily, and bodyweight was assessed weekly.
This protocol conforms to the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health) and was approved by the Ethics Committee of University of São Paulo.
Plasma lipids and blood cell counting
After overnight fasting, blood samples were taken for determination of total and high-density lipoprotein (HDL) cholesterol and triglycerides by commercial kits (Labtest, São Paulo) and for blood cell count.
Preparation of LDE and association of etoposide to LDE
LDE was prepared according to the method described by Maranhão et al.19 A lipid mixture of 40 mg cholesteryl oleate, 20 mg egg phosphatidylcholine, 1 mg triolein, and 0.5 mg cholesterol was emulsified in aqueous medium by prolonged ultrasonic irradiation for 3 hours. A two-step ultracentrifugation (195,000 × g for 30 minutes followed by 195,000 × g for 120 minutes in a TH 641 [Thermo Scientific, Waltham, MA] rotor at 4°C) of the crude emulsion with density adjustment by addition of solid KBr was done to obtain the LDE nanoemulsion. LDE was dialyzed overnight against 2 L of 10 mM tris-HCl buffer pH 8.0 for KBr extraction, and sterilized by passing through a 0.22 μm filter.
Etoposide (6 mg) was associated to nanoemulsion (1 mL, 30 mg of total lipids) by solubilization of etoposide in ethanol (10% v:v).20 Etoposide was added to the emulsion. The solution was sonicated for 1 hour at 70°C using a Sonifier® 450 (Branson Ultrasonics, Danbury, CT), equipped with a 1 cm flat titanium probe. The resultant mixture was centrifuged at 3,500 rpm for 15 minutes to separate free etoposide. Etoposide loaded nanoemulsion was then passed through 0.22 μm pore filter and kept at 4°C. The amount of drug associated to the nanoemulsion was always measured by high-performance liquid chromatography before injection. The yield of association was 85% and the particle diameter, measured by laser light scattering, was about 60 nm.
Analysis of lesion areas
Aorta was excised from the aortic arch to the abdominal artery, opened longitudinally, washed with saline, and placed in 10% buffered formalin. After fixation, the aorta was stained in Scarlet R, and pictures were taken to measure the lesions. The aortic arch was sectioned in 5 mm segments and embedded in paraffin. Sections taken for each segment were stained in hematoxylin-eosin for total area measurement. Additional sections were stained with anti-LRP-1 (Calbiochem, Darmstadt, Germany), anti-LDL receptor (Lifespan, Seattle, WA), anti-rabbit macrophage (RAM-11 clone) (Dako, Carpinteria, CA), anti-matrix metallopeptidase 9 (MMP9) (AbCam, Cambridge, MA), anti-tumor necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN), anti-interleukin (IL)-1β (R&D Systems), anti-tubulin (AbCam), anti-scavenger receptor class B member 1 (SR-B1) (Millipore, Billerica, MA), anti-proliferating cell nuclear antigen (PCNA) (AbCam), anti-cluster of differentiation 36 (CD36) (Genetex, Irvine, CA), anti-α actin (Dako), and anti-topoisomerase IIα (AbCam). All measurements were performed using Leica QWin Image Analysis Software (Leica Microsystems, Wetzlar, Germany).
Statistical analysis
Comparison between groups of lipid and hematological profiles and bodyweight was assessed by one-way analysis of variance with Tukey post test. All other analysis was assessed using the Student’s t-test. In all analysis, difference of two-tailed P below 0.05 was considered statistically significant. Values were expressed as mean ± SD.
Results
Plasma lipids
As shown in Table 1, total cholesterol concentration in the cholesterol fed rabbits increased 20-fold in the group treated with LDE-etoposide and tenfold in the control group from baseline to the end of the 8th week of the cholesterol feeding period. In contrast, HDL cholesterol was unchanged in both groups. After the 8-week period, triglyceride values were increased by the diet about tenfold in both groups. It is worthwhile to point out that there were no differences in the plasma lipids when controls and LDE-etoposide treated rabbits were compared at baseline and at 8 weeks.
Table 1.
Serum lipids of rabbits treated with LDE-etoposide (6 mg/kg body weight/week) or saline solution (control group), for 8 weeks
| Control (n = 9) | LDE-etoposide (n = 9) | |||
|---|---|---|---|---|
| Baseline | 8th week | Baseline | 8th week | |
| Total Cholesterol | 42 ± 29 | 1369 ± 279a | 36 ± 25 | 2250 ± 321a,b |
| HDL-Cholesterol | 13 ± 8 | 18 ± 8 | 12 ± 4 | 19 ± 6 |
| Triglycerides | 53 ± 33 | 354 ± 231c | 35 ± 48 | 694 ± 234a |
Notes: Data are expressed as mean ± SD.
P < 0.001 versus baseline;
P < 0.001 versus 8th week control;
P < 0.05 versus baseline.
Toxicity evaluation
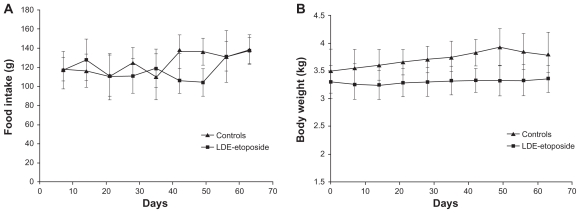
Compared with baseline, after the 8-week experimental period the food intake increased by 15% in both LDE-etoposide and control groups (Figure 1), but the bodyweight was unchanged.
Figure 1.
Food intake (A) and bodyweight (B) of rabbits treated with LDE-etoposide or saline solution (control group) over an 8-week period.
Abbreviation: LDE, cholesterol-rich nanoemulsion.
The red blood cell count of the animals treated with LDE-etoposide fell by 50% and was not significantly altered in the control group. On the other hand, leukocyte count did not diminish in both LDE-etoposide and control groups as shown in Table 2.
Table 2.
Hematological profile of rabbits treated with LDE-etoposide (6 mg/kg body weight/week) or saline solution (control group), for 8 weeks
| Control (n = 9) | LDE-etoposide (n = 9) | |||
|---|---|---|---|---|
| Baseline | 8th week | Baseline | 8th week | |
| Red blood cells (109/mL) | 5.7 ± 1.0 | 4.4 ± 1.6 | 6.0 ± 1.0 | 2.9 ± 0.8a,b |
| Leuckocytes (106/mL) | 6.6 ± 1.0 | 7.6 ± 3.1 | 7.6 ± 3.5 | 6.9 ± 2.9 |
| Lymphocytes (%) | 72.8 ± 4.7 | 62.7 ± 11.6 | 68.2 ± 7.9 | 73.7 ± 9.9 |
| Monocytes (%) | 5.6 ± 5.0 | 10.8 ± 5.4 | 7.0 ± 3.9 | 5.9 ± 3.7 |
| Neutrophils (%) | 18.9 ± 9.9 | 26.6 ± 10.2 | 25.9 ± 7.4 | 20.4 ± 9.3 |
Notes: Data are expressed as mean ± SD.
P < 0.001 versus baseline;
P < 0.001 versus 8th week control.
Evaluation of atherosclerotic lesions
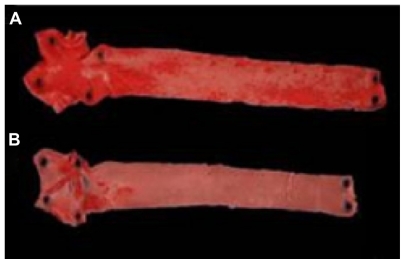
Figure 2 shows the aorta of a rabbit treated with LDE-etoposide and that of a control rabbit. By comparing against the controls, LDE-etoposide treatment reduced the lesions by 85% as analyzed by macroscopic morphometry, as shown in Table 3.
Figure 2.
Aortas of rabbits treated with (A) saline solution (control group) or (B) LDE-etoposide, for 8 weeks, stained by Scarlet Red.
Abbreviation: LDE, cholesterol-rich nanoemulsion.
Table 3.
Macroscopic morphometry of aorta in rabbits treated with LDE-etoposide (6 mg/kg body weight/week) or saline solution (control group), for 8 weeks
| Control (n = 9) | LDE-etoposide (n = 9) | |
|---|---|---|
| Total area of aorta (pixel2 × 106) | 0.54 ± 0.44 | 1.05 ± 0.17 |
| Lesion area (pixel2 × 106) | 0.40 ± 0.32 | 0.06 ± 0.04 |
| Lesion/total area | 0.68 ± 0.17 | 0.06 ± 0.04a |
Notes: Data are expressed as mean ± SD.
P < 0.001 versus control.
Table 4 presents the results of the microscopic analysis of segments of the aortic arch of rabbits treated with LDE-etoposide and those of controls. The computer-assisted microscopic morphometric analysis showed that the LDE-etoposide reduced the intima layer area by 75%. LDE-etoposide treatment did not affect the area of the media layer.
Table 4.
Microscopic morphometry (intima and media layers) of aorta of rabbits treated with LDE-etoposide (6 mg/kg body weight/week) or saline solution (control group), for 8 weeks
| Control (n = 9) | LDE-etoposide (n = 9) | |
|---|---|---|
| Total area (μm2) (×103) | 0.40 ± 0.05 | 0.30 ± 0.02a |
| Media layer area (μm2) (×103) | 0.30 ± 0.04 | 0.28 ± 0.01 |
| Intima layer area (μm2) (×103) | 0.10 ± 0.01 | 0.03 ± 0,01b |
| Intima/media area | 0.30 ± 0.04 | 0.10 ± 0.03a |
Notes: Data are expressed as mean ± SD.
P < 0.01;
P < 0.001 versus control.
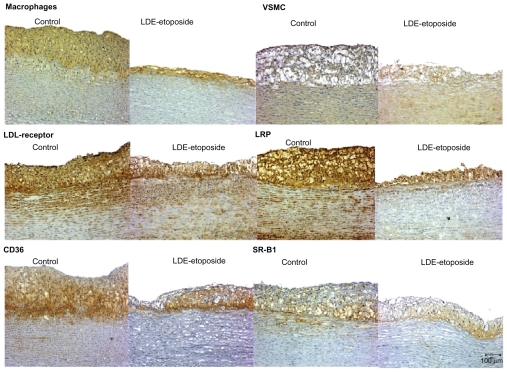
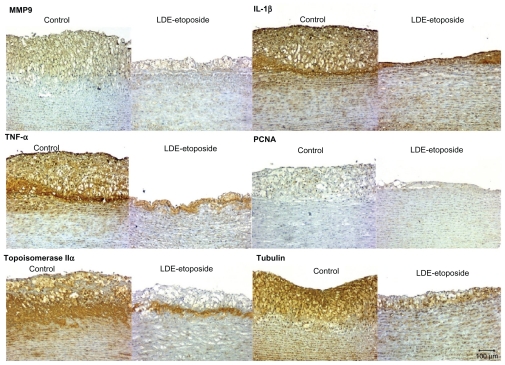
Figures 3 and 4 show the photomicrographs of stained intimal areas of aortic arch tissues stained for different antibodies. In Table 5, there are the percentages of stained areas of each one of the antibody labels calculated by a computer assisted morphometry technique.
Figure 3.
Immunohistochemistry of artery tissues from rabbits treated with saline solution (control group) or LDE-etoposide. Photomicrographs of diaminobenzidine chromogen immunostaining for macrophages, VSMC, LDL receptor, LRP-1, CD36, and SR-B1. Magnifications: 100×.
Abbreviations: CD36, cluster of differentiation 36; LDE, cholesterol-rich nanoemulsion; LDL, low-density lipoprotein; LRP, LDL-related protein; SR-B1, scavenger receptor class B member 1; VSMC, vascular smooth muscle cells.
Figure 4.
Immunohistochemistry of artery tissues from rabbits treated with saline solution (control group) or LDE-etoposide. Photomicrographs of diaminobenzidine chromogen immunostaining for MMP9, IL-1β, TNF-α, PCNA, topoisomerase IIα, tubulin. Magnifications: 100×.
Abbreviations: IL, interleukin; LDE, cholesterol-rich nanoemulsion; MMP9, matrix metallopeptidase 9; PCNA, proliferating cell nuclear antigen; TNF, tumor necrosis factor.
Table 5.
Protein expression (%) of inflammation and proliferation markers and lipoprotein receptors, evaluated by immunohistochemistry of aorta of rabbits treated with LDE-etoposide (6 mg/kg body weight/week) or saline solution (control group), for 8 weeks
| Control (n = 9) | LDE-etoposide (n = 9) | |
|---|---|---|
| RAM-11 | 67.73 ± 13.03 | 26.96 ± 28.46b |
| VSMC | 27.52 ± 10.63 | 7.89 ± 9.06a |
| IL-1β | 55.41 ± 17.82 | 24.82 ± 22.69b |
| TNF-α | 20.04 ± 15.77 | 5.57 ± 6.42c |
| MMP-9 | 14.98 ± 8.59 | 6.44 ± 8.41a |
| PCNA | 3.53 ± 3.90 | 4.51 ± 4.55 |
| Topoisomerase IIα | 42.46 ± 15.77 | 8.51 ± 10.35c |
| Tubulin | 47.40 ± 15.38 | 16.01 ± 19.82b |
| LDL receptor | 35.13 ± 20.13 | 11.28 ± 16.80a |
| LRP-1 | 41.94 ± 21.35 | 5.11 ± 9.92c |
| CD-36 | 40.13 ± 15.26 | 8.60 ± 13.54c |
| SR-B1 | 19.86 ± 8.26 | 4.87 ± 8.65b |
Notes: Data are expressed as mean ± SD.
P < 0.05 versus control;
P < 0.01 versus control;
P < 0.001 versus control.
LDE-etoposide treatment reduced macrophage presence by 50% (67.73% ± 13.03% of stained area in controls and 26.96% ± 28.46% in LDE-etoposide treated rabbits, P < 0.01). The percentage of stained area by vascular smooth muscle cells (VSMCs) was reduced by 60% (27.52% ± 10.63% in the control group and 7.89% ± 9.06% in the LDE-etoposide group, P < 0.05).
LDE-etoposide also reduced the expression of the LDL receptors by 60% (35.13% ± 20.13% in the control group and 11.28% ± 16.80% in the LDE-etoposide group, P < 0.05) and of the LRP-1 receptor by 90% (41.94% ± 21.35% in the control group and 5.11% ± 9.92% in the LDE-etoposide group, P < 0.001). In respect to the expression of scavenger receptors, LDE-etoposide reduced CD36 by 80% (40.13% ± 15.26% in the control group and 8.60% ± 13.54% in the LDE-etoposide group, P < 0.001) and SR-B1 by 80% (19.86% ± 8.26% in the controls and 4.87% ± 8.65% in the LDE-etoposide treated rabbits, P < 0.01).
Regarding the expression of inflammatory cytokines, IL-1β protein was reduced by 50% (55.41% ± 17.82% in the control group and 24.82% ± 22.69% in the LDE-etoposide group, P < 0.01) and TNF-α by 75% (20.04% ± 15.77% in the control group and 5.57% ± 6.42% in the LDE-etoposide group, P < 0.001). MMP9 protein expression was reduced by 50% (14.98% ± 8.59% in the control group and 6.44% ± 8.41% in the LDE-etoposide group, P < 0.05). As to the expression of proteins related with cell proliferation, tubulin was reduced by 80% (47.40% ± 15.38% in the control group and 16.01% ± 19.82% in the LDE-etoposide group, P < 0.01) and topoisomerase IIα by 60% (42.46% ± 15.77% in the control group and 8.51% ± 10.35% of stained area in the LDE-etoposide group, P < 0.001); PCNA was equal in LDE-etoposide treated and control animals.
Discussion
In this study, it was shown that in aortas of cholesterol-fed rabbits, LDE-etoposide at the 6 mg/kg bw weekly dose for 4 weeks was able to reduce by 85% the total lesion area as measured by macroscopic morphometry. De la Llera-Moya et al21 reported that the treatment of cholesterol-fed rabbits with a conventional commercial etoposide preparation at a much larger dose (40 mg/kg bw/week dose administered for 8 weeks) than that used in this present study resulted in a 50% lesion area reduction, which is a very marked difference in effectiveness of the treatments.
Differently from the protocol used in this present study, the etoposide treatment was introduced 1 week before the beginning of the cholesterol feeding, which leads to a preventive rather than curative treatment approach. Anyhow, the largely known toxicity spectrum of etoposide, which encompasses myelosuppression, gastrointestinal symptoms, hypotension, and allergic reactions prohibits the introduction of this drug in the vehicle used in the commercial formulation in the cardiology field.22
In this study, anemia was the only observed toxicity documented in the animals after the LDE-etoposide treatment. Other toxicities under scrutiny here, such as weight loss, diminished food intake, leukopenia, and clinically observable alterations, were absent. In fact, in the classical toxicological tests conducted in mice, the toxicity of etoposide was pronouncedly reduced by association with LDE. The novel formulation showed maximum tolerance dose and 50% lethal dose roughly five times that of the commercial etoposide formulation.12,20 Moreover, the toxicity of LDE-etoposide was also tested in a small safety study in two patients with refractory lymphoma. In the etoposide, 300 mg/m2 dose/3 weeks, administered over 6 cycles.17 This dose scheme corresponds to those routinely used in the clinical oncology practice. During the treatment, no clinical and laboratorial toxicities, including anemia or other myelotoxicities, were observed in both patients. Interestingly, anemia not accompanied by leucopenia was the only prominent toxicity found in atherosclerotic rabbits treated with commercial etoposide reported by De la Llera-Moya et al.21 Apparently, this may be a species-related toxicity trait that, as we have shown in our previous studies, did not appear in our LDE-etoposide treated patients.17
In patients with ovarian carcinoma, the plasma decay curve of etoposide associated with LDE was markedly slower than that of the commercial formulation, as shown by Azevedo et al.23 In that study, it was shown that etoposide oleate did not dissociate from LDE while in the blood stream. LDE-etoposide pharmacokinetic parameters favor the novel formulation, since extension of half-life of etoposide achieved by the association to LDE may be an interesting feature in favoring the pharmacological action in chronic diseases.
The antiatherosclerotic effects of LDE-etoposide, at the order of 85% lesion area reduction, were apparently greater than those of LDE-paclitaxel described in a previous study. LDE-paclitaxel achieved a 60% reduction of the lesion area.14 By performing statistical analysis with the data obtained in the two studies, this difference was found significant at P = 0.02.
Similarly to the LDE-paclitaxel experiment in a previous study by the same authors,14 LDE-etoposide could not only inhibit the VSMC proliferation and invasion of the intima but also the massive macrophage presence in the intima. This results in the reduction of the intima width that was statistically equal to that observed in the LDE-paclitaxel treatments. In respect to the media, thickening of this arterial layer is not involved in atherogenesis, so the finding that LDE-etoposide treatment did not change media width was indeed expected.
The finding of overall reduction of the protein expression of the receptors tested here, namely LDL receptor, LRP-1, CD36, and SR-B1, by LDE-etoposide may simply mirror the decrease in the cellularity of the intima, rather than diminution of receptor number/cell. Apparently, the effect of treatment was greater on the expression of LRP-1 than on the expression of the other receptors, a finding that could suggest a selective effect on the receptor rather than diminution of cell number. LDL receptors are typically found in VSMCs, whereas CD36 is characteristically a macrophage receptor. 24 The fact that the protein expression of both receptors diminished equally reflects the post-treatment diminution of both VSMC and macrophages that was also equal.
The evaluation of pro-inflammatory cytokines IL-1β and TNF-α showed that LDE-etoposide treatment was effective in impairing the protein expression of both. IL-1β is primarily a macrophage-secreted cytokine, whereas TNF-α is produced by diverse cell lines present in the inflammation site.25 Impairment of cytokine production can be also related with the diminution of their secreting cells by treatment. MMP9 protein expression was also diminished by the treatment. It is produced by macrophages during inflammation. The secretion of inflammatory cytokines impairs the production of collagen and other structural proteins by VMSC and increases the expression of MMP9 and other metalloproteinases.2 Therefore, the treatment with the novel etoposide formulation promotes an overall inhibition of pro-inflammatory proteins.
The protein expression of two of the three cell proliferation markers analyzed here was inhibited by LDE-etoposide treatment, namely the structural protein tubulin that is overexpressed during mitosis26 and topoisomerase IIα, that is overexpressed during DNA synthesis.27 Those results convey the strong effect of the treatment that consisted in the inhibition of the massive invasion of the intima by macrophages and VSMCs. Unexpectedly, the treatment did not inhibit PCNA which is a classical marker of DNA synthesis.28 It was worthwhile to point out, however, that the expression of this protein was low in the control group.
Etoposide stabilizes the complex formed between DNA and topoisomerase II, which leads to accumulation of strand breaks in DNA. Etoposide treatment prevents cells from entering mitosis, thus slowing down the progression of the cell cycle, with arrest in G2 phase.29 However, the effects of the drug in nonneoplastic proliferating tissues and inflammatory sites are largely unexplored. The antiproliferative effect of etoposide may account for the decrease in atheroma formation in the treated rabbits by proliferation inhibition of smooth muscle cells, macrophages, and macrophage-derived foam cells.21
In conclusion, LDE-etoposide was effective in reducing the rabbit atherosclerotic lesions by strongly diminishing the proliferation in the intima of VSMC and macrophages, decreasing the production of pro-inflammatory factors such as IL-1β, TNF-α, and MMP9. Lipoprotein receptors and proliferation markers were also inhibited by the treatment. Because the safety of LDE-etoposide treatment has already been shown not only in animal experiments but also in a pilot clinical study, with excellent tolerability, this novel formulation of etoposide can offer a promising strategy for the treatment of atherosclerotic cardiovascular disease.
Acknowledgments
This study was supported by Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP), São Paulo, Brazil. Dr Maranhão has a Research Award from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasilia, Brazil.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;14;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010;74(2):213–220. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- 4.Kolodgie FD, John M, Khurana C, Farb A, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002;106(10):1195–1198. doi: 10.1161/01.cir.0000032141.31476.15. [DOI] [PubMed] [Google Scholar]
- 5.Maranhão RC, Roland IA, Toffoletto O, et al. Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids. 1997;32(6):627–633. doi: 10.1007/s11745-997-0080-6. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz J, Kouiavskaia D, Migliorini M, et al. The apoE isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res. 2005;46(8):1721–1731. doi: 10.1194/jlr.M500114-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Graziani SR, Igreja FA, Hegg R, et al. Uptake of a cholesterol-rich emulsion by breast cancer. Ginecol Oncol. 2002;85:493–497. doi: 10.1006/gyno.2002.6654. [DOI] [PubMed] [Google Scholar]
- 8.Ades A, Carvalho JP, Graziani SR, et al. Uptake of a cholesterol-rich emulsion by neoplasic ovarian tissues. Gynecol Oncol. 2001;81(1):84–87. doi: 10.1006/gyno.2001.6203. [DOI] [PubMed] [Google Scholar]
- 9.Maranhão RC, Garicochea B, Silva EL, et al. Plasma kinetics and biodistribution of a lipid emulsion resembling low-density lipoprotein in patients with acute leukemia. Cancer Res. 1994;54:4660–4666. [PubMed] [Google Scholar]
- 10.Hungria VT, Latrilha MC, Rodrigues DG, Bydlowski SP, Chiattone CS, Maranhão RC. Metabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and a preliminary clinical study of LDE as a drug vehicle for treatment of the disease. Cancer Chemother Pharmacol. 2004;53:51–60. doi: 10.1007/s00280-003-0692-y. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira RS, Curi R, Maranhão RC. Effects on Walker 256 tumor of carmustine associated with a cholesterol-rich microemulsion (LDE) J Pharm Pharmacol. 2004;56(7):909–914. doi: 10.1211/0022357023826. [DOI] [PubMed] [Google Scholar]
- 12.Lo Prete A, Maria DA, Rodrigues DG, Valduga CJ, Ibanez OCM, Maranhão RC. Evaluation in melanoma-bearing mice of an etoposide derivative associated to a cholesterol-rich nanoemulsion. J Pharm Pharmacol. 2006;58(6):801–808. doi: 10.1211/jpp.58.6.0010. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues DG, Maria DA, Fernandes DC, et al. Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol. 2005;55(6):565–576. doi: 10.1007/s00280-004-0930-y. [DOI] [PubMed] [Google Scholar]
- 14.Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD. Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis. 2008;197(2):959–966. doi: 10.1016/j.atherosclerosis.2007.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Bujo H, Yamazaki H, et al. Enhanced expression of the LDL receptor family member LR11 increases migration of smooth muscle cells in vitro. Circulation. 2002;105(15):1830–1836. doi: 10.1161/01.cir.0000014413.91312.ef. [DOI] [PubMed] [Google Scholar]
- 16.Pires LA, Hegg R, Valduga CJ, Graziani SR, Rodrigues DG, Maranhão RC. Use of cholesterol-rich nanoparticles that bind to lipoprotein receptors as a vehicle to paclitaxel in the treatment of breast cancer: pharmacokinetics, tumor uptake and a pilot clinical study. Cancer Chemother Pharmacol. 2009;63(2):281–287. doi: 10.1007/s00280-008-0738-2. [DOI] [PubMed] [Google Scholar]
- 17.Pinheiro KV, Hungria VT, Ficker ES, Valduga CJ, Mesquita CH, Maranhão RC. Plasma kinetics of a cholesterol-rich microemulsion (LDE) in patients with Hodgkin’s and non-Hodgkin’s lymphoma and a preliminary study on the toxicity of etoposide associated with LDE. Cancer Chemother Pharmacol. 2006;57(5):624–630. doi: 10.1007/s00280-005-0090-8. [DOI] [PubMed] [Google Scholar]
- 18.Engel R, Valkov NI, Gump JL, Hazlehurst L, Dalton WS, Sullivan DM. The cytoplasmic trafficking of DNA topoisomerase II alpha correlates with etoposide resistance in human myeloma cells. Exp Cell Res. 2004;295(2):421–431. doi: 10.1016/j.yexcr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Maranhão RC, Cesar TB, Pedroso-Mariani SR, Hirata MH, Mesquita CH. Metabolic behavior in rats of a non-protein microemulsion resembling low density lipoprotein. Lipids. 1993;28:691–696. doi: 10.1007/BF02535988. [DOI] [PubMed] [Google Scholar]
- 20.Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CH, Rodrigues DG, Maranhão RC. Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol. 2003;55(12):1615–1622. doi: 10.1211/0022357022232. [DOI] [PubMed] [Google Scholar]
- 21.de la Llera-Moya M, Rothblat GH, Glick JM, England JM. Etoposide treatment suppresses atherosclerotic plaque development in cholesterolfed rabbits. Arterioscler Thromb. 1992;12(11):1363–1370. doi: 10.1161/01.atv.12.11.1363. [DOI] [PubMed] [Google Scholar]
- 22.Kancherla RR, Nair JS, Ahmed T, et al. Evaluation of topotecan and etoposide for non-Hodgkin lymphoma: correlation of topoisomerase-DNA complex formation with clinical response. Cancer. 2001;91(3):463–471. doi: 10.1002/1097-0142(20010201)91:3<463::aid-cncr1023>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 23.Azevedo CH, Carvalho JP, Valduga CJ, Maranhão RC. Plasma kinetics and uptake by the tumor of a cholesterol-rich microemulsion (LDE) associated to etoposide oleate in patients with ovarian carcinoma. Gynecol Oncol. 2005;97(1):178–182. doi: 10.1016/j.ygyno.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res. 2007;75(3):468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79(3):360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez EA. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol Cancer Ther. 2009;8(8):2086–2095. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- 27.Willman JH, Holden JA. Immunohistochemical staining for DNA topoisomerase II-alpha in benign, premalignant, and malignant lesions of the prostate. Prostate. 2000;42(4):280–286. doi: 10.1002/(sici)1097-0045(20000301)42:4<280::aid-pros5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 28.Beresford MJ, Wilson GD, Makris A. Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res. 2006;8(6):216. doi: 10.1186/bcr1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meresse P, Dechaux E, Monneret C, Bertounesque E. Etoposide: discovery and medicinal chemistry. Curr Med Chem. 2004;11(18):2443–2466. doi: 10.2174/0929867043364531. [DOI] [PubMed] [Google Scholar]